Topical Administration of a Mixed Microbial Culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cerevisiae Significantly Inhibits the Development of Atopic Dermatitis in a Mouse Model Through IL-10 Overexpression by Dendritic Cells
Abstract
1. Introduction
2. Materials and Methods
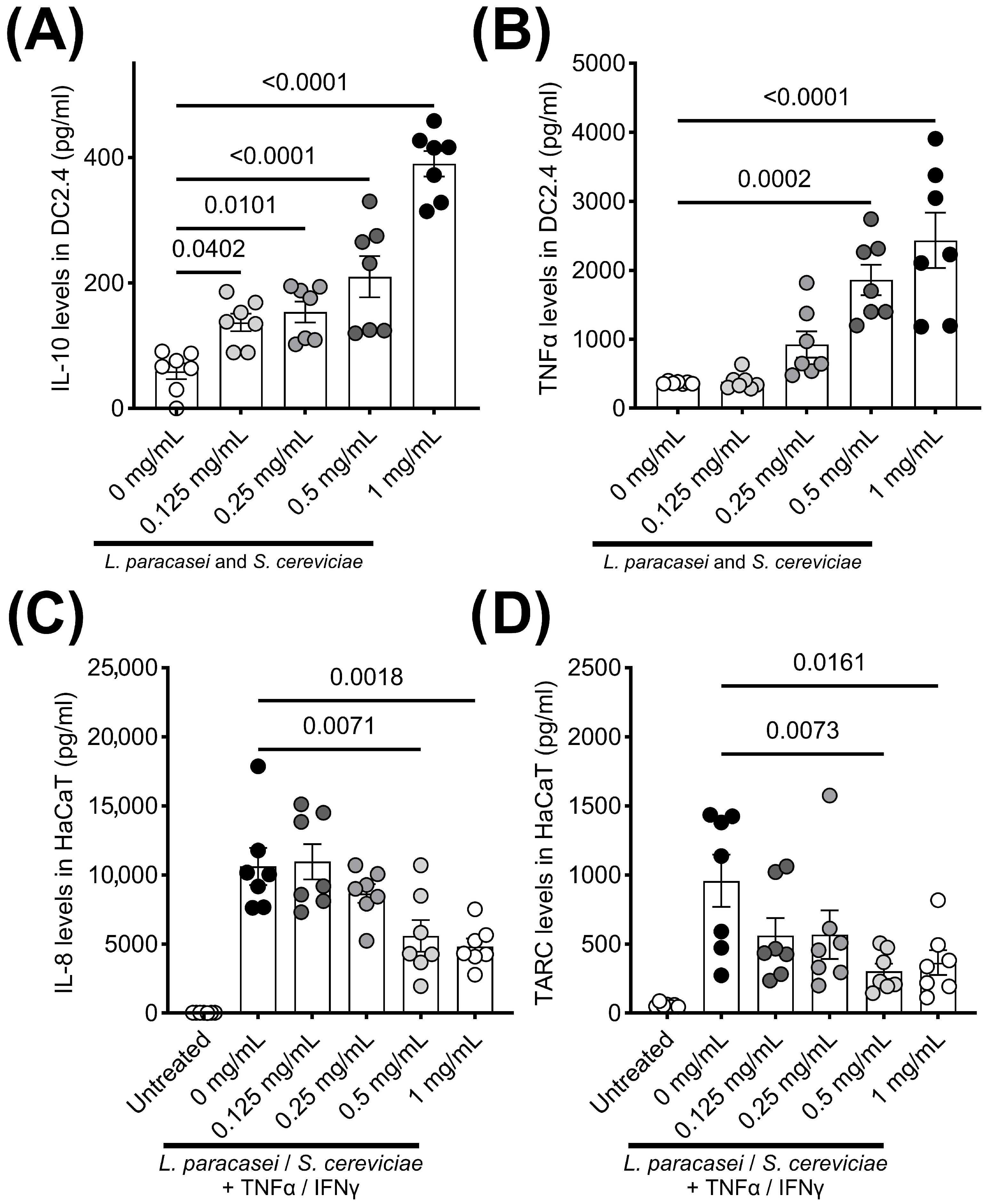
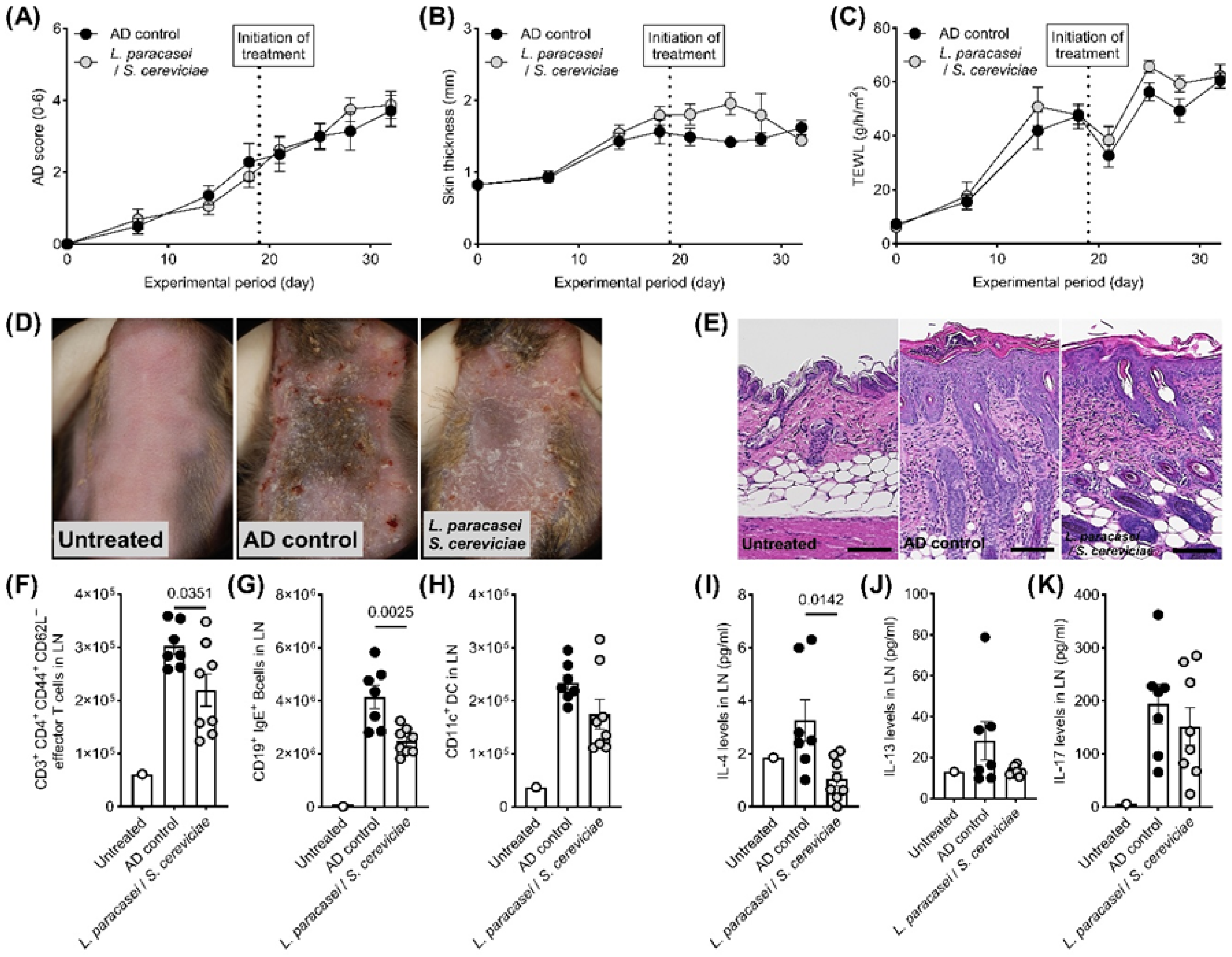
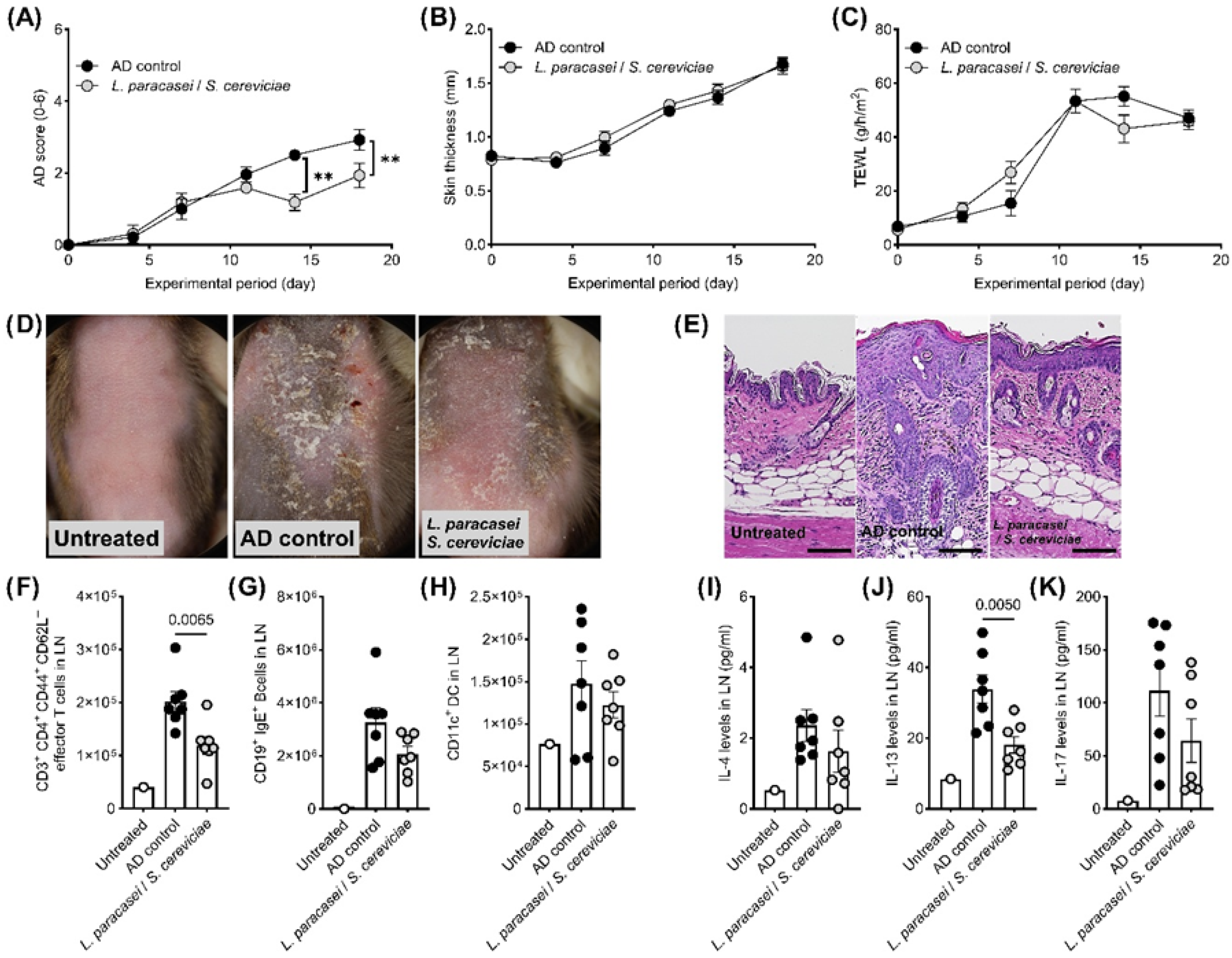
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| ANOVA | Analysis of variance |
| DCs | Dendritic cells |
| FCS | Fetal calf serum |
| HaCaT | Human epidermal keratinocytes |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| LN | Lymph nodes |
| LS | Mixed microbial culture of Lactobacillus paracasei, Pichia membranifaciens, and Saccharomyces cerevisiae |
| SEM | Standard error of the mean |
| TARC | Thymus and activation-regulated chemokine |
| TEWL | Transepidermal water loss |
| TNF | Tumor necrosis factor |
References
- Li, W.; Li, A. Exploring the causal relationship between gut microbiota and atopic dermatitis: A Mendelian randomization study. Medicine 2024, 103, e40193. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Qu, L. The changes of intestinal flora and metabolites in atopic dermatitis mice. Front. Microbiol. 2024, 15, 1462491. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Kunstner, A.; Wohlers, I.; Olbrich, M.; Lenfers, T.; Osumi, T.; Shimazaki, Y.; Nishifuji, K.; Ibrahim, S.M.; Watson, A.; et al. A comprehensive analysis of gut and skin microbiota in canine atopic dermatitis in Shiba Inu dogs. Microbiome 2023, 11, 232. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L. The impact of prebiotics, probiotics and synbiotics on the prevention and treatment of atopic dermatitis in children: An umbrella meta-analysis. Front. Pediatr. 2025, 13, 1498965. [Google Scholar] [CrossRef]
- Xi, Z.; Fenglin, X.; Yun, Z.; Chunrong, L. Efficacy of probiotics in the treatment of allergic diseases: A meta-analysis. Front. Nutr. 2025, 12, 1502390. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Steinhoff, M. Meta-analysis on preventive and therapeutic effects of probiotic supplementation in infant atopic dermatitis. J. Dtsch. Dermatol. Ges. 2023, 21, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Puisto, R.; Gomez-Gallego, C.; Collado, M.C.; Turta, O.; Isolauri, E.; Rautava, S. The Role of Infant Gut Microbiota Modulation by Perinatal Maternal Probiotic Intervention in Atopic Eczema Risk Reduction. Neonatology 2025, 122, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Park, H.J.; Kim, Y.K.; Choi, Y.; Park, H.S. Lactobacillus paracasei-derived extracellular vesicles alleviate neutrophilic asthma by inhibiting the JNK pathway in airway epithelium. Allergol. Int. 2024, 73, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Song, X.; Shu, T.; Zhang, S.; Zhang, Z.; Hu, C.; Pan, J.; Dai, X.; Hao, H.; Xiao, G.; et al. Prevention and alleviation of allergic rhinitis by oral administration of Lacticaseibacillus paracasei GOLDGUT-Lpc969. Front. Immunol. 2024, 15, 1444778. [Google Scholar] [CrossRef]
- Li, Y.; Aoki, T.; Iwabuchi, S.; Arai, S.; Iwabuchi, N.; Motobayashi, H.; Tanaka, M.; Hashimoto, S. Immunomodulatory activity of heat-killed Lacticaseibacillus paracasei MCC1849 based on the activation of plasmacytoid dendritic cells in the peripheral blood of healthy adults. Food Sci. Nutr. 2024, 12, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Arai, S.; Sato, S.; Iwabuchi, N.; Takara, T.; Tanaka, M. Effects of Heat-Killed Lacticaseibacillus paracasei MCC1849 on Immune Parameters in Healthy Adults-A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2024, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Morikawa, M.; Yamamoto-Fujimura, M.; Iwata, A.; Maki, A.; Kato-Nagaoka, N.; Oana, K.; Kiyoshima-Shibata, J.; Matsuura, Y.; Kaji, R.; et al. Diverse impact of a probiotic strain, Lacticaseibacillus paracasei Shirota, on peripheral mononuclear phagocytic cells in healthy Japanese office workers: A randomized, double-blind, controlled trial. Biosci Microbiota Food Health 2023, 42, 65–72. [Google Scholar] [CrossRef]
- Wiese-Szadkowska, M.; Helmin-Basa, A.; Eljaszewicz, A.; Gackowska, L.; Januszewska, M.; Motyl, I.; Andryszczyk, M.; Wieczynska, J.; Michalkiewicz, J. Selected commensal bacteria change profiles of Helicobacter pylori-induced T cells via dendritic cell modulation. Helicobacter 2019, 24, e12614. [Google Scholar] [CrossRef] [PubMed]
- Mileti, E.; Matteoli, G.; Iliev, I.D.; Rescigno, M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: Prediction for in vivo efficacy. PLoS ONE 2009, 4, e7056. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Segre, J.A. Skin microbiome and dermatologic disorders. J. Clin. Investig. 2025, 135, e184315. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Blanchet-Rethore, S.; Bourdes, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef]
- La Colla, L.; Mangano, A.; Mangano, A.; Albertin, A. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled clinical study. Does this make a real difference? Br. J. Dermatol. 2009, 161, 477–478; author reply 478–479. [Google Scholar] [CrossRef]
- Gueniche, A.; Knaudt, B.; Schuck, E.; Volz, T.; Bastien, P.; Martin, R.; Rocken, M.; Breton, L.; Biedermann, T. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled clinical study. Br. J. Dermatol. 2008, 159, 1357–1363. [Google Scholar] [CrossRef]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef]
- Flint, E.; Ahmad, N.; Rowland, K.; Hildebolt, C.; Raskin, D. Topical Probiotics Reduce Atopic Dermatitis Severity: A Systematic Review and Meta-Analysis of Double-Blind, Randomized, Placebo-Controlled Trials. Cureus 2024, 16, e70001. [Google Scholar] [CrossRef]
- Kodama, H.; Nakamura, H.; Kashima, M.; Iwasaki, T.; Togase, H. Protection against atypical Aeromonas salmonicida infection in common carp, Cyprinus carpio L., by oral administration of a mixed microbial culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cereviciae. J. Vet. Med. Sci. 2011, 73, 1319–1325. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alonso, A.; Marquina, D.; Santos, A. The Biology of Pichia membranifaciens Killer Toxins. Toxins 2017, 9, 112. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Beppu, Y.; Izumo, T.; Horii, Y.; Shen, J.; Fujisaki, Y.; Nakashima, T.; Tsuruoka, N.; Nagai, K. Effects of culture supernatant from Lactobacillus pentosus strain S-PT84 on autonomic nerve activity in rats. In Vivo 2012, 26, 355–359. [Google Scholar] [PubMed]
- Cho, B.S.; Kim, S.B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Attenuate Atopic Dermatitis. Animals 2023, 13, 2215. [Google Scholar] [CrossRef] [PubMed]
- Kaneki, M.; Ohira, C.; Takahashi, M.; Iwashita, N.; Takagi, Y.; Nagane, M.; Uchiyama, J.; Fukuyama, T. Therapeutic potential of ozone water treatment in alleviating atopic dermatitis symptoms in mouse models: Exploring its bactericidal and direct anti-inflammatory properties. Int. Immunopharmacol. 2023, 124, 110920. [Google Scholar] [CrossRef]
- Aihara, R.; Ookawara, T.; Morimoto, A.; Iwashita, N.; Takagi, Y.; Miyasaka, A.; Kushiro, M.; Miyake, S.; Fukuyama, T. Acute and subacute oral administration of mycotoxin deoxynivalenol exacerbates the pro-inflammatory and pro-pruritic responses in a mouse model of allergic dermatitis. Arch. Toxicol. 2020, 94, 4197–4207. [Google Scholar] [CrossRef] [PubMed]
- Ookawara, T.; Aihara, R.; Morimoto, A.; Iwashita, N.; Kurata, K.; Takagi, Y.; Miyasaka, A.; Kushiro, M.; Miyake, S.; Fukuyama, T. Acute and subacute oral toxicity of deoxynivalenol exposure in a Dermatophagoides farinae induced murine asthma model. Toxicol. Sci. 2020, 179, 229–240. [Google Scholar] [CrossRef]
- Joo, H.; Baert, L.; Yang, A.; Duluc, D.; Yi, J.; Oh, S. Dendritic cells in the human vaginal mucosa can direct CD4(+) T cell responses by expressing surface OX40L. Front. Immunol. 2025, 16, 1657115. [Google Scholar] [CrossRef]
- Nakane, A.; Subsomwong, P.; Takahashi, T.; Ito, K.; Asano, K. Salmon nasal cartilage proteoglycan up-regulates Listeria monocytogenes-mediated immune response in mice. Curr. Res. Microb. Sci. 2025, 9, 100465. [Google Scholar] [CrossRef] [PubMed]
| Untreated (n = 1) | AD Control (n = 7) | L. paracasei and S. cereviciae (n = 8) | |
|---|---|---|---|
| Therapeutic setting | |||
| Epidermis | |||
| Parakeratosis | 0.00 ± 0.00 | 1.00 ± 0.00 | 0.75 ± 0.16 |
| Hyperplasia in keratinized layer | 0.00 ± 0.00 | 2.43 ± 0.20 | 1.75 ± 0.14 p = 0.0205 |
| Crust | 0.00 ± 0.00 | 2.29 ± 0.29 | 0.75 ± 0.16 p = 0.0003 |
| Hyperplasia in non-keratinized layer | 0.00 ± 0.00 | 2.14 ± 0.14 | 2.00 ± 0.00 |
| Ulcer | 0.00 ± 0.00 | 0.86 ± 0.34 | 0.50 ± 0.19 |
| Dermis | |||
| Inflammatory cell infiltration | 0.00 ± 0.00 | 2.86 ± 0.14 | 2.13 ± 0.40 |
| Preventive setting | |||
| Epidermis | |||
| Parakeratosis | 0.00 ± 0.00 | 1.29 ± 0.18 | 0.63 ± 0.26 |
| Hyperplasia in keratinized layer | 0.00 ± 0.00 | 1.86 ± 0.26 | 0.88 ± 0.23 p = 0.0135 |
| Crust | 0.00 ± 0.00 | 2.14 ± 0.26 | 0.63 ± 0.38 p = 0.0066 |
| Hyperplasia in non-keratinized layer | 0.00 ± 0.00 | 2.00 ± 0.00 | 1.88 ± 0.13 |
| Ulcer | 0.00 ± 0.00 | 1.00 ± 0.31 | 0.25 ± 0.25 |
| Dermis | |||
| Inflammatory cell infiltration | 0.00 ± 0.00 | 3.00 ± 0.00 | 1.00 ± 0.38 p = 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneki, M.; Ohira, C.; Magami, T.; Hamauzu, A.; Inaba, Y.; Togase, H.; Fukuyama, T. Topical Administration of a Mixed Microbial Culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cerevisiae Significantly Inhibits the Development of Atopic Dermatitis in a Mouse Model Through IL-10 Overexpression by Dendritic Cells. Biomedicines 2025, 13, 2536. https://doi.org/10.3390/biomedicines13102536
Kaneki M, Ohira C, Magami T, Hamauzu A, Inaba Y, Togase H, Fukuyama T. Topical Administration of a Mixed Microbial Culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cerevisiae Significantly Inhibits the Development of Atopic Dermatitis in a Mouse Model Through IL-10 Overexpression by Dendritic Cells. Biomedicines. 2025; 13(10):2536. https://doi.org/10.3390/biomedicines13102536
Chicago/Turabian StyleKaneki, Mao, Chiharu Ohira, Tensei Magami, Aika Hamauzu, Yukari Inaba, Hideo Togase, and Tomoki Fukuyama. 2025. "Topical Administration of a Mixed Microbial Culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cerevisiae Significantly Inhibits the Development of Atopic Dermatitis in a Mouse Model Through IL-10 Overexpression by Dendritic Cells" Biomedicines 13, no. 10: 2536. https://doi.org/10.3390/biomedicines13102536
APA StyleKaneki, M., Ohira, C., Magami, T., Hamauzu, A., Inaba, Y., Togase, H., & Fukuyama, T. (2025). Topical Administration of a Mixed Microbial Culture of Lactobacillus paracasei, Pichia membranifaciens and Saccharomyces cerevisiae Significantly Inhibits the Development of Atopic Dermatitis in a Mouse Model Through IL-10 Overexpression by Dendritic Cells. Biomedicines, 13(10), 2536. https://doi.org/10.3390/biomedicines13102536






