From Steatosis to Immunosenescence: The Impact of Metabolic Dysfunction on Immune Aging in HIV and Non-HIV Populations
Abstract
1. Introduction
Search Strategy
2. Metabolic Dysfunction and Low-Grade Chronic Inflammation
2.1. MASLD/MAFLD: Epidemiology and Pathophysiology
2.2. Type 2 Diabetes Mellitus: Insulin Resistance and Immune Response
- Chronic hepatometabolic inflammation, sustaining continuous release of pro-inflammatory cytokines (IL-6, TNF-α) and ROS, thereby perpetuating insulin resistance and metabolic impairment [48];
- Depletion and dysfunction of regulatory T cells (Tregs), driven by both HIV infection and hepatic inflammation, which limits the capacity to restrain immune activation and facilitates persistent sterile inflammation [49];
- Reduced immunological resilience, with progressive immunosenescence manifesting as phenotypic alterations (decreased CD4/CD8 ratio, expansion of exhausted T cells) and chronic activation biomarkers (sCD14, IL-6), intensified by the coexistence of metabolic and viral comorbidities [50].
2.3. Metaflammation and Chronic Systemic Inflammation
3. Immunosenescence: Cellular and Molecular Mechanisms
3.1. Senescence of the Innate and Adaptive Immune System
3.2. Molecular Markers and Cellular Phenotypes of Immune Aging
3.3. MicroRNAs as Emerging Biomarkers
3.4. Inflammaging: Origins, Systemic Impact, and Predisposing Factors
4. Immunometabolism: The Link Between Metabolism and Immune Senescence
4.1. Cellular Metabolism of Immune Cells Under Physiological Conditions
- Reduced immune metabolic plasticity, with limited flexibility to shift between glycolysis and OXPHOS;
- Increased immunosenescence, with accumulation of senescent T lymphocytes (CD28− CD57+) associated with chronic inflammation and loss of immune memory;
- Dysregulation of peripheral tolerance, resulting in a systemic pro-inflammatory state, further exacerbated by intestinal dysbiosis and visceral adiposity.
4.2. Metabolic Dysfunctions of Immune Cells in MASLD and Type 2 Diabetes Mellitus
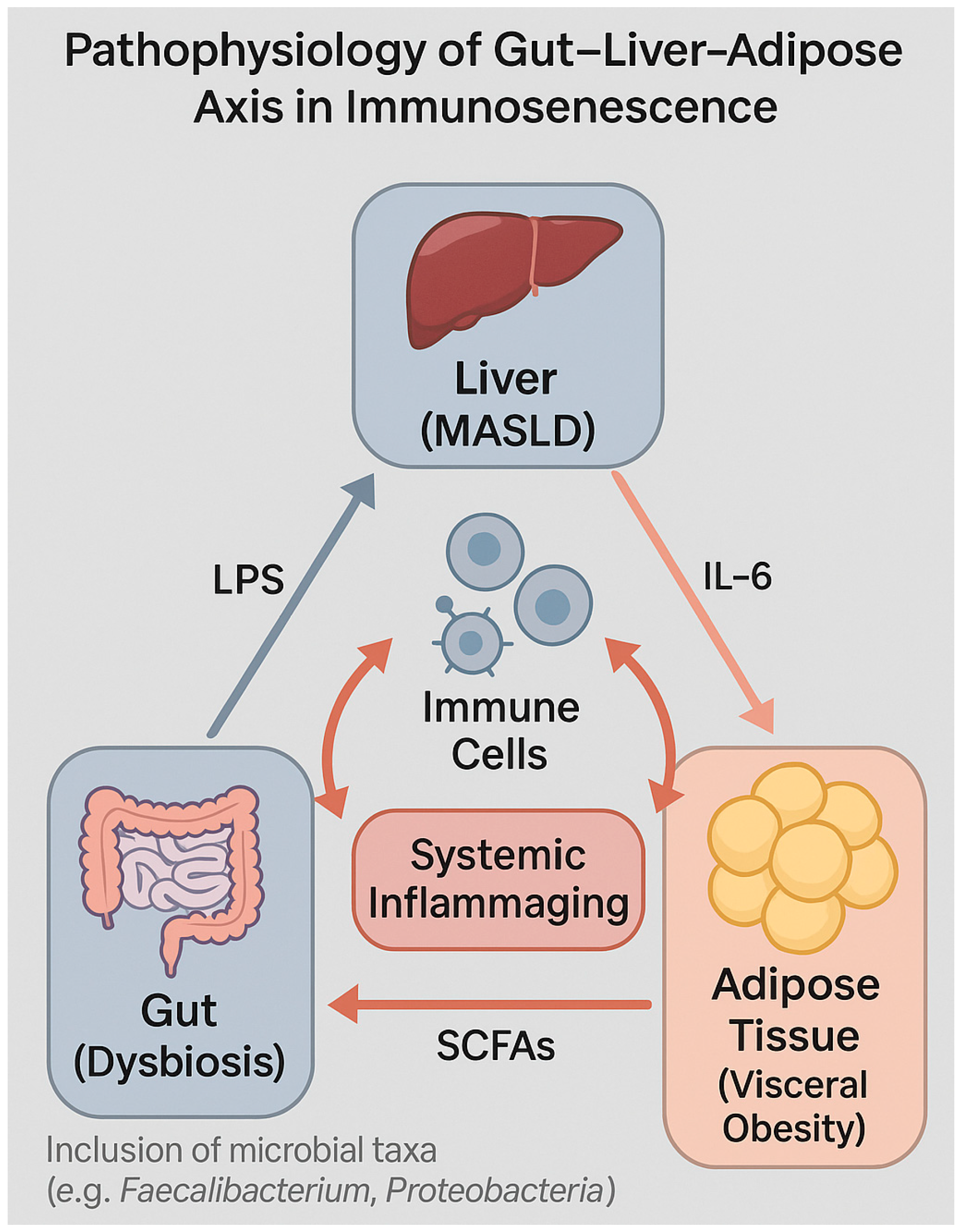
4.3. Role of Adipocytes, Cytokines, and the Microbiota–Gut–Liver Axis
4.4. Gut Permeability and Systemic Inflammation in HIV and Non-HIV Individuals
5. HIV and Accelerated Immune Aging
5.1. Chronic Immune Activation and Immunological Dysregulation in PLWH
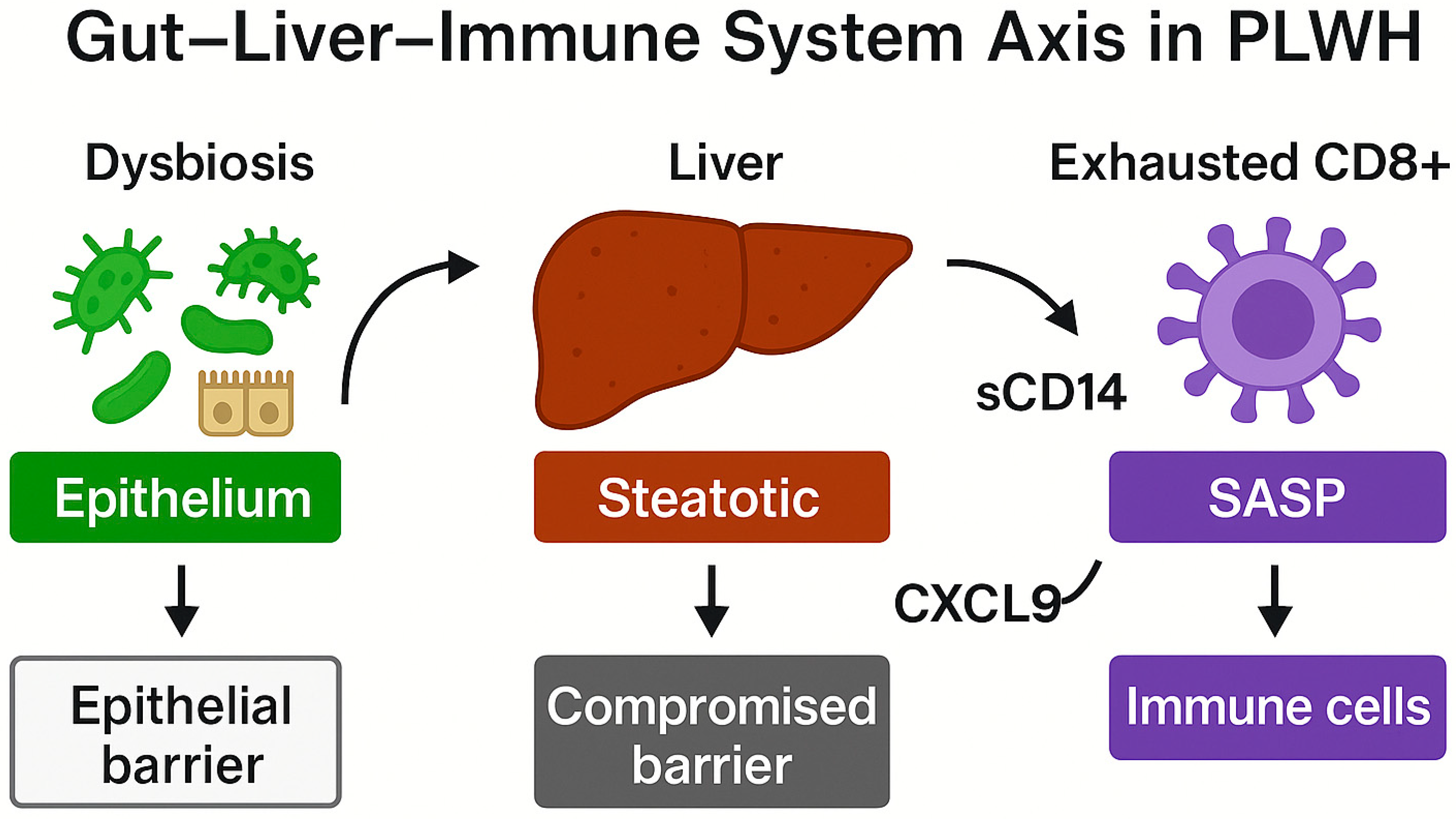
5.2. Microbial Translocation, Intestinal Barrier, and Persistent Inflammation
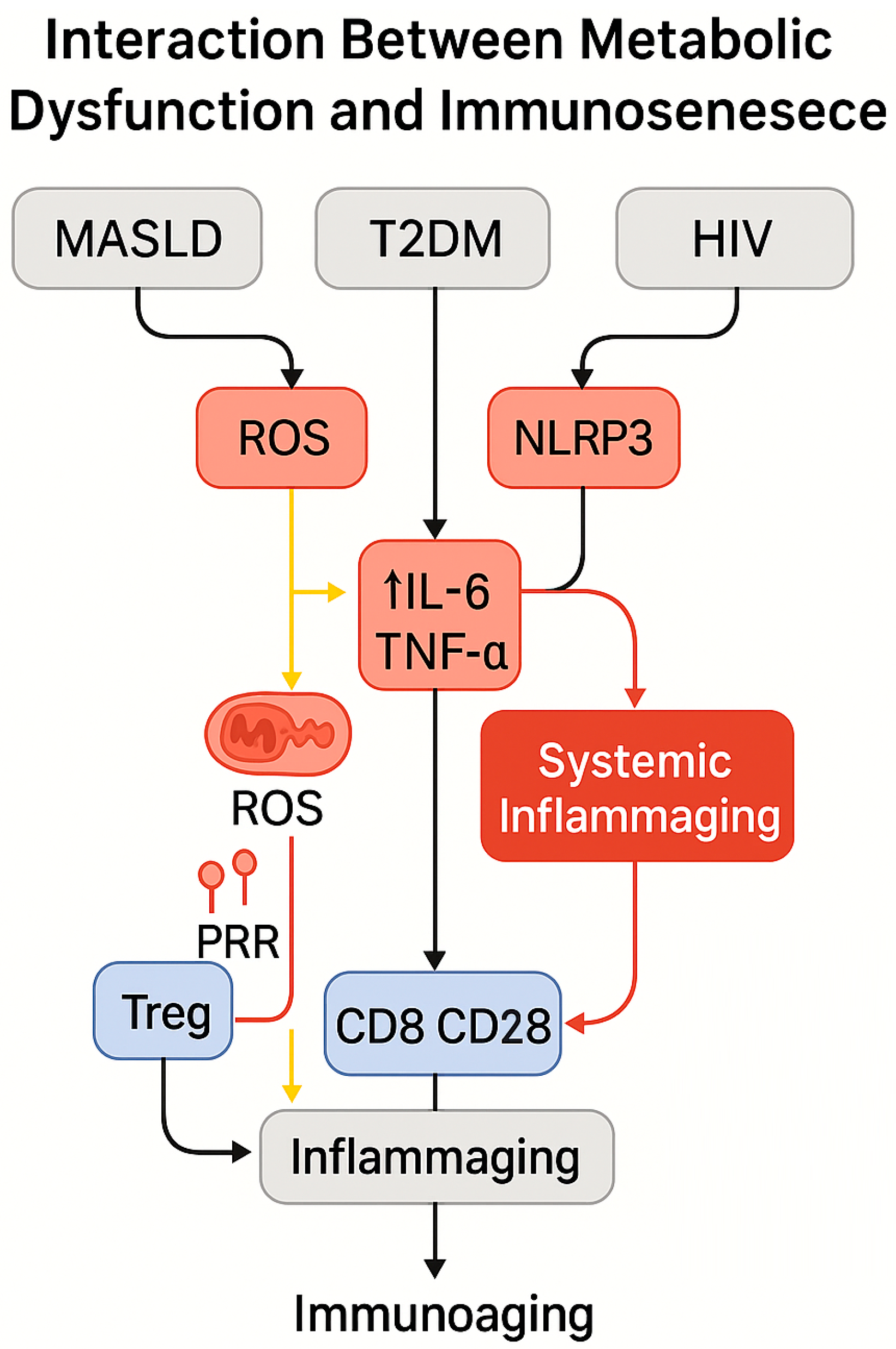
5.3. Interactions Between Metabolic Dysfunction and Immunosenescence in HIV-Positive Individuals
5.4. Comparison with HIV-Negative Populations: Physiological vs. Accelerated Aging
6. Clinical Implications
6.1. Increased Infectious Risk: Recurrent and Severe Infections in Older Adults and PLWH
6.2. Reduced Vaccine Response: Evidence from Influenza, Pneumococcal, and SARS-CoV-2 Vaccines
6.3. Healthy Aging and Immune Resilience: Predictive Models and Preventive Strategies
7. Future Perspectives and Research Directions
Integrated Models for Immunometabolic Risk Assessment
8. Conclusions
Limitations of the Review
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Shimi, G.; Sohouli, M.H.; Ghorbani, A.; Shakery, A.; Zand, H. The interplay between obesity, immunosenescence, and insulin resistance. Immun. Ageing 2024, 21, 13. [Google Scholar] [CrossRef]
- Martin, J.; Volberding, P. HIV and premature aging: A field still in its infancy. Ann. Intern. Med. 2010, 153, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jiang, T.; Deng, W.; Wang, S.; Li, X.; Zhang, Z.; Chen, Y.; Liu, H.; Zhao, Q.; Sun, L.; et al. Insulin resistance has closer correlation with the occurrence of metabolic dysfunction-associated steatotic liver disease diagnosed by liver biopsy. Front. Med. 2024, 11, 1384927. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Adipose tissue, immune aging and cellular senescence. Semin. Immunopathol. 2020, 42, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, M.; Sauce, D. Mechanisms of immune aging in HIV. Clin. Sci. 2022, 136, 61–80. [Google Scholar] [CrossRef]
- He, X.Y.; Wang, X.W.; Li, S.; Li, N.; Li, W.; Hu, Y.; Guo, N.; Zheng, Y.T.; Zheng, H.Y.; Su, B. Immunosenescence and its related comorbidities in older people living with HIV. Infect. Dis. Immun. 2025, 5, 44–55. [Google Scholar] [CrossRef]
- Zheng, H.; Sechi, L.A.; Navarese, E.P.; Casu, G.; Vidili, G. Metabolic dysfunction-associated steatotic liver disease and cardiovascular risk: A comprehensive review. Cardiovasc. Diabetol. 2024, 23, 346. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Talavera-Rodríguez, A.; Díaz-Álvarez, J.; Rosas Cancio-Suárez, M.; Rodríguez, J.M.; Alba, C.; Montes, M.L.; Martín-Mateos, R.; Burgos-Santamaría, D.; Moreno, S.; et al. A gut microbiome signature for HIV and metabolic dysfunction-associated steatotic liver disease. Front. Immunol. 2023, 14, 1297378. [Google Scholar] [CrossRef]
- Acierno, C.; Nevola, R.; Rinaldi, L.; Sasso, F.C.; Adinolfi, L.E.; Caturano, A. The intestinal thread of fate: How the microbiota shapes the story of liver disease. Livers 2025, 5, 17. [Google Scholar] [CrossRef]
- McCrory, C.; Fiorito, G.; Hernandez, B.; Polidoro, S.; O’Halloran, A.M.; Hever, A.; Ni Cheallaigh, C.; Lu, A.T.; Horvath, S.; Vineis, P.; et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 741–749. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, C.; Pan, J.; Zhou, Y.; Pan, X.; Kang, J.; Ren, L.; Gong, L.; Li, Y. CXCL9, IL2RB, and SPP1, potential diagnostic biomarkers in the co-morbidity pattern of atherosclerosis and non-alcoholic steatohepatitis. Sci. Rep. 2024, 14, 16364. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Canivet, C.M.; Boursier, J.; Loomba, R. New nomenclature for nonalcoholic fatty liver disease: Understanding metabolic dysfunction-associated steatotic liver disease, metabolic dysfunction- and alcohol-associated liver disease, and their implications in clinical practice. Semin. Liver Dis. 2024, 44, 35–42. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Brandman, D. A clinical update on MASLD. JAMA Intern. Med. 2025, 185, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Acierno, C.; Barletta, F.; Caturano, A.; Nevola, R.; Sasso, F.C.; Adinolfi, L.E.; Rinaldi, L. Alcohol consumption and liver metabolism in the era of MASLD: Integrating nutritional and pathophysiological insights. Nutrients 2025, 17, 2229. [Google Scholar] [CrossRef]
- Jiang, M.; Butt, A.S.; Cua, I.H.; Pan, Z.; Al-Busafi, S.A.; Méndez-Sánchez, N.; Eslam, M. MAFLD vs. MASLD: A year in review. Expert Rev. Endocrinol. Metab. 2025, 20, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2025, 31, S32–S50. [Google Scholar] [CrossRef]
- Michel, M.; Labenz, C.; Armandi, A.; Kaps, L.; Kremer, W.M.; Galle, P.R.; Grimm, D.; Sprinzl, M.; Schattenberg, J.M. Metabolic dysfunction-associated fatty liver disease in people living with HIV. Sci. Rep. 2023, 13, 9158. [Google Scholar] [CrossRef]
- Carli, F.; Della Pepa, G.; Sabatini, S.; Vidal Puig, A.; Gastaldelli, A. Lipid metabolism in MASLD and MASH: From mechanism to the clinic. JHEP Rep. 2024, 6, 101185. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-alcoholic fatty liver disease: From pathogenesis to clinical impact. Processes 2021, 9, 135. [Google Scholar] [CrossRef]
- Meyer, M.; Schwärzler, J.; Jukic, A.; Tilg, H. Innate immunity and MASLD. Biomolecules 2024, 14, 476. [Google Scholar] [CrossRef]
- Li, H.; Xia, N. The multifaceted roles of B lymphocytes in metabolic dysfunction–associated steatotic liver disease. Front. Immunol. 2024, 15, 1447391. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.M.; Lee, J.Y.; Ryu, T. Chronic inflammation and immune dysregulation in metabolic-dysfunction-associated steatotic liver disease progression: From steatosis to hepatocellular carcinoma. Biomedicines 2025, 13, 1260. [Google Scholar] [CrossRef]
- Mao, T.; Yang, R.; Luo, Y.; He, K. Crucial role of T cells in NAFLD-related disease: A review and prospect. Front. Endocrinol. 2022, 13, 1051076. [Google Scholar] [CrossRef]
- van Welzen, B.J.; Mudrikova, T.; El Idrissi, A.; Hoepelman, A.I.M.; Arends, J.E. A review of non-alcoholic fatty liver disease in HIV-infected patients: The next big thing? Infect. Dis. Ther. 2019, 8, 33–50. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Allende, D.S.; Cummings, O.; Sternberg, A.L.; Behling, C.A.; Carpenter, D.; Gill, R.M.; Guy, C.D.; Yeh, M.M.; Gawrieh, S.; Sterling, R.K.; et al. MASLD in people with HIV exhibits higher fibrosis stage despite lower disease activity than in matched controls. Aliment. Pharmacol. Ther. 2024, 60, 1351–1360. [Google Scholar] [CrossRef]
- Yan, H.; Chen, S.; Gao, X.; Jiang, Y.; Liang, G.; Peng, J.; Cai, S. Association between TyG index, liver steatosis and immunosenescence in people living with HIV. Infect. Drug Resist. 2024, 17, 5049–5059. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 diabetes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 9 June 2025).
- Lau, E.Y.M.; Carroll, E.C.; Callender, L.A.; Hood, G.A.; Berryman, V.; Pattrick, M.; Finer, S.; Hitman, G.A.; Ackland, G.L.; Henson, S.M. Type 2 diabetes is associated with the accumulation of senescent T cells. Clin. Exp. Immunol. 2019, 197, 205–213. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, hepatokines and myokines: Focus on their role and molecular mechanisms in adipose tissue inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Type 2 diabetes—An autoinflammatory disease driven by metabolic stress. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3805–3823. [Google Scholar] [CrossRef]
- Guo, L.; Liu, X.; Su, X. The role of TEMRA cell-mediated immune senescence in the development and treatment of HIV disease. Front. Immunol. 2023, 14, 1284293. [Google Scholar] [CrossRef]
- Laphanuwat, P.; Gomes, D.C.O.; Akbar, A.N. Senescent T cells: Beneficial and detrimental roles. Immunol. Rev. 2023, 316, 160–175. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Dixit, V.D. Nlrp3 inflammasome activation in type 2 diabetes: Is it clinically relevant? Diabetes 2013, 62, 22–24. [Google Scholar] [CrossRef]
- Lu, S.; Li, Y.; Qian, Z.; Zhao, T.; Feng, Z.; Weng, X.; Yu, L. Role of the inflammasome in insulin resistance and type 2 diabetes mellitus. Front. Immunol. 2023, 14, 1052756. [Google Scholar] [CrossRef]
- Pedro, M.N.; Rocha, G.Z.; Guadagnini, D.; Santos, A.; Magro, D.O.; Assalin, H.B.; Oliveira, A.G.; Pedro, R.d.J.; Saad, M.J.A. Insulin resistance in HIV-patients: Causes and consequences. Front. Endocrinol. 2018, 9, 514. [Google Scholar] [CrossRef]
- Ripa, M.; Chiappetta, S.; Tambussi, G. Immunosenescence and hurdles in the clinical management of older HIV-patients. Virulence 2017, 8, 508–528. [Google Scholar] [CrossRef][Green Version]
- Sciety. Evaluation of Triglyceride-Glucose (TyG) Index in Individuals Living with HIV Under Antiretroviral Therapy (ART). 2025. Available online: https://sciety.org/articles/activity/10.21203/rs.3.rs-6314815/v1 (accessed on 9 June 2025).
- Lee, E.Y.; Yang, H.K.; Lee, J.; Kang, B.; Yang, Y.; Lee, S.-H.; Ko, S.-H.; Ahn, Y.-B.; Cha, B.Y.; Yoon, K.-H.; et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016, 15, 155. [Google Scholar] [CrossRef]
- Mazzuti, L.; Turriziani, O.; Mezzaroma, I. The many faces of immune activation in HIV-1 infection: A multifactorial interconnection. Biomedicines 2023, 11, 159. [Google Scholar] [CrossRef]
- Sokoya, T.; Steel, H.C.; Nieuwoudt, M.; Rossouw, T.M. HIV as a cause of immune activation and immunosenescence. Mediat. Inflamm. 2017, 2017, 6825493. [Google Scholar] [CrossRef]
- Deguit, C.D.T.; Hough, M.; Hoh, R.; Krone, M.; Pilcher, C.D.; Martin, J.N.; Deeks, S.G.; McCune, J.M.; Hunt, P.W.; Rutishauser, R.L. Some aspects of CD8+ T-cell exhaustion are associated with altered T-cell mitochondrial features and ROS content in HIV infection. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 82, 211–219. [Google Scholar] [CrossRef]
- Facciolà, A.; D’Amato, S.; Calimeri, S.; Di Giudice, L.; Micali, C.; Russotto, Y.; Rullo, E.V.; Nunnari, G.; Squeri, R.; Pellicanò, G.F. Efficacy of COVID-19 vaccination in people living with HIV: A public health fundamental tool for the protection of patients and the correct management of infection. Infect. Dis. Rep. 2022, 14, 784–793. [Google Scholar] [CrossRef]
- Parmigiani, A.; Alcaide, M.L.; Freguja, R.; Pallikkuth, S.; Frasca, D.; Fischl, M.A.; Pahwa, S.; Sambhara, S. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS ONE 2013, 8, e79816. [Google Scholar] [CrossRef]
- Loste, C.; Trigueros, M.; Muñoz-López, F.; Urrea, V.; Martínez, A.; González, S.; Puig, J.; Martín, M.; Bonjoch, A.; Echeverría, P.; et al. Immunoaging at early ages could drive a higher comorbidity burden in people with HIV on antiretroviral therapy compared with the uninfected population. Int. J. Mol. Sci. 2024, 25, 10930. [Google Scholar] [CrossRef]
- Schleh, M.W.; Caslin, H.L.; Garcia, J.N.; Mashayekhi, M.; Srivastava, G.; Bradley, A.B.; Hasty, A.H. Metaflammation in obesity and its therapeutic targeting. Sci. Transl. Med. 2023, 15, eADF9382. [Google Scholar] [CrossRef]
- Kanbay, M.; Yerlikaya, A.; Sag, A.A.; Ortiz, A.; Kuwabara, M.; Covic, A.; Wiecek, A.; Stenvinkel, P.; Afsar, B. A journey from microenvironment to macroenvironment: The role of metaflammation and epigenetic changes in cardiorenal disease. Clin. Kidney J. 2019, 12, 861–870. [Google Scholar] [CrossRef]
- Malla, S.; Shahreen, N.; Saha, R. Immunometabolism at the crossroads of infection: Mechanistic and systems-level perspectives from host and pathogen. arXiv 2025, arXiv:2506.02236. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, F.; Yin, Q.; Wang, D.; Han, W.; Zhang, Y. Reactive oxygen species interact with NLRP3 inflammasomes and are involved in the inflammation of sepsis: From mechanism to treatment of progression. Front. Physiol. 2020, 11, 571810. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-induced TNFα and IL-6 signaling: The missing link between obesity and inflammation–driven liver and colorectal cancers. Cancers 2019, 11, 24. [Google Scholar] [CrossRef]
- Yu, W.; Li, C.; Zhang, D.; Li, Z.; Xia, P.; Liu, X.; Cai, X.; Yang, P.; Ling, J.; Zhang, J.; et al. Advances in T cells based on inflammation in metabolic diseases. Cells 2022, 11, 3554. [Google Scholar] [CrossRef]
- Lee, K.A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune senescence, immunosenescence and aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef]
- Nga, H.T.; Nguyen, T.L.; Yi, H.-S. T-cell senescence in human metabolic diseases. Diabetes Metab. J. 2024, 48, 864–881. [Google Scholar] [CrossRef]
- Masyuko, S.J.; Page, S.T.; Polyak, S.J.; Kinuthia, J.; Osoti, A.O.; Otieno, F.C.; Kibachio, J.M.; Mogaka, J.N.; Macharia, P.M.; Chohan, B.H.; et al. Human immunodeficiency virus is associated with higher levels of systemic inflammation among Kenyan adults despite viral suppression. Clin. Infect. Dis. 2020, 73, e2034–e2042. [Google Scholar] [CrossRef]
- Acierno, C.; Nevola, R.; Barletta, F.; Rinaldi, L.; Sasso, F.C.; Adinolfi, L.E.; Caturano, A. Multidrug-resistant infections and metabolic syndrome: An overlooked bidirectional relationship. Biomedicines 2025, 13, 1343. [Google Scholar] [CrossRef]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef]
- Ruderman, S.A.; Hunt, P.W.; Beck-Engeser, G.; Ambayec, G.; Willig, A.L.; Saag, M.S.; Napravnik, S.; Cachay, E.; Bamford, L.; Landay, A.; et al. Biomarkers of microbial translocation and generalized inflammation are associated with frailty among people with HIV. AIDS 2025, 39, 153–161. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Mohanta, S.K.; Heron, C.; Klaus-Bergmann, A.; Horstmann, H.; Brakenhielm, E.; Giannarelli, C.; Habenicht, A.J.R.; Gerhardt, H.; Weber, C. Metabolic and immune crosstalk in cardiovascular disease. Circ. Res. 2025, 136, 1433–1453. [Google Scholar] [CrossRef]
- Kalopitas, G.; Arvanitakis, K.; Tsachouridou, O.; Malandris, K.; Koufakis, T.; Metallidis, S.; Germanidis, G. Metabolic dysfunction-associated steatotic liver disease in people living with HIV—Limitations on antiretroviral therapy selection. Life 2024, 14, 742. [Google Scholar] [CrossRef]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative stress and cardiovascular complications in type 2 diabetes: From pathophysiology to lifestyle modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Latchney, S.E.; Calvi, L.M. The aging hematopoietic stem cell niche: Phenotypic and functional changes and mechanisms that contribute to hematopoietic aging. Semin. Hematol. 2017, 54, 25–32. [Google Scholar] [CrossRef]
- Saitoh, T.; Akira, S. Regulation of inflammasomes by autophagy. J. Allergy Clin. Immunol. 2016, 138, 28–36. [Google Scholar] [CrossRef]
- Nasi, M.; De Biasi, S.; Gibellini, L.; Bianchini, E.; Pecorini, S.; Bacca, V.; Guaraldi, G.; Mussini, C.; Pinti, M.; Cossarizza, A. Ageing and inflammation in patients with HIV infection. Clin. Exp. Immunol. 2017, 187, 44–52. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S.; Pawelec, G. The immune system and its dysregulation with aging. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Book Series: Subcellular Biochemistry (SCBI); Springer: Singapore, 2019; Volume 91, pp. 21–43. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhang, Z.; Zhang, Y. Characteristics and mechanisms of T-cell senescence: A potential target for cancer immunotherapy. Eur. J. Immunol. 2024, 54, e2451093. [Google Scholar] [CrossRef]
- Ma, S.; Wang, C.; Mao, X.; Hao, Y. B cell dysfunction associated with aging and autoimmune diseases. Front. Immunol. 2019, 10, 318. [Google Scholar] [CrossRef]
- Rubtsova, K.; Rubtsov, A.V.; Cancro, M.P.; Marrack, P. Age-associated B cells: A T-bet dependent effector with roles in protective and pathogenic immunity. J. Immunol. 2015, 195, 1933–1937. [Google Scholar] [CrossRef]
- Vassallo, M.; Durant, J.; Addou, S.; Ticchioni, M.; Fabre, R.; Chirio, D.; Naqvi, A.; Cua, E.; Ameil, L.; Godemert, M.; et al. Immunosenescence markers in T- and NK-cells according to the CD4/CD8 ratio in successfully treated people living with HIV. Front. Med. 2025, 12, 1562537. [Google Scholar] [CrossRef]
- Hove-Skovsgaard, M.; Zhao, Y.; Tingstedt, J.L.; Hartling, H.J.; Thudium, R.F.; Benfield, T.; Afzal, S.; Nordestgaard, B.; Ullum, H.; Gerstoft, J.; et al. Impact of age and HIV status on immune activation, senescence and apoptosis. Front. Immunol. 2020, 11, 583569. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The impact of immune system aging on infectious diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef]
- Theodorakis, N.; Feretzakis, G.; Hitas, C.; Kreouzi, M.; Kalantzi, S.; Spyridaki, A.; Kollia, Z.; Verykios, V.S.; Nikolaou, M. Immunosenescence: How aging increases susceptibility to bacterial infections and virulence factors. Microorganisms 2024, 12, 2052. [Google Scholar] [CrossRef]
- Pangrazzi, L.; Meryk, A. Molecular and cellular mechanisms of immunosenescence: Modulation through interventions and lifestyle changes. Biology 2024, 14, 17. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; Bendall, M.L.; Dwaraka, V.; Pang, A.P.; Dopkins, N.; Carreras, N.; Smith, R.; Nixon, D.F.; Corley, M.J. Retroelement-age clocks: Epigenetic age captured by human endogenous retrovirus and LINE-1 DNA methylation states. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence study of T cells: A systematic review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef]
- AApetroaei, M.-M.; Baliou, S.; Ioannou, P.; Fragkiadaki, P.; Ștefan, G.; Nedea, M.I.; Burcea-Dragomiroiu, G.-T.-A.; Velescu, B.Ș.; Docea, A.O.; Udeanu, D.I.; et al. The hallmarks of ageing in human immunodeficiency virus infection and the impact of antiretroviral therapy on telomeres: A molecular perspective. Curr. Issues Mol. Biol. 2025, 47, 273. [Google Scholar] [CrossRef]
- Mouat, I.C.; Horwitz, M.S. Age-associated B cells in viral infection. PLoS Pathog. 2022, 18, e1010297. [Google Scholar] [CrossRef]
- Xie, G.; Chen, X.; Gao, Y.; Yang, M.; Zhou, S.; Lu, L.; Wu, H.; Lu, Q. Age-associated B cells in autoimmune diseases: Pathogenesis and clinical implications. Clin. Rev. Allergy Immunol. 2025, 68, 18. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Mendy, A.; Mersha, T.B. Epigenetic age acceleration and mortality risk prediction in US adults. GeroScience 2025, 47, 6029–6038. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Hussain, M.A.; Moore, D.J.; Horvath, S.; Lin, D.T.S.; Kobor, M.S.; Kobor, M.S.; Levine, A.; HNRP Group. Inflammation-related genes are associated with epigenetic aging in HIV. J. Neurovirol. 2019, 25, 853–865. [Google Scholar] [CrossRef]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 2021, 1, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Fauce, S.R.; Effros, R.B. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 2008, 22, 600–605. [Google Scholar] [CrossRef]
- Gabrielson, M.; Björklund, M.; Carlson, J.; Shoshan, M. Expression of mitochondrial regulators PGC1α and TFAM as putative markers of subtype and chemoresistance in epithelial ovarian carcinoma. PLoS ONE 2014, 9, e107109. [Google Scholar] [CrossRef]
- Lima-Silva, M.L.; Torres, K.C.L.; Mambrini, J.V.M.; Brot, N.C.; Santos, S.O.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Lima-Costa, M.F.; Peixoto, S.V. A nationwide study on immunosenescence biomarkers profile in older adults: ELSI-Brazil. Exp. Gerontol. 2024, 191, 112433. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. T cell immunosenescence in aging, obesity, and cardiovascular disease. Cells 2021, 10, 2435. [Google Scholar] [CrossRef]
- Sim, B.C.; Kang, Y.E.; You, S.K.; Lee, S.E.; Nga, H.T.; Lee, H.Y.; Nguyen, T.L.; Moon, J.S.; Tian, J.; Jang, H.J.; et al. Hepatic T-cell senescence and exhaustion are implicated in the progression of fatty liver disease in patients with type 2 diabetes and mouse model with nonalcoholic steatohepatitis. Cell Death Dis. 2023, 14, 618. [Google Scholar] [CrossRef]
- Guerville, F.; De Souto Barreto, P.; Ader, I.; Andrieu, S.; Casteilla, L.; Dray, C.; Fazilleau, N.; Guyonnet, S.; Langin, D.; Liblau, R.; et al. Revisiting the hallmarks of aging to identify markers of biological age. J. Prev. Alzheimer’s Dis. 2020, 7, 56–64. [Google Scholar] [CrossRef]
- Bresciani, E.; Squillace, N.; Orsini, V.; Piolini, R.; Rizzi, L.; Molteni, L.; Meanti, R.; Soria, A.; Lapadula, G.; Bandera, A.; et al. miRNA expression profiling in subcutaneous adipose tissue of monozygotic twins discordant for HIV infection: Validation of differentially expressed miRNA and bioinformatic analysis. Int. J. Mol. Sci. 2022, 23, 3486. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Alabasi, G.; Sandaltzopoulos, R.; Marcu, K.B.; Kolettas, E. Roles of NF-κB signaling in the regulation of miRNAs impacting on inflammation in cancer. Biomedicines 2018, 6, 40. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Yang, Y.; Cho, W.C.; Flynn, R.J.; Harandi, M.F.; Song, H.; Luo, X.; Zheng, Y. Interplays of liver fibrosis-associated microRNAs: Molecular mechanisms and implications in diagnosis and therapy. Genes. Dis. 2022, 10, 1457–1469. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Zeng, X.; Yang, P.; Liu, X.; Zhao, X.; Liang, S. Roles of microRNA on cancer cell metabolism. J. Transl. Med. 2012, 10, 228. [Google Scholar] [CrossRef]
- Yu, F.; Guo, Y.; Chen, B.; Dong, P.; Zheng, J. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab. Investig. 2015, 95, 781–789. [Google Scholar] [CrossRef]
- Kitano, M.; Bloomston, P.M. Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J. Clin. Med. 2016, 5, 38. [Google Scholar] [CrossRef]
- Ha, T.Y. The role of microRNAs in regulatory T cells and in the immune response. Immune Netw. 2011, 11, 11–41. [Google Scholar] [CrossRef]
- Raisch, J.; Darfeuille-Michaud, A.; Nguyen, H.T.T. Role of microRNAs in the immune system, inflammation and cancer. World J. Gastroenterol. 2013, 19, 2985–2996. [Google Scholar] [CrossRef]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the diagnosis of digestive diseases: A comprehensive review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef]
- López-Sánchez, G.N.; Dóminguez-Pérez, M.; Uribe, M.; Chávez-Tapia, N.C.; Nuño-Lámbarri, N. Non-alcoholic fatty liver disease and microRNAs expression, how it affects the development and progression of the disease. Ann. Hepatol. 2021, 21, 100212. [Google Scholar] [CrossRef]
- Xiao, X.; Mao, X.; Chen, D.; Yu, B.; He, J.; Yan, H.; Wang, J. miRNAs can affect intestinal epithelial barrier in inflammatory bowel disease. Front. Immunol. 2022, 13, 868229. [Google Scholar] [CrossRef]
- Rome, S. Use of miRNAs in biofluids as biomarkers in dietary and lifestyle intervention studies. Genes. Nutr. 2015, 10, 33. [Google Scholar] [CrossRef]
- Mall, C.; Rocke, D.M.; Durbin-Johnson, B.; Weiss, R.H. Stability of miRNA in human urine supports its biomarker potential. Biomark. Med. 2013, 7, 623–631. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Santaella, F.J.; Rodrigues, M.A.M.; Sassaki, L.Y.; Di Stasi, L.C. MicroRNAs expression influence in ulcerative colitis and Crohn’s disease: A pilot study for the identification of diagnostic biomarkers. World J. Gastroenterol. 2021, 27, 7801–7812. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, W.-D.; Wang, Y.-D. The roles of the gut microbiota–miRNA interaction in the host pathophysiology. Mol. Med. 2020, 26, 101. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of aging: The birth of inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Lu, B.; Huang, L.; Cao, J.; Li, L.; Wu, W.; Chen, X.; Ding, C. Adipose tissue macrophages in aging-associated adipose tissue function. J. Physiol. Sci. 2021, 71, 38. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between inflammageing and immunosenescence during ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Barhoumi, T.; Bouchareb, R.; Alroqi, F.; Nefzi, A.; Todryk, S. Editorial: Inflammaging and immunosenescence: Role in aging-associated cardiovascular diseases. Front. Cardiovasc. Med. 2025, 12, 1616623. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Kovtonyuk, L.V.; Fritsch, K.; Feng, X.; Manz, M.G.; Takizawa, H. Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment. Front. Immunol. 2016, 7, 502. [Google Scholar] [CrossRef]
- Teer, E.; Mukonowenzou, N.C.; Essop, M.F. HIV, inflammation, and immunometabolism: A model of the inflammatory theory of disease. Viruses 2025, 17, 839. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Patankar, V.; Kitchen, S.; Zhen, A. Examining chronic inflammation, immune metabolism, and T cell dysfunction in HIV infection. Viruses 2024, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Thurman, M.; Johnson, S.; Acharya, A.; Pallikkuth, S.; Mahesh, M.; Byrareddy, S.N. Biomarkers of activation and inflammation to track disparity in chronological and physiological age of people living with HIV on combination antiretroviral therapy. Front. Immunol. 2020, 11, 583934. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef]
- Grund, B.; Baker, J.V.; Deeks, S.G.; Wolfson, J.; Wentworth, D.; Cozzi-Lepri, A.; Cohen, C.J.; Phillips, A.; Lundgren, J.D.; Neaton, J.D.; et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS ONE 2016, 11, e0155100. [Google Scholar] [CrossRef]
- Wan, X.; Xu, C.; Yu, C.; Li, Y. Role of NLRP3 inflammasome in the progression of NAFLD to NASH. Can. J. Gastroenterol. Hepatol. 2016, 2016, 6489012. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Schrock, J.M. Accelerated aging in people living with HIV: The neuroimmune feedback model. Brain Behav. Immun. Health 2024, 36, 100737. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Lee, J.Y.; Seo, H.; Hwang, S.S.; Lee, C.K.; Lee, G.R. Modulation of immune responses by metabolic reprogramming: The key role of immunometabolism. Immune Netw. 2025, 25, e15. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.-H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef]
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic instruction of immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, X.; Yuan, Z.; Wang, H. Metabolic reprogramming in immune response and tissue inflammation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1990–2001. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The role of HIF in immunity and inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Yang, K.; Chi, H. mTOR and metabolic pathways in T cell quiescence and functional activation. Semin. Immunol. 2012, 24, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Miao, C. Metabolic reprogram and T cell differentiation in inflammation: Current evidence and future perspectives. Cell Death Discov. 2025, 11, 123. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef]
- Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Macut, D.; Mladenović, D. The interconnection between hepatic insulin resistance and metabolic dysfunction-associated steatotic liver disease—The transition from an adipocentric to liver-centric approach. Curr. Issues Mol. Biol. 2023, 45, 9084–9102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Jindal, A.; Locatelli, I.; Vacchiano, M.; Gigliotti, L.; Bozzola, C.; Albano, E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 2014, 59, 886–897. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.A.; Guillén-Nieto, G.E.; Rodríguez-Rodríguez, N.; Mendoza-Mari, Y.; Bringas-Vega, M.L.; Berlanga-Saez, J.O.; Herrera, D.G.d.B.; Martinez-Jimenez, I.; Hernandez-Gutierrez, S.; Valdés-Sosa, P.A. Cellular senescence as the pathogenic hub of diabetes-related wound chronicity. Front. Endocrinol. 2020, 11, 573032. [Google Scholar] [CrossRef]
- Palatella, M.; Guillaume, S.M.; Linterman, M.A.; Huehn, J. The dark side of Tregs during aging. Front. Immunol. 2022, 13, 940705. [Google Scholar] [CrossRef]
- Uti, D.E.; Atangwho, I.J.; Omang, W.A.; Alum, E.U.; Obeten, U.N.; Udeozor, P.A.; Agada, S.A.; Bawa, I.; Ogbu, C.O. Cytokines as key players in obesity low grade inflammation and related complications. Obes. Med. 2025, 54, 100585. [Google Scholar] [CrossRef]
- Khan, S.; Chan, Y.T.; Revelo, X.S.; Winer, D.A. The immune landscape of visceral adipose tissue during obesity and aging. Front. Endocrinol. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Torriani, M.; Srinivasa, S.; Fitch, K.V.; Thomou, T.; Wong, K.; Petrow, E.; Kahn, C.R.; Cypess, A.M.; Grinspoon, S.K. Dysfunctional subcutaneous fat with reduced Dicer and brown adipose tissue gene expression in HIV-infected patients. J. Clin. Endocrinol. Metab. 2016, 101, 1225–1234. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Dunham, R.M.; Iwai, S.; Maher, M.C.; Albright, R.G.; Broadhurst, M.J.; Hernandez, R.D.; Lederman, M.M.; Huang, Y.; Somsouk, M.; et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013, 5, 193ra91. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, Z.; Nagavci, B.; Szabó, B.G.; Lakatos, B. Gut microbiome alteration in HIV/AIDS and the role of antiretroviral therapy—A scoping review. Microorganisms 2024, 12, 2221. [Google Scholar] [CrossRef]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Luo, R.; Yao, Y.; Chen, Z.; Sun, X. An examination of the LPS-TLR4 immune response through the analysis of molecular structures and protein–protein interactions. Cell Commun. Signal. 2025, 23, 142. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Sasu, S.M.; Todor, S.B. Key insights into gut alterations in metabolic syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef] [PubMed]
- Crakes, K.R.; Jiang, G. Gut microbiome alterations during HIV/SIV infection: Implications for HIV cure. Front. Microbiol. 2019, 10, 1104. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wu, N.; Jin, C. Intestinal microbiota dysbiosis promotes mucosal barrier damage and immune injury in HIV-infected patients. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 3080969. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.C.; Silveira, E.A.; Falco, M.O.; Nery, M.W.; Turchi, M.D. Effectiveness of nutritional treatment and synbiotic use on gastrointestinal symptoms reduction in HIV-infected patients: Randomized clinical trial. Clin. Nutr. 2017, 36, 680–685. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J.; Mi, J. Clinical effectiveness of fecal microbial transplantation for metabolic syndrome: Advances in clinical efficacy and multi-omics research. Curr. Res. Microb. Sci. 2025, 9, 100415. [Google Scholar] [CrossRef]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef]
- Hunt, P.W.; Brenchley, J.; Sinclair, E.; McCune, J.M.; Roland, M.; Page-Shafer, K.; Hsue, P.; Emu, B.; Krone, M.; Lampiris, H.; et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J. Infect. Dis. 2008, 197, 126–133. [Google Scholar] [CrossRef]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef]
- Mavilio, D.; Benjamin, J.; Daucher, M.; Lombardo, G.; Kottilil, S.; Planta, M.A.; Marcenaro, E.; Bottino, C.; Moretta, L.; Moretta, A.; et al. Natural killer cells in HIV-1 infection: Dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. USA 2003, 100, 15011–15016. [Google Scholar] [CrossRef]
- Manches, O.; Frleta, D.; Bhardwaj, N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. 2014, 35, 114–122. [Google Scholar] [CrossRef]
- Shin, H.; Wherry, E.J. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007, 19, 408–415. [Google Scholar] [CrossRef]
- Kuller, L.H.; Tracy, R.; Belloso, W.; De Wit, S.; Drummond, F.; Lane, H.C.; Ledergerber, B.; Lundgren, J.; Neuhaus, J.; Nixon, D.; et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008, 5, e203. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
- Estes, J.D.; Harris, L.D.; Klatt, N.R.; Tabb, B.; Pittaluga, S.; Paiardini, M.; Barclay, G.R.; Smedley, J.; Pung, R.; Oliveira, K.M.; et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010, 6, e1001052. [Google Scholar] [CrossRef]
- Tenorio, A.R.; Zheng, Y.; Bosch, R.J.; Krishnan, S.; Rodriguez, B.; Hunt, P.W.; Plants, J.; Seth, A.; Wilson, C.C.; Deeks, S.G.; et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis. 2014, 210, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Li, M.; Campbell, T.B.; Flores, S.C.; Linderman, D.; Gebert, M.J.; Knight, R.; Fontenot, A.P.; Palmer, B.E. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 2013, 14, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Dock, J.N.; Effros, R.B. Role of CD8 T cell replicative senescence in human aging and in HIV-mediated immunosenescence. Aging Dis. 2011, 2, 382–397. [Google Scholar] [PubMed]
- Klatt, N.R.; Chomont, N.; Douek, D.C.; Deeks, S.G. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol. Rev. 2013, 254, 326–342. [Google Scholar] [CrossRef]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; Cole, S.R.; Li, X.; Kingsley, L.A.; Palella, F.J.; Riddler, S.A.; Visscher, B.R.; Margolick, J.B.; Dobs, A.S. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch. Intern. Med. 2005, 165, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Dubrow, R.; Silverberg, M.J.; Park, L.S.; Crothers, K.; Justice, A.C. HIV infection, aging, and immune function: Implications for cancer risk and prevention. Curr. Opin. Oncol. 2012, 24, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Koethe, J.R.; Hulgan, T.; Niswender, K. Adipose tissue and immune function: A review of evidence relevant to HIV infection. J. Infect. Dis. 2013, 208, 1194–1201. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Duarte, M.J.; Tien, P.C.; Somsouk, M.; Price, J.C. The human microbiome and gut–liver axis in people living with HIV. Curr. HIV/AIDS Rep. 2023, 20, 170–180. [Google Scholar] [CrossRef]
- Moretti, S.; Schietroma, I.; Sberna, G.; Maggiorella, M.T.; Sernicola, L.; Farcomeni, S.; Giovanetti, M.; Ciccozzi, M.; Borsetti, A. HIV-1–host interaction in gut-associated lymphoid tissue (GALT): Effects on local environment and comorbidities. Int. J. Mol. Sci. 2023, 24, 12193. [Google Scholar] [CrossRef]
- Kolte, L. Thymic function in HIV-infection. Dan. Med. J. 2013, 60, B4622. [Google Scholar]
- Li, N.; Zheng, H.-Y.; Li, W.; He, X.-Y.; Zhang, M.; Li, X.; Tian, R.-R.; Dong, X.-Q.; Shen, Z.-Q.; Zheng, Y.-T. Limited restoration of T cell subset distribution and immune function in older people living with HIV-1 receiving HAART. Immun. Ageing 2025, 22, 3. [Google Scholar] [CrossRef]
- Vallejo, A.N.; Weyand, C.M.; Goronzy, J.J. T-cell senescence: A culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol. Med. 2004, 10, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W. HIV and inflammation: Mechanisms and consequences. Curr. HIV/AIDS Rep. 2012, 9, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Oblak, L.; van der Zaag, J.; Higgins-Chen, A.T.; Levine, M.E.; Boks, M.P. A systematic review of biological, social and environmental factors associated with epigenetic clock acceleration. Ageing Res. Rev. 2021, 69, 101348. [Google Scholar] [CrossRef]
- Akusjärvi, S.S.; Neogi, U. Biological aging in people living with HIV on successful antiretroviral therapy: Do they age faster? Curr. HIV/AIDS Rep. 2023, 20, 42–50. [Google Scholar] [CrossRef]
- Weiskopf, D.; Weinberger, B.; Grubeck-Loebenstein, B. The aging of the immune system. Transpl. Int. 2009, 22, 1041–1050. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretić, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered mitochondrial function in MASLD: Key features and promising therapeutic approaches. Antioxidants 2024, 13, 906. [Google Scholar] [CrossRef]
- Merino, J.; Martínez-González, M.A.; Rubio, M.; Inogés, S.; Sánchez-Ibarrola, A.; Subirá, M.L. Progressive decrease of CD8high+ CD28+ CD57− cells with ageing. Clin. Exp. Immunol. 1998, 112, 48–51. [Google Scholar] [CrossRef]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [CrossRef] [PubMed]
- Appay, V.; Sauce, D. Immune activation and inflammation in HIV-1 infection: Causes and consequences. J. Pathol. 2008, 214, 231–241. [Google Scholar] [CrossRef]
- Leng, S.X.; Margolick, J.B. Understanding frailty, aging, and inflammation in HIV infection. Curr. HIV/AIDS Rep. 2015, 12, 25–32. [Google Scholar] [CrossRef]
- Guaraldi, G.; Palella, F.J. Clinical implications of aging with HIV infection: Perspectives and the future medical care agenda. AIDS 2017, 31 (Suppl. S2), S129–S135. [Google Scholar] [CrossRef] [PubMed]
- High, K.P.; Brennan-Ing, M.; Clifford, D.B.; Cohen, M.H.; Currier, J.; Deeks, S.G.; Deren, S.; Effros, R.B.; Gebo, K.; Goronzy, J.J.; et al. HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J. Acquir. Immune Defic. Syndr. 2012, 60 (Suppl. S1), S1–S18. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of fame among pro-inflammatory cytokines: Interleukin-6 gene and its transcriptional regulation mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Shan, L.; Huang, H.; Haupt, C.; Bessell, C.; Canaday, D.H.; Zhang, H.; Ho, Y.-C.; Powell, J.D.; Oelke, M.; et al. Sprouty-2 regulates HIV-specific T cell polyfunctionality. J. Clin. Investig. 2014, 124, 198–208. [Google Scholar] [CrossRef]
- Buseyne, F.; Rivière, Y. HIV-specific CD8+ T-cell immune responses and viral replication. AIDS 1993, 7 (Suppl. S2), S81–S86. [Google Scholar] [CrossRef]
- Li, Q.; Duan, L.; Estes, J.D.; Ma, Z.M.; Rourke, T.; Wang, Y.; Reilly, C.; Carlis, J.; Miller, C.J.; Haase, A.T. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 2005, 434, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Hazenberg, M.D.; Otto, S.A.; van Benthem, B.H.; Roos, M.T.; Coutinho, R.A.; Lange, J.M.; Hamann, D.; Prins, M.; Miedema, F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003, 17, 1881–1888. [Google Scholar] [CrossRef]
- Byakwaga, H.; Boum, Y., II; Huang, Y.; Muzoora, C.; Kembabazi, A.; Weiser, S.D.; Bennett, J.; Cao, H.; Haberer, J.E.; Deeks, S.G.; et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J. Infect. Dis. 2014, 210, 383–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vujkovic-Cvijin, I.; Somsouk, M. HIV and the gut microbiota: Composition, consequences, and avenues for amelioration. Curr. HIV/AIDS Rep. 2019, 16, 204–213. [Google Scholar] [CrossRef]
- Dillon, S.M.; Frank, D.N.; Wilson, C.C. The gut microbiome and HIV-1 pathogenesis: A two-way street. AIDS 2016, 30, 2737–2751. [Google Scholar] [CrossRef] [PubMed]
- Talathi, R.; Anekwe, C.V.; Toribio, M. Epidemiology of obesity among people with HIV. Curr. Opin. HIV AIDS 2024, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
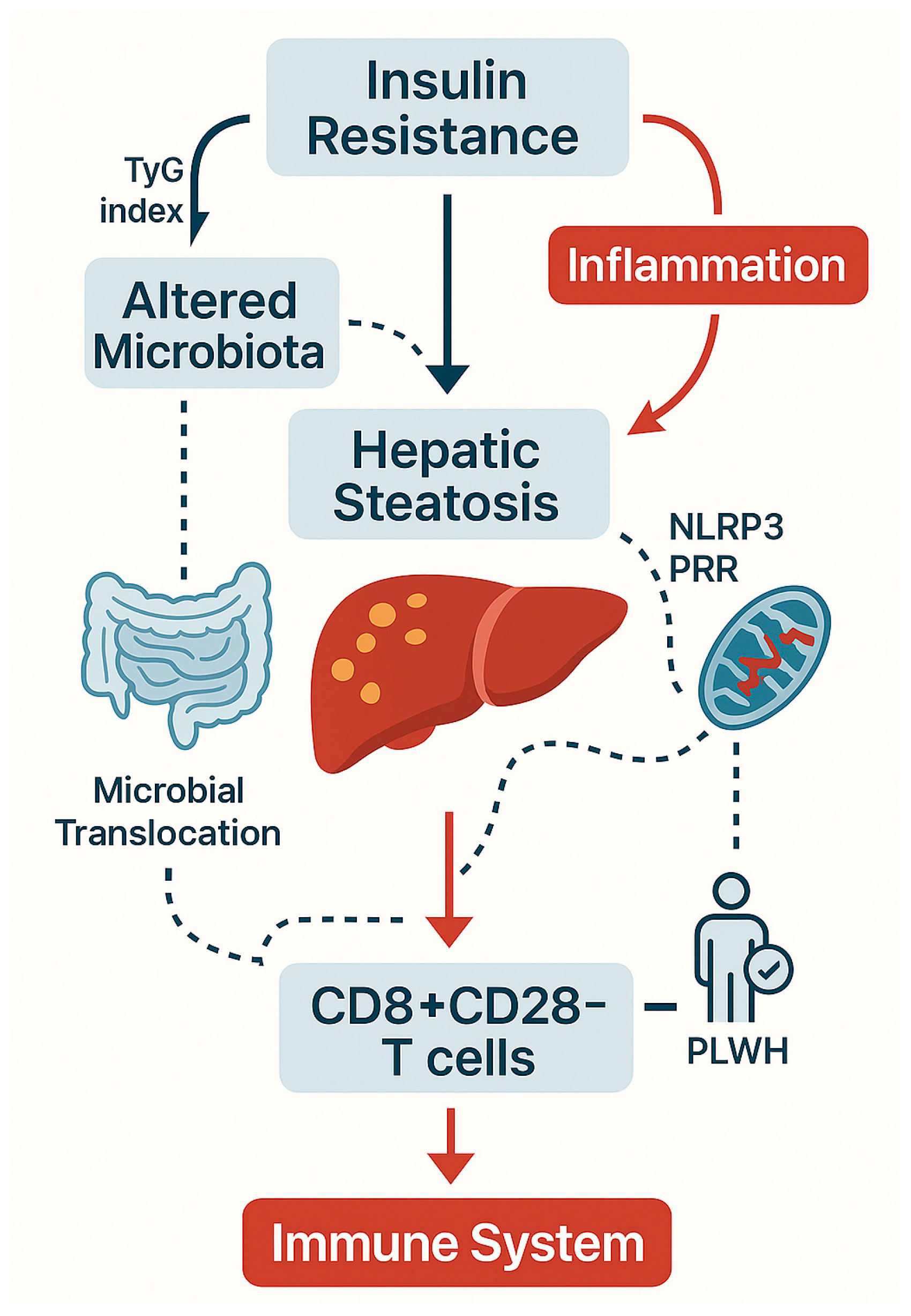
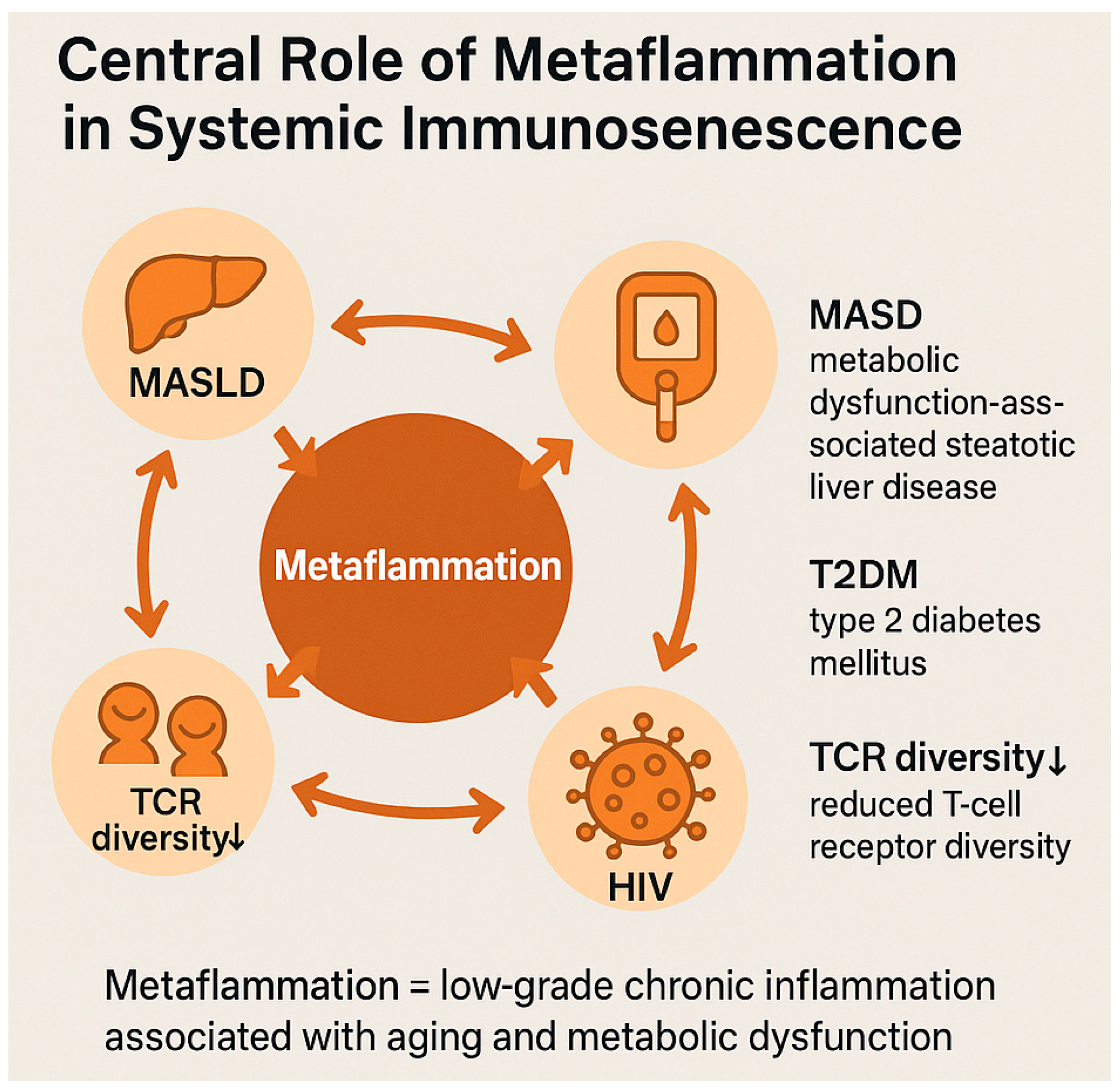
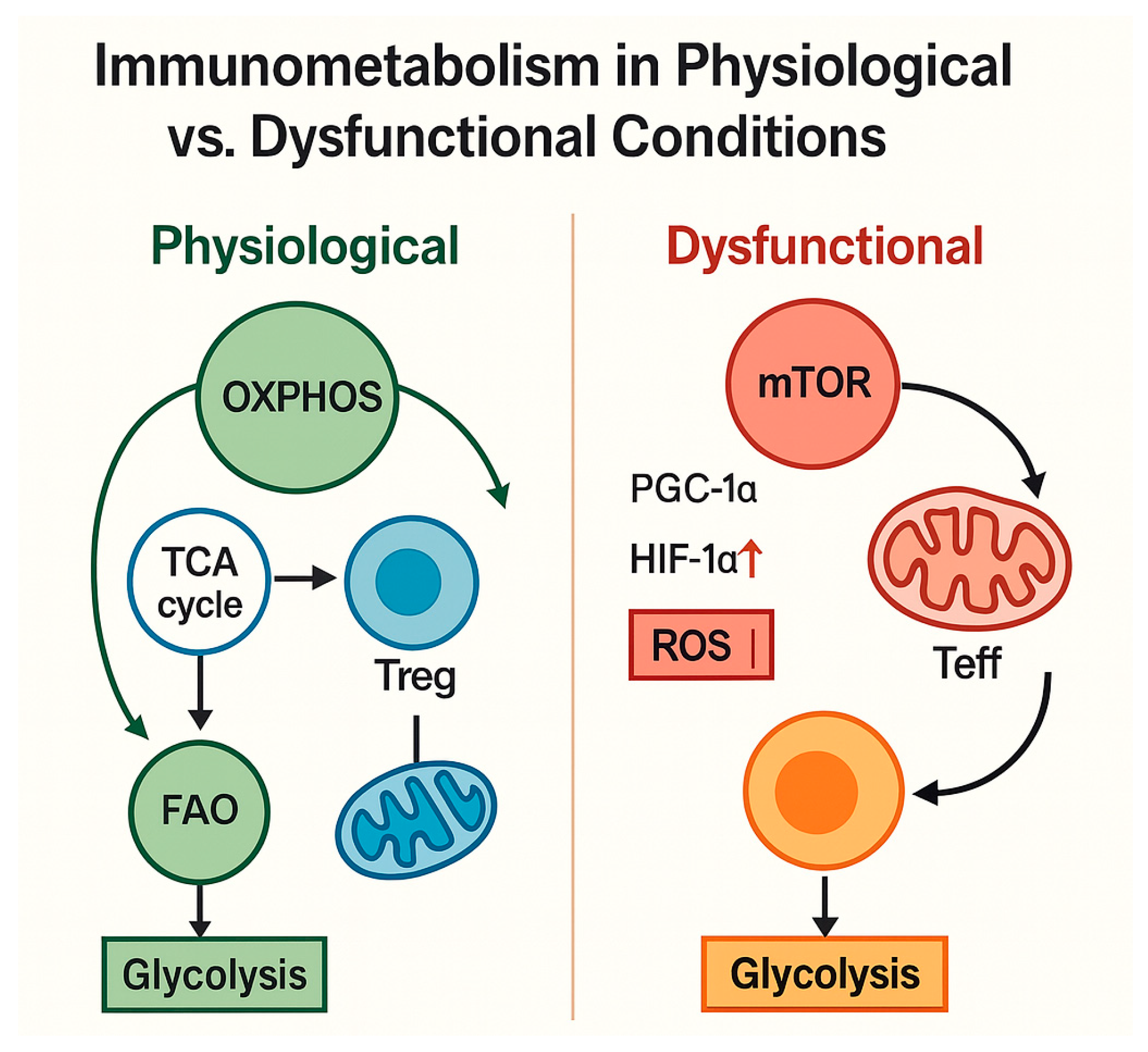
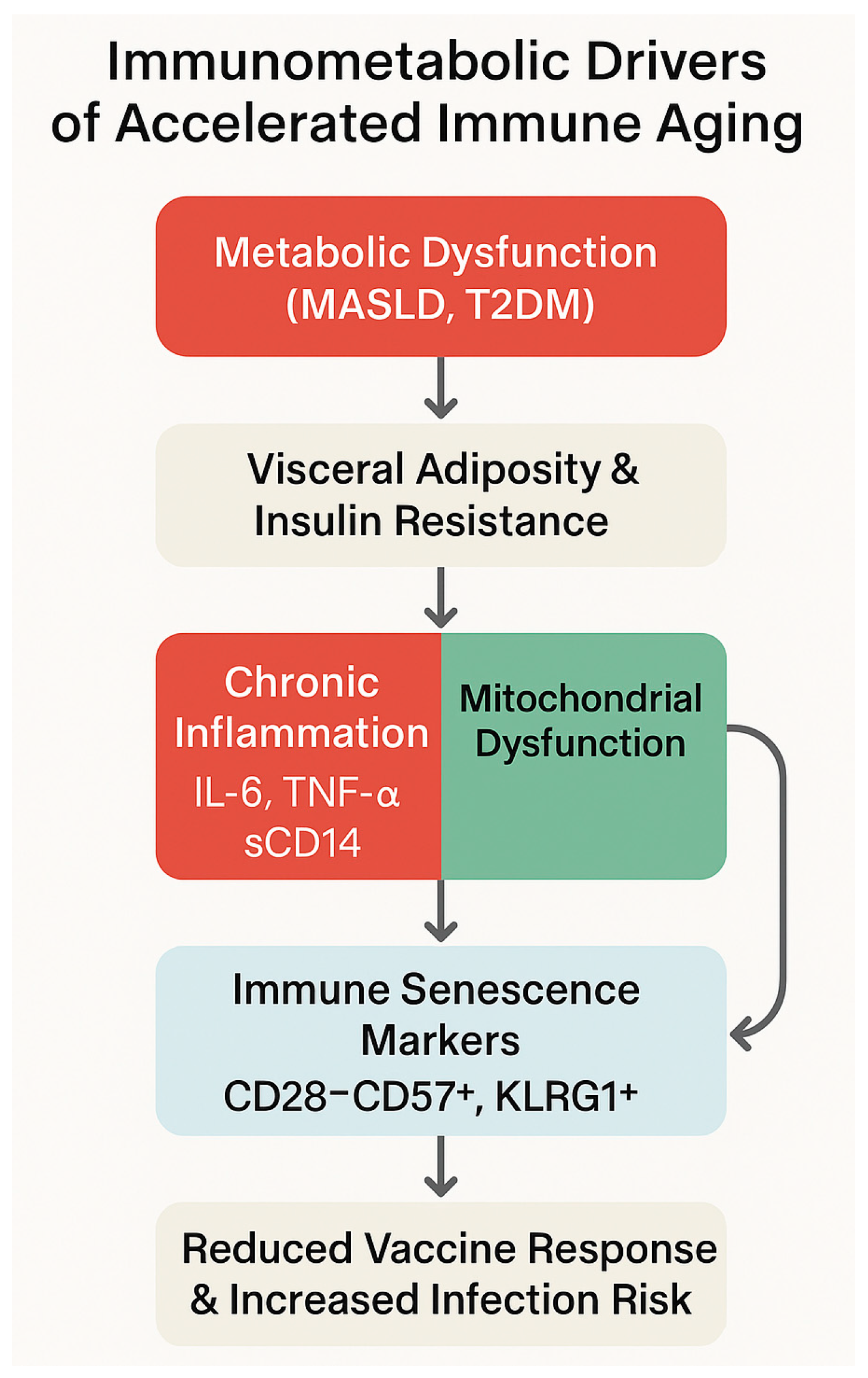
| Pathogenic Driver | Description | Observations in PLWH | References |
|---|---|---|---|
| Metabolic dysfunction | Includes insulin resistance, visceral obesity, MASLD, T2DM | Highly prevalent despite viral suppression | [2,3,4] |
| Visceral adipose tissue | Pro-inflammatory secretome (IL-6, TNF-α, leptin); ROS and DAMPs | Induces senescent immune activation | [5] |
| HIV-related immune remodeling | CD4/CD8 inversion, CD8+CD28− accumulation, naive T-cell loss | Persistent despite effective ART | [6,7] |
| Gut–liver–immune axis | Microbial translocation, chronic hepatic inflammation | Altered mucosal barrier, liver dysfunction | [9,10] |
| Immune aging biomarkers | Epigenetic clocks, IMM-AGE, TyG index | Predict frailty and cardiometabolic risk | [11,12] |
| Condition | Pathophysiological Features | Immune Consequences in PLWH | References |
|---|---|---|---|
| MASLD | FFA overload, lipotoxicity, ROS, NLRP3 activation | CD8+ and NKT senescence; CD4/CD8 ratio decline | [13,14,15,16,17,18,24,25] |
| Mitochondrial dysfunction | Impaired biogenesis (PGC-1α, TFAM), increased oxidative stress | Reduced immune metabolic flexibility | [26,27] |
| T2DM | Chronic hyperglycemia, adipokine imbalance, systemic metaflammation | Expansion of CD28−/CD57+ T cells, naive T-cell loss | [30,31,32,33,34,35,36] |
| Metaflammation mediators | DAMPs, mtDNA, ATP, ROS activating PRRs and inflammasomes | Amplifies systemic senescence in PLWH | [37,38,39,40] |
| TyG index | Surrogate for insulin resistance; correlates with senescent T cells | Proposed immunometabolic risk biomarker | [29,43,44] |
| Immune Component | Senescent Features | HIV-Related Amplification | References |
|---|---|---|---|
| Innate immunity | Reduced neutrophil chemotaxis, impaired NK cytotoxicity | Chronic activation, low IFN-γ output | [73,74,75] |
| Adaptive T cells | CD8+CD28−CD57+KLRG1+ expansion, TCR loss, apoptosis resistance | Persistent even with ART | [76,77,78,79,80] |
| B cells | Decreased naive pool, increase in ABCs, poor antibody response | Impaired vaccination efficacy | [78,79] |
| Molecular markers | Epigenetic clocks, telomere shortening, IMM-AGE, iAge, TyG | Accelerated epigenetic aging in PLWH | [87,91,92,93,94] |
| Functional consequences | Reduced immune coordination (“integrated immunocompetence”) | Elevated frailty, multimorbidity | [83,85] |
| Metabolic Disruption | Immune Impact | Observations in PLWH with MASLD/T2DM | References |
|---|---|---|---|
| Loss of immune plasticity | Impaired glycolysis–OXPHOS switch; ATP deficit | Promotes senescent, exhausted phenotypes | [120,121,122,123] |
| Steatotic liver environment | ROS, IL-1β/IL-6 secretion, NLRP3 activation | Dysfunctional CD8+/NKT cells with PD-1+/CD57+ | [130,131,132] |
| Mitochondrial dysfunction | Reduced PGC-1α, TFAM, mitophagy dysregulation | Metabolic exhaustion of immune cells | [27,131] |
| T2DM-induced reprogramming | Persistent mTOR/HIF-1α activation; glycolytic overload | SASP phenotypes, Treg depletion | [133,134] |
| TyG index | Captures interplay of hepatic steatosis and insulin resistance | Tracks CD4/CD8 inversion and senescent T cells | [29] |
| Axis Component | Dysregulation Mechanism | Immunological Repercussions in PLWH | References |
|---|---|---|---|
| Adipose tissue | Leptin/resistin ↑, adiponectin ↓; M1 macrophage recruitment | Chronic metaflammation and cytokine storm | [135,136,137] |
| Dysbiosis | SCFAs depletion, pro-inflammatory taxa enrichment | Loss of mucosal tolerance, immune senescence | [139,140] |
| Microbial translocation | LPS and PAMPs reach liver, activate PRRs | Exacerbates hepatic and systemic inflammation | [140,141] |
| SCFA modulation | Butyrate activates GPR41/43 on immune cells | T-cell memory and regulatory circuits affected | [142] |
| ART effects | Induces lipodystrophy, worsens metabolic and microbiota balance | Accelerates immunometabolic decline | [138] |
| Mechanism | Immunological Consequences | Clinical Relevance | References |
|---|---|---|---|
| Persistent immune activation | CD4+ depletion, CD8+ hyperactivation (CD28−CD57+), IFN-γ ↑, TNF-α ↑ | Senescence phenotype despite ART | [145,146,147] |
| B- and NK-cell dysfunction | Loss of memory B cells, ABCs ↑; NK receptor downregulation | Impaired surveillance and vaccine response | [148,149] |
| Dendritic cell impairment | Decreased numbers, poor antigen presentation | Deficient priming of naive T cells | [151] |
| CMV co-infection | Clonal expansion of senescent CD8+ cells | Immune exhaustion and frailty | [152] |
| Microbial translocation | Sustains inflammation (↑ sCD14, LBP, I-FABP) | Predictor of mortality and comorbidity | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acierno, C.; Frontuto, M.; De Stefano, G.F.; Erezanu, A.; Limone, A.; Morella, S.; Picaro, F.; Palazzo, D.; Gilio, M. From Steatosis to Immunosenescence: The Impact of Metabolic Dysfunction on Immune Aging in HIV and Non-HIV Populations. Biomedicines 2025, 13, 2513. https://doi.org/10.3390/biomedicines13102513
Acierno C, Frontuto M, De Stefano GF, Erezanu A, Limone A, Morella S, Picaro F, Palazzo D, Gilio M. From Steatosis to Immunosenescence: The Impact of Metabolic Dysfunction on Immune Aging in HIV and Non-HIV Populations. Biomedicines. 2025; 13(10):2513. https://doi.org/10.3390/biomedicines13102513
Chicago/Turabian StyleAcierno, Carlo, Maria Frontuto, Giulio Francesco De Stefano, Ana Erezanu, Andrea Limone, Simona Morella, Francesco Picaro, Donatella Palazzo, and Michele Gilio. 2025. "From Steatosis to Immunosenescence: The Impact of Metabolic Dysfunction on Immune Aging in HIV and Non-HIV Populations" Biomedicines 13, no. 10: 2513. https://doi.org/10.3390/biomedicines13102513
APA StyleAcierno, C., Frontuto, M., De Stefano, G. F., Erezanu, A., Limone, A., Morella, S., Picaro, F., Palazzo, D., & Gilio, M. (2025). From Steatosis to Immunosenescence: The Impact of Metabolic Dysfunction on Immune Aging in HIV and Non-HIV Populations. Biomedicines, 13(10), 2513. https://doi.org/10.3390/biomedicines13102513






