Exploring Cannabidiol’s Role in Regenerative Medicine: Focus on Neural and Skeletal Tissues
Abstract
1. Introduction
2. Nerve and Bone Diseases Associated with Aging
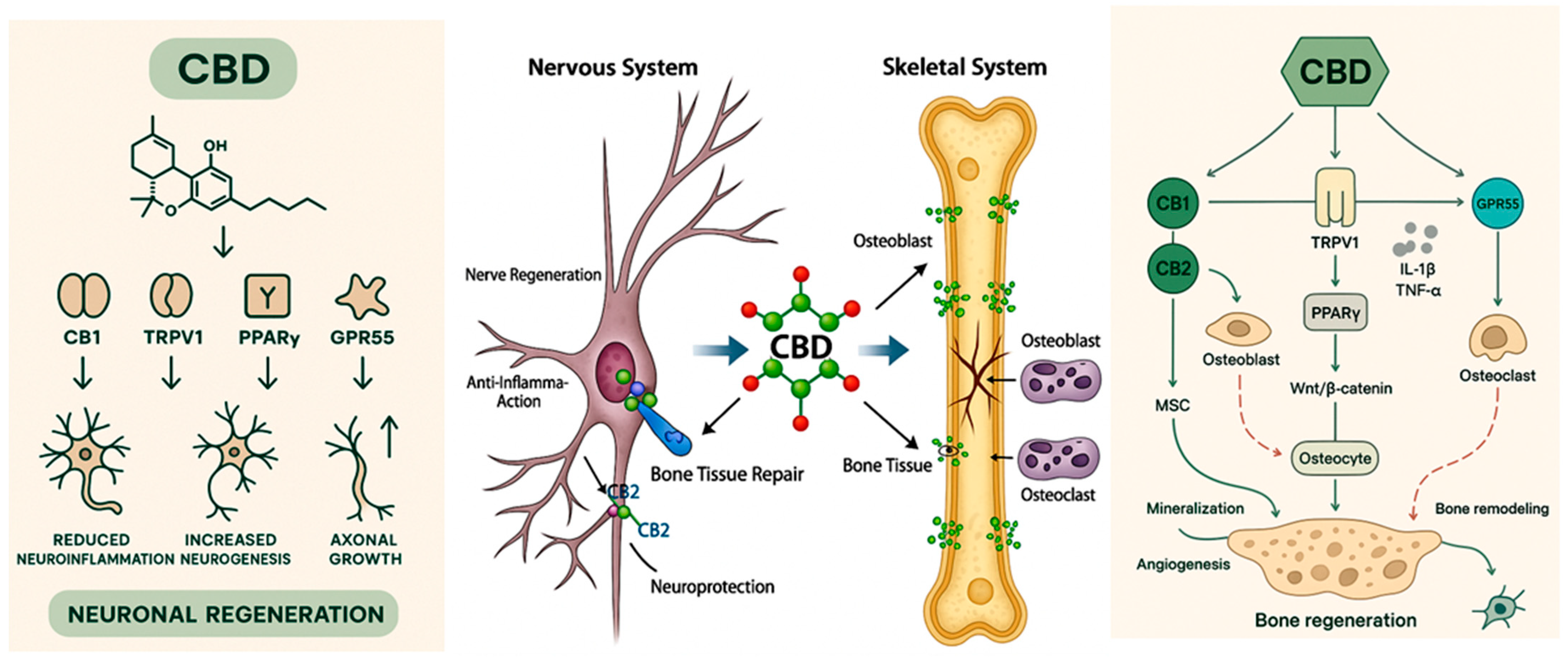
3. Molecular Mechanisms of Cannabidiol
4. Nerve Regeneration
5. Cannabidiol Associated with Neurological Diseases
5.1. Central Nervous System (CNS)
5.1.1. Epilepsy and Seizures
5.1.2. Main Neurodegenerative Diseases
Alzheimer
Parkinson
Multiple Sclerosis
5.2. Peripheral Nervous System (PNS)
5.2.1. Neuropathic Pain
5.2.2. Chronic Pain
6. Bone Regeneration
7. Cannabidiol and Bone Disease
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguiar, D.D.; da Costa Oliveira, C.; Fonseca, F.C.S.; de Almeida, D.L.; Campos Pereira, W.V.; Guimarães, F.S.; Perez, A.C.; Duarte, I.D.G.; Romero, T.R.L. Peripherally Injected Canabidiol Reduces Neuropathic Pain in Mice: Role of the 5-HT1A and TRPV1 Receptors. Biochem. Biophys. Res. Commun. 2023, 660, 58–64. [Google Scholar] [CrossRef]
- David, C.; Elizalde-Hernández, A.; Barboza, A.S.; Cardoso, G.C.; Santos, M.B.F.; Moraes, R.R. Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2022, 10, 193. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Austrich-Olivares, A.; Sala, F.; Manzanares, J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules 2020, 10, 1575. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Liu, Q.; Chen, B.; Liu, X.; Pathak, J.L.; Watanabe, N.; Li, J. Dental Pulp Stem Cells-Derived Cannabidiol-Treated Organoid-like Microspheroids Show Robust Osteogenic Potential via Upregulation of WNT6. Commun. Biol. 2024, 7, 972. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for Neurodegenerative Disorders: A Comprehensive Review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef]
- Aychman, M.M.; Goldman, D.L.; Kaplan, J.S. Cannabidiol’s Neuroprotective Properties and Potential Treatment of Traumatic Brain Injuries. Front. Neurol. 2023, 14, 1087011. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Zhao, M.; Cai, M.; Lei, F.; Zeng, X.; Zhu, B. A Bibliometrics and Visualization Analysis of Cannabidiol Research from 2004 to 2021. Front. Pharmacol. 2022, 13, 969883. [Google Scholar] [CrossRef]
- Cásedas, G.; de Yarza-Sancho, M.; López, V. Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain. Pharmaceuticals 2024, 17, 1438. [Google Scholar] [CrossRef] [PubMed]
- Capano, A.; Weaver, R.; Burkman, E. Evaluation of the Effects of CBD Hemp Extract on Opioid Use and Quality of Life Indicators in Chronic Pain Patients: A Prospective Cohort Study. Postgrad. Med. 2020, 132, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Campana, M.D.; de Paolis, G.; Sammartino, G.; Bucci, P.; Aliberti, A.; Gasparro, R. Cannabinoids: Therapeutic Perspectives for Management of Orofacial Pain, Oral Inflammation and Bone Healing—A Systematic Review. Int. J. Mol. Sci. 2025, 26, 3766. [Google Scholar] [CrossRef]
- Sierakowska-Byczek, A.; Gałuszka, A.; Janus, Ł.; Radwan-Pragłowska, J. Bioactive Three-Dimensional Chitosan-Based Scaffolds Modified with Poly(Dopamine)/CBD@Pt/Au/PVP Nanoparticles as Potential NGCs Applicable in Nervous Tissue Regeneration—Preparation and Characterization. Molecules 2024, 29, 25. [Google Scholar] [CrossRef]
- Schrot, R.J.; Hubbard, J.R. Cannabinoids: Medical Implications. Ann. Med. 2016, 48, 128–141. [Google Scholar] [CrossRef]
- Porter, B.; Marie, B.S.; Milavetz, G.; Herr, K. Cannabidiol (CBD) Use by Older Adults for Acute and Chronic Pain. J. Gerontol. Nurs. 2021, 47, 6–15. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23, 18–41. [Google Scholar] [CrossRef]
- Raïch, I.; Lillo, J.; Rivas-Santisteban, R.; Rebassa, J.B.; Capó, T.; Santandreu, M.; Cubeles-Juberias, E.; Reyes-Resina, I.; Navarro, G. Potential of CBD Acting on Cannabinoid Receptors CB1 and CB2 in Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 6708. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Vari, C.E.; Rusz, C.M.; Tero-Vescan, A.; Pușcaș, A. Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance. Antioxidants 2023, 12, 485. [Google Scholar] [CrossRef]
- Saito, V.M.; Wotjak, C.T.; Moreira, F.A. Exploração Farmacológica Do Sistema Endocanablnolde: Novas Perspectivas Para o Tratamento de Transtornos de Ansiedade e Depressão? Rev. Bras. Psiquiatr. 2010, 32, 7–14. [Google Scholar] [CrossRef]
- Barbosa, F.C.; Tadine, R.M.; Rezende, J.D.P.; Lopes, G.C.D.; Shiozawa, P. Neurociência Do Canabidiol—Aspectos Neurocognitivos, Terapêuticos e Comportamentais. Obs. La Econ. Latinoam. 2023, 21, 8891–8914. [Google Scholar] [CrossRef]
- Sadri, F.; Rezaei, Z.; Fereidouni, M. The Significance of the SDF-1/CXCR4 Signaling Pathway in the Normal Development. Mol. Biol. Rep. 2022, 49, 3307–3320. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Lemmer, Y.; Mason, S. A Narrative Review of the Therapeutic and Remedial Prospects of Cannabidiol with Emphasis on Neurological and Neuropsychiatric Disorders. J. Cannabis Res. 2024, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Karuppagounder, V.; Nowak, I.; Sepulveda, D.E.; Lewis, G.S.; Norbury, C.C.; Raup-Konsavage, W.M.; Vrana, K.E.; Kamal, F.; Elbarbary, R.A. Cannabidiol and Cannabigerol, Nonpsychotropic Cannabinoids, as Analgesics That Effectively Manage Bone Fracture Pain and Promote Healing in Mice. J. Bone Miner. Res. 2023, 38, 1560–1576. [Google Scholar] [CrossRef]
- Zhao, J.; Han, Z.; Ding, L.; Wang, P.; He, X.; Lin, L. The Molecular Mechanism of Aging and the Role in Neurodegenerative Diseases. Heliyon 2024, 10, e24751. [Google Scholar] [CrossRef]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, e24751. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, H. Cellular Senescence in Health, Disease, and Lens Aging. Pharmaceuticals 2025, 18, 244. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [CrossRef] [PubMed]
- Cosco, T.D.; Howse, K.; Brayne, C. Healthy Ageing, Resilience and Wellbeing. Epidemiol. Psychiatr. Sci. 2017, 26, 579–583. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Mukherjee, S.K.; Biswas, S.K.; Obrycki, J.F.; Ekramullah, S.M.; Arman, D.M.; Islam, J.; Christiani, D.C.; Mazumdar, M.M.; Chen, W.J.; et al. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence from Clinical Trials and Human Laboratory Studies. HHS Public Access. Environ. Health Perspect. 2021, 8, 1–20. [Google Scholar] [CrossRef]
- Rao, I.Y.; Hanson, L.R.; Ii, W.H.F. Brain Glucose Hypometabolism and Brain Iron Accumulation as Therapeutic Targets for Alzheimer’ s Disease and Other CNS Disorders. Pharmaceuticals 2025, 18, 271. [Google Scholar] [CrossRef]
- Marrone, G.; Urciuoli, S.; Di Lauro, M.; Cornali, K.; Masci, C.; Tesauro, M.; Vignolini, P.; Noce, A. The Possible Role of Plant-Based Bars Consumption in CKD Geriatric Patients. Pharmaceuticals 2024, 17, 1689. [Google Scholar] [CrossRef]
- Wada, Y.; Inoko, M.; Ishihara, K.; Fukumoto, K.; Tsurudome, Y.; Horiguchi, M.; Fujimura, A.; Ushijima, K. Aging Reduces ATP-Binding Cassette Transporter Expression in Brain Microvessels of Mice. Pharmaceuticals 2025, 18, 191. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Oliveira, A.I.; Cruz, A.; Ribeiro, O.; Afreixo, V.; Pimentel, F. Complexity of the Therapeutic Regimen in Older Adults with Cancer: Associated Factors. Pharmaceuticals 2024, 17, 1541. [Google Scholar] [CrossRef]
- Ni, B.; Liu, Y.; Dai, M.; Zhao, J.; Liang, Y.; Yang, X.; Han, B.; Jiang, M. The Role of Cannabidiol in Aging. Biomed. Pharmacother. 2023, 165, 115074. [Google Scholar] [CrossRef]
- Philpott, H.T.; O’Brien, M.; McDougall, J.J. Attenuation of Early Phase Inflammation by Cannabidiol Prevents Pain and Nerve Damage in Rat Osteoarthritis. Pain 2017, 158, 2442–2451. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, S.Q. Antiosteoporosis Effects, Pharmacokinetics, and Drug Delivery Systems of Icaritin: Advances and Prospects. Pharmaceuticals 2022, 15, 397. [Google Scholar] [CrossRef]
- Stromsnes, K.; Fajardo, C.M.; Soto-Rodriguez, S.; Kajander, E.R.U.; Lupu, R.I.; Pozo-Rodriguez, M.; Boira-Nacher, B.; Font-Alberich, M.; Gambini-Castell, M.; Olaso-Gonzalez, G.; et al. Osteoporosis: Causes, Mechanisms, Treatment and Prevention: Role of Dietary Compounds. Pharmaceuticals 2024, 17, 1697. [Google Scholar] [CrossRef]
- Dong, H.; Tang, F.; Zhao, Z.; Huang, W.; Wan, X.; Hong, Z.; Liu, Y.; Dong, X.; Chen, S. The Bioactive Compounds of Epimedium and Their Potential Mechanism of Action in Treating Osteoporosis: A Network Pharmacology and Experimental Validation Study. Pharmaceuticals 2024, 17, 706. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Lee, J.; Lee, D. Emerging Roles of Natural Compounds in Osteoporosis: Regulation, Molecular Mechanisms and Bone Regeneration. Pharmaceuticals 2024, 17, 984. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zeng, L.; Zhang, Z.; Zhu, G.; Xu, Z.; Xia, J.; Weng, J.; Li, J.; Pathak, J.L. Cannabidiol Rescues TNF-α-Inhibited Proliferation, Migration, and Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells. Biomolecules 2023, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Boix, F.; Hasselstrøm, J.B.; Sørensen, L.K.; Kjolby, M.; Gustavsen, S.; Hansen, R.M.; Petersen, T.; Sellebjerg, F.; Kasch, H.; et al. Pharmacokinetics and Pharmacodynamics of Cannabis-Based Medicine in a Patient Population Included in a Randomized, Placebo-Controlled, Clinical Trial. Clin. Transl. Sci. 2024, 17, e13685. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhao, P.Y.; Yang, X.P.; Li, H.; Hu, S.D.; Xu, Y.X.; Du, X.H. Cannabidiol Regulates Apoptosis and Autophagy in Inflammation and Cancer: A Review. Front. Pharmacol. 2023, 14, 1094020. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis Sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. Biomed Res. Int. 2018, 2018, 15. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Mai, Y.; Zhang, Z.; Li, S.; Lin, H.; Wei, F.; Chen, Y. CBD-Conjugated BMP-Inhibiting Exosomes on Collagen Scaffold Dual-Target Achilles Tendon Repair: Synergistic Regeneration and Heterotopic Ossification Prevention. Mater. Today Bio 2025, 32, 101790. [Google Scholar] [CrossRef]
- Gross, C.; Ramirez, D.A.; McGrath, S.; Gustafson, D.L. Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells. Front. Pharmacol. 2021, 12, 725136. [Google Scholar] [CrossRef]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Front. Vet. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; Elsohly, M.A. Minor Oxygenated Cannabinoids from High Potency Cannabis Sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef]
- Beers, J.L.; Zhou, Z.; Jackson, K.D. Advances and Challenges in Modeling Cannabidiol Pharmacokinetics and Hepatotoxicity. Drug Metab. Dispos. 2024, 52, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.W.; Bardhi, K.; Fort, G.; Chen, G.; Lazarus, P. Inhibition of UDP-Glucuronosyltransferase Enzymes by Major Cannabinoids and Their Metabolites. Drug Metab. Dispos. 2021, 49, 1081–1089. [Google Scholar] [CrossRef]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Russo, E.B. Practical Considerations in Medical Cannabis Administration and Dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef]
- Bow, E.W.; Rimoldi, J.M. The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect. Medicin. Chem. 2016, 8, 17–39. [Google Scholar] [CrossRef]
- Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules 2023, 28, 5980. [Google Scholar] [CrossRef]
- Finn, D. Vanilloid Receptors. xPharm Compr. Pharmacol. Ref. 2007, 405, 1–3. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Blangetti, M.; Namdar, D.; Koltai, H. Structure-Activity Relationship of Cannabis Derived Compounds for the Treatment of Neuronal Activity-Related Diseases. Molecules 2018, 23, 1526. [Google Scholar] [CrossRef] [PubMed]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABAA Receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Drysdale, A.J.; Ryan, D.; Pertwee, R.G.; Platt, B. Cannabidiol-Induced Intracellular Ca2+ Elevations in Hippocampal Cells. Neuropharmacology 2006, 50, 621–631. [Google Scholar] [CrossRef]
- Zeng, W.; Yang, F.; Shen, W.L.; Zhan, C.; Zheng, P.; Hu, J. Interactions between Central Nervous System and Peripheral Metabolic Organs. Sci. China Life Sci. 2022, 65, 1929–1958. [Google Scholar] [CrossRef]
- Murtazina, A.; Adameyko, I. The Peripheral Nervous System. Development 2023, 150, dev201164. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Barone, D.G.; Wronowski, F.; Malliaras, G.G.; Fawcett, J.W.; Franze, K. Regenerative Capacity of Neural Tissue Scales with Changes in Tissue Mechanics Post Injury. Biomaterials 2023, 303, 122393. [Google Scholar] [CrossRef]
- Huebner, E.A.; Strittmatter, S.M. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009, 48, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central Nervous System Regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef]
- Ferrari, E.A.d.M.; Toyoda, M.S.S.; Faleiros, L.; Cerutti, S.M. Plasticidade Neural: Relações Com o Comportamento e Abordagens Experimentais. Psicol. Teor. Pesqui. 2001, 17, 187–194. [Google Scholar] [CrossRef]
- Acklin, S.E.; Nicholls, J.G. Intrinsic and Extrinsic Factors Influencing Properties and Growth Patterns of Identified Leech Neurons in Culture. J. Neurosci. 1990, 10, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, Neuroprotection and Neuropsychiatric Disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Marzola, P.; Melzer, T.; Pavesi, E.; Gil-Mohapel, J.; Brocardo, P.S. Exploring the Role of Neuroplasticity in Development, Aging, and Neurodegeneration. Brain Sci. 2023, 13, 1610. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Structural Plasticity of the Adult Brain. Dialogues Clin. Neurosci. 2004, 6, 135–141. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Kang, E.K.; Ji, G.Y.; Kim, Y.; Choi, I.S. In Vitro Studies on Therapeutic Effects of Cannabidiol in Neural Cells: Neurons, Glia, and Neural Stem Cells. Molecules 2021, 26, 6077. [Google Scholar] [CrossRef]
- Elia, I.; Schmieder, R.; Christen, S.; Fendt, S.-M. Organ-Specific Cancer Metabolism and Its Potential for Therapy Ilaria: Adipokines and the Endocrine Role of Adipose Tissues. Handb. Exp. Pharmacol. 2015, 1, 251–263. [Google Scholar]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective Effect of Cannabidiol in Rats Exposed to Doxorubicin Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Cannabinoids in the Management of Difficult to Treat Pain. Ther. Clin. Risk Manag. 2008, 4, 245–259. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Arena, A.; Zimmer, T.S.; van Scheppingen, J.; Korotkov, A.; Anink, J.J.; Mühlebner, A.; Jansen, F.E.; van Hecke, W.; Spliet, W.G.; van Rijen, P.C.; et al. Oxidative Stress and Inflammation in a Spectrum of Epileptogenic Cortical Malformations: Molecular Insights into Their Interdependence. Brain Pathol. 2019, 29, 351–365. [Google Scholar] [CrossRef]
- Parsons, A.L.M.; Bucknor, E.M.V.; Castroflorio, E.; Soares, T.R.; Oliver, P.L.; Rial, D. The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants 2022, 11, 157. [Google Scholar] [CrossRef]
- Lima, I.V.d.A.; Bellozi, P.M.Q.; Batista, E.M.; Vilela, L.R.; Brandão, I.L.; Ribeiro, F.M.; Moraes, M.F.D.; Moreira, F.A.; de Oliveira, A.C.P. Cannabidiol Anticonvulsant Effect Is Mediated by the PI3Kγ Pathway. Neuropharmacology 2020, 176, 108156. [Google Scholar] [CrossRef]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, Diagnostics, and Therapeutics for Alzheimer’s Disease: Breaking the Memory Barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Wang, H. Advancements in Targeting Ion Channels for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1462. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’ s Disease. HHS Public Access 2022, 397, 1577–1590. [Google Scholar] [CrossRef]
- Trojan, V.; Landa, L.; Šulcová, A.; Slíva, J.; Hřib, R. The Main Therapeutic Applications of Cannabidiol (CBD) and Its Potential Effects on Aging with Respect to Alzheimer’s Disease. Biomolecules 2023, 13, 1446. [Google Scholar] [CrossRef]
- Watt, G.; Karl, T. In Vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer’s Disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Kaszyńska, A.A. Cannabinoids: Potential for Modulation and Enhancement When Combined with Vitamin B12 in Case of Neurodegenerative Disorders. Pharmaceuticals 2024, 17, 813. [Google Scholar] [CrossRef]
- Lee, H.; Elkamhawy, A.; Rakhalskaya, P.; Lu, Q.; Nada, H.; Quan, G.; Lee, K. Small Molecules in Parkinson’s Disease Therapy: From Dopamine Pathways to New Emerging Targets. Pharmaceuticals 2024, 17, 1688. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation—An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.u.A. Parkinson’s Disease: Mechanisms, Translational Models and Management Strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Joaquim, H.P.G.; Pedrazzi, J.F.C.; Pain, A.d.O.; Duque, G.; Aprahamian, I. Cannabinoids in Late Life Parkinson’s Disease and Dementia: Biological Pathways and Clinical Challenges. Brain Sci. 2022, 12, 1596. [Google Scholar] [CrossRef]
- Kikegawa, M.; Sone, H. Comprehensive Analysis of Drug-Induced Parkinson-like Events. Pharmaceuticals 2024, 17, 1099. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Jones, É.; Vlachou, S. A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and Their Combination in Multiple Sclerosis Treatment. Molecules 2020, 25, 4930. [Google Scholar] [CrossRef]
- Häusler, D.; Weber, M.S. Towards Treating Multiple Sclerosis Progression. Pharmaceuticals 2024, 17, 1474. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, J.; Lublin, F.; Klineova, S.; Berwaerts, J.; Chinnapongse, R.; Checketts, D.; Javaid, S.; Steinerman, J.R. Efficacy of Nabiximols Oromucosal Spray on Spasticity in People with Multiple Sclerosis: Treatment Effects on Spasticity Numeric Rating Scale, Muscle Spasm Count, and Spastic Muscle Tone in Two Randomized Clinical Trials. Mult. Scler. Relat. Disord. 2023, 75, 104745. [Google Scholar] [CrossRef] [PubMed]
- Afridi, B.; Khan, H.; Akkol, E.K.; Aschner, M. Pain Perception and Management: Where do We Stand? J. Curr. Mol. Pharmacol. 2021, 14, 678–688. [Google Scholar] [CrossRef]
- Pacifico, P.; Coy-Dibley, J.S.; Miller, R.J.; Menichella, D.M. Peripheral Mechanisms of Peripheral Neuropathic Pain. Front. Mol. Neurosci. 2023, 16, 1252442. [Google Scholar] [CrossRef]
- Lovaglio, A.C.; Socolovsky, M.; Di Masi, G.; Bonilla, G. Treatment of Neuropathic Pain after Peripheral Nerve and Brachial Plexus Traumatic Injury. Neurol. India 2019, 67, S32–S37. [Google Scholar] [CrossRef]
- Silva-Cardoso, G.K.; Lazarini-Lopes, W.; Primini, E.O.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Garcia-Cairasco, N.; Leite-Panissi, C.R.A. Cannabidiol Modulates Chronic Neuropathic Pain Aversion Behavior by Attenuation of Neuroinflammation Markers and Neuronal Activity in the Corticolimbic Circuit in Male Wistar Rats. Behav. Brain Res. 2023, 452, 114588. [Google Scholar] [CrossRef]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic Pain: Diagnosis, Pathophysiological Mechanisms, and Treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Quintero, J.M.; Pulido, G.; Giraldo, L.F.; Leon, M.X.; Diaz, L.E.; Bustos, R.H. A Systematic Review on Cannabinoids for Neuropathic Pain Administered by Routes Other than Oral or Inhalation. Plants 2022, 11, 807–819. [Google Scholar] [CrossRef]
- Matos, C.; Pereira, A.T.; Jo, M.; Sousa, C.; Vinha, A.F.; Moutinho, C.; Carvalho, M. Cannabis for Chronic Pain: Mechanistic Insights and Therapeutic Challenges. Stresses 2025, 5, 7. [Google Scholar] [CrossRef]
- Santos, A.d.M.; Carvalho, H.d.O.; Gonçalves, D.E.S.; Gomes, L.P.; Colares, N.N.D.; Santos, A.V.T.d.L.T.d.; dos Santos, A.Y.S.; Teixeira, T.A.; Carvalho, J.C.T. Synergistic Pain-Reducing Effects of Bixa Orellana (Chronic® and Chronic In®) and Cannabidiol-Rich Cannabis Sativa Extracts in Experimental Pain Models. Pharmaceuticals 2024, 17, 1710. [Google Scholar] [CrossRef] [PubMed]
- Macêdo-Souza, C.; Maisonnette, S.S.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Landeira-Fernandez, J.; Leite-Panissi, C.R.A. Systemic Chronic Treatment with Cannabidiol in Carioca High- and Low-Conditioned Freezing Rats in the Neuropathic Pain Model: Evaluation of Pain Sensitivity. Pharmaceuticals 2023, 16, 1003. [Google Scholar] [CrossRef]
- Chaves, C.; Bittencourt, P.C.T.; Pelegrini, A. Ingestion of a THC-Rich Cannabis Oil in People with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pain Med. 2020, 21, 2212–2218. [Google Scholar] [CrossRef]
- Moore, A.; Straube, S.; Fisher, E.; Eccleston, C. Cannabidiol (CBD) Products for Pain: Ineffective, Expensive, and With Potential Harms. J. Pain 2024, 25, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Park, S.Y.; Ethiraj, L.P.; Singh, P.; Raj, G.; Quek, J.; Prasadh, S.; Choo, Y.; Goh, B.T. Role of Adipose-Derived Mesenchymal Stem Cells in Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 6805. [Google Scholar] [CrossRef] [PubMed]
- Paulini, M.R.; Aimone, M.; Feldman, S.; Buchaim, D.V.; Buchaim, R.L.; Issa, M. Relationship of Chronic Stress and Hypertension with Bone Resorption. J. Funct. Morphol. Kinesiol 2025, 10, 21. [Google Scholar] [CrossRef]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of Iron on Bone Homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, H. Pathophysiology and Therapeutic Management of Bone Loss in Patients with Critical Illness. Pharmaceuticals 2023, 16, 1718. [Google Scholar] [CrossRef]
- Ballout, N.; Toumieux, S.; Darwiche, W.; Gomila, C.; Trécherel, E.; Accadbled, F.; Laurencin-dalicieux, S.; Gennero, I.; Kovensky, J.; Boullier, A.; et al. Enhancement of In Vivo Bone Regeneration by the Carbohydrate Derivative DP2. Pharmaceuticals 2025, 18, 215. [Google Scholar] [CrossRef] [PubMed]
- Sieiński, W. Endometrial Stromal Neoplasms of the Uterus. A Clinicopathologic Study. Patol. Pol. 1992, 43, 30–34. [Google Scholar]
- Li, L.; Feng, J.; Sun, L.; Xuan, Y.W.; Wen, L.; Li, Y.X.; Yang, S.; Zhu, B.; Tian, X.Y.; Li, S.; et al. Cannabidiol Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in the Inflammatory Microenvironment via the CB2-Dependent P38 MAPK Signaling Pathway. Int. J. Stem Cells 2022, 15, 405–414. [Google Scholar] [CrossRef]
- Simei, J.L.Q.; Souza, J.D.R.; Pedrazzi, J.F.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.; Hallak, J.E.C.; Crippa, J.A.S. Research and Clinical Practice Involving the Use of Cannabis Products, with Emphasis on Cannabidiol: A Narrative Review. Pharmaceuticals 2024, 17, 1644. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’sullivan, S.E. Towards Better Delivery of Cannabidiol (Cbd). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Filipiuc, L.E.; Ştefănescu, R.; Solcan, C.; Ciorpac, M.; Szilagyi, A.; Cojocaru, D.; Stanciu, G.D.; Creangă, I.; Caratașu, C.C.; Ababei, D.C.; et al. Acute Toxicity and Pharmacokinetic Profile of an EU-GMP-Certified Cannabis Sativa L. in Rodents. Pharmaceuticals 2023, 16, 694. [Google Scholar] [CrossRef]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S.; et al. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607. [Google Scholar] [CrossRef]
- Łazarczyk, M.; Skiba, D.; Mickael, M.; Jaskuła, K.; Nawrocka, A.; Religa, P.; Sacharczuk, M. Opioid System and Epithelial—Mesenchymal Transition. Pharmaceuticals 2025, 18, 120. [Google Scholar]
- Li, D.; Lin, Z.; Meng, Q.; Wang, K.; Wu, J.; Yan, H. Cannabidiol Administration Reduces Sublesional Cancellous Bone Loss in Rats with Severe Spinal Cord Injury. Eur. J. Pharmacol. 2017, 809, 13–19. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Kulpa, J.; Harrison, A.; Rudolph, L.; Eglit, G.M.L.; Turcotte, C.; Bonn-Miller, M.O.; Peters, E.N. Oral Cannabidiol Treatment in Two Postmenopausal Women with Osteopenia: A Case Series. Cannabis Cannabinoid Res. 2023, 8, S83–S89. [Google Scholar] [CrossRef] [PubMed]
- Van De Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; Van Velzen, M. An Experimental Randomized Study on the Analgesic Effects of Pharmaceutical-Grade Cannabis in Chronic Pain Patients with Fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef]
- Kulpa, J.; Eglit, G.; Hill, M.L.; MacNair, L.; Yardley, H.; Ware, M.A.; Bonn-Miller, M.O.; Peters, E.N. Serum Markers of Bone Turnover Following Controlled Administration of Two Medical Cannabis Products in Healthy Adults. Cannabis Cannabinoid Res. 2024, 9, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, D.; Deshaies, A.A.; Williamson, D.; Bernard, F.; Arbour, C.; Pinard, A.M.; Rouleau, D.; De Beaumont, L. Impact of an Acute 1-Month Cannabidiol Treatment on Pain and Inflammation after a Long Bone Fracture: A Triple-Blind Randomised, Placebo-Controlled, Clinical Trial Protocol. BMJ Open 2025, 15, e092919. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Berkovic, S.F.; French, J.A.; Messenheimer, J.A.; Sebree, T.B.; Bonn-Miller, M.O.; Gutterman, D.L. Adjunctive Transdermal Cannabidiol for Adults with Focal Epilepsy: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, E2220189. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Zheng, T.; BouSaba, J.; Taylor, A.; Dilmaghani, S.; Busciglio, I.; Carlson, P.; Torres, M.; Ryks, M.; Burton, D.; Harmsen, W.S.; et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin. Gastroenterol. Hepatol. 2023, 21, 3405–3414.e4. [Google Scholar] [CrossRef]
- Chrepa, V.; Villasenor, S.; Mauney, A.; Kotsakis, G.; Macpherson, L. Cannabidiol as an Alternative Analgesic for Acute Dental Pain. J. Dent. Res. 2024, 103, 235–242. [Google Scholar] [CrossRef]
- Umpreecha, C.; Bhalang, K.; Charnvanich, D.; Luckanagul, J. Efficacy and Safety of Topical 0.1% Cannabidiol for Managing Recurrent Aphthous Ulcers: A Randomized Controlled Trial. BMC Complement. Med. Ther. 2023, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Spinella, T.C.; Stewart, S.H.; Naugler, J.; Yakovenko, I.; Barrett, S.P. Evaluating Cannabidiol (CBD) Expectancy Effects on Acute Stress and Anxiety in Healthy Adults: A Randomized Crossover Study. Psychopharmacology 2021, 238, 1965–1977. [Google Scholar] [CrossRef] [PubMed]





| Article | Kind Study | Objective | CNS | CBD | Mainly Outcomes |
|---|---|---|---|---|---|
| Taylor et al., 2018 [42] | Clinical Study | This investigation examined the safety, tolerability and pharmacokinetics of orally administered CBD in healthy volunteers. | Neuroprotection. | CBD | Results indicate that oral doses of CBD up to 6000 mg are well tolerated and are swiftly and extensively metabolized into the active compound 7-OH-CBD. |
| Lima et al., 2020 [80] | Experimental In vitro and animal study | Investigate the mediation pathways of the anticonvulsant and neuroprotective effects of CBD through an experimental study. | Neuroregeneration and neuroprotection. | CBD | CBD induced an anticonvulsant effect, in addition to reducing neurodegeneration in vivo and neuronal death in cell cultures. |
| Troján et al., 2023 [84] | Review | Conduct a review on the beneficial effects of CBD use and explore its potential applications in clinical practice. | Neuroprotections and Alzheimer. | CBD | More clinical studies are needed to build stronger evidence; however, in most cases, CBD induced beneficial effects on Alzheimer’s and in the treatment of other conditions in elderly individuals. |
| Costa et al., 2022 [90] | Review | Explore the role of CBD as an adjunctive therapy in the treatment of PD and dementia in the elderly. | Neuroprotection and Parkinson’s Disease. | CBD | Improvement of sleep quality, anxiety, and tremors caused by it, indicating an improvement in the quality of life of patients with PD. |
| Nicolau et al., 2023 [96] | Clinical Study | To deliver an extensive analysis of nabiximols spray (CBD+THC) therapy for spasticity in MS across two clinical studies. | Neuroprotection and Multiple Sclerosis. | CBD and THC | The sustained spasticity reduction observed with nabiximols over 12 weeks underscores its potential as a clinically meaningful intervention, as reflect in spasticity scores, spasm counts and muscle assessments. |
| Article | Kind Study | Objective | PNS | CBD | Mainly Outcomes |
|---|---|---|---|---|---|
| Silva-Carsoso et al., 2023 [100] | Experimental Animal study | Evaluate whether sub chronic CBD treatment could be associated with conditioned pain reversal through a study in rodents. | Pain modulation and Neuroprotection. | CBD | Systemic treatment with CBD had a positive effect on the modulation of pain or the emotional behavior associated with pain, as well as reversing the expression of proteins that may hinder neuronal regeneration and synaptic plasticity. |
| Capano et al., 2019 [10] | Clinical Study | Examine the effects of full-spectrum hemp-derived CBD on opioid consumption and quality of life outcomes in individuals with chronic pain. | Pain Modulation and Chronic Pain. | CBD and opioid | The use of CBD may contribute to a reduction in opioid intake and provide improvements in chronic pain management as well as sleep quality among individuals dependent on opioid therapy. |
| Chaves et al., 2020 [106] | Clinical Study | To investigate the potential therapeutic benefits of a THC-rich, CBD-containing cannabis oil on pain modulation and quality of life among individual with fibromyalgia. | Pain Modulation and Chronic Pain. | CBD and THC | Treatment with cannabis derivatives was associated with reduced pain scores relative to placebo, indicating their potential as an affordable and well-tolerated option for improving symptoms and quality of life in chronic pain patients. |
| Macêdo-Souza et al., 2023 [105] | Experimental Animal study | Investigate the effects of chronic systemic CBD treatment on pain alteration in rats subjected to neuropathic pain. | Pain Modulation. | CBD | The treatment results indicated the efficacy of systemic CBD treatment on the sensory aspects of chronic neuropathic pain. |
| De Vita et al., 2021 [107] | Clinical Study | This study aimed to experimentally assess the impact of CBD and the influence of expectancy on pain responses in humans. | Pain Modulation and Analgesia. | CBD | The pilot findings suggest that CBD and analgesic expectations independently and jointly reduce pain discomfort, underscoring the need for further research on the mechanisms driving CBD analgesia. |
| Article | Kind Study | Objective | Bone Regeneration | CBD | Main Outcomes |
|---|---|---|---|---|---|
| Khajuria et al., 2023 [23] | Experimental Animal study | To investigate the impact of CBD and CBG on the different stages of healing of a bone injury. | Bone fracture in mouse | CBD and CBG | CBD and CBG intensify the supervision of bone cells, this fact causes an increase in bone and mineral volume, boosting the mineralization of fibrocartilaginous heat. |
| Liu et al., 2024 [5] | Experimental In situ | To develop DPSCs based osteogenic microspheroids for the treatment of bone regeneration using CBD as osteoinduction. | Dental pulp stem cells | CBD | Due to the upregulation of WN6, DPSCs treated with CBD showed satisfactory bone regenerative potential. |
| Petrescu et al., 2020 [119] | Experimental In vitro | To determine a differentiation protocol using CBD and Vit. D3 for osteogenic differentiation of mesenchymal stem cells derived from dental tissue. | Osteogenic Differentiation | CBD and Vit. D3 | CBD and vitamin D3 enhance osteogenic differentiation potential in dental tissue derived mesenchymal stem cells. |
| Li et al., 2022 [115] | Experimental In vitro | To investigate the efficiency of CBD in the osteogenic differentiation of BMSCs in the inflammatory microenvironment. | Bone marrow mesenchymal stem cells | CBD | CBD performed osteogenic differentiation of BMSCs by CB2/p38 MAPK signaling in the inflammatory microenvironment. |
| Yu et al., 2023 [40] | Experimental In vitro | To analyze the effect of CBD on the minority, migration and mineralization of DPSCs. | Dental Pulp Stem Cells | CBD | The application of CBD in DPSCs resulted in the osteogenic differentiation of the cells in question and inhibited the action of pro-inflammatory cytokines. |
| Kulpa et al., 2023 [123] | Clinical Study | To explore the effects of oral CBD administration on bone remodeling in two postmenopausal women with osteopenia | bone remodeling | CBD | CBD was well tolerated after 12 weeks of twice-daily oral administration and was associated with a reduction in bone turnover markers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchaim, R.L.; Dias, L.C.; de Sousa, F.G.C.P.; Morais, S.d.S.; Jacintho, A.J.; Paulini, M.R.; Issa, J.P.M.; Buchaim, D.V. Exploring Cannabidiol’s Role in Regenerative Medicine: Focus on Neural and Skeletal Tissues. Biomedicines 2025, 13, 2490. https://doi.org/10.3390/biomedicines13102490
Buchaim RL, Dias LC, de Sousa FGCP, Morais SdS, Jacintho AJ, Paulini MR, Issa JPM, Buchaim DV. Exploring Cannabidiol’s Role in Regenerative Medicine: Focus on Neural and Skeletal Tissues. Biomedicines. 2025; 13(10):2490. https://doi.org/10.3390/biomedicines13102490
Chicago/Turabian StyleBuchaim, Rogerio Leone, Livia Cristina Dias, Fabiana Gomes Cardoso Pereira de Sousa, Samuel de Sousa Morais, Alexandre José Jacintho, Marina Ribeiro Paulini, João Paulo Mardegan Issa, and Daniela Vieira Buchaim. 2025. "Exploring Cannabidiol’s Role in Regenerative Medicine: Focus on Neural and Skeletal Tissues" Biomedicines 13, no. 10: 2490. https://doi.org/10.3390/biomedicines13102490
APA StyleBuchaim, R. L., Dias, L. C., de Sousa, F. G. C. P., Morais, S. d. S., Jacintho, A. J., Paulini, M. R., Issa, J. P. M., & Buchaim, D. V. (2025). Exploring Cannabidiol’s Role in Regenerative Medicine: Focus on Neural and Skeletal Tissues. Biomedicines, 13(10), 2490. https://doi.org/10.3390/biomedicines13102490








