Exosomes in Osteoarthritis: Breakthrough Innovations and Advanced Tissue Engineering for Cartilage Regeneration Since 2020
Abstract
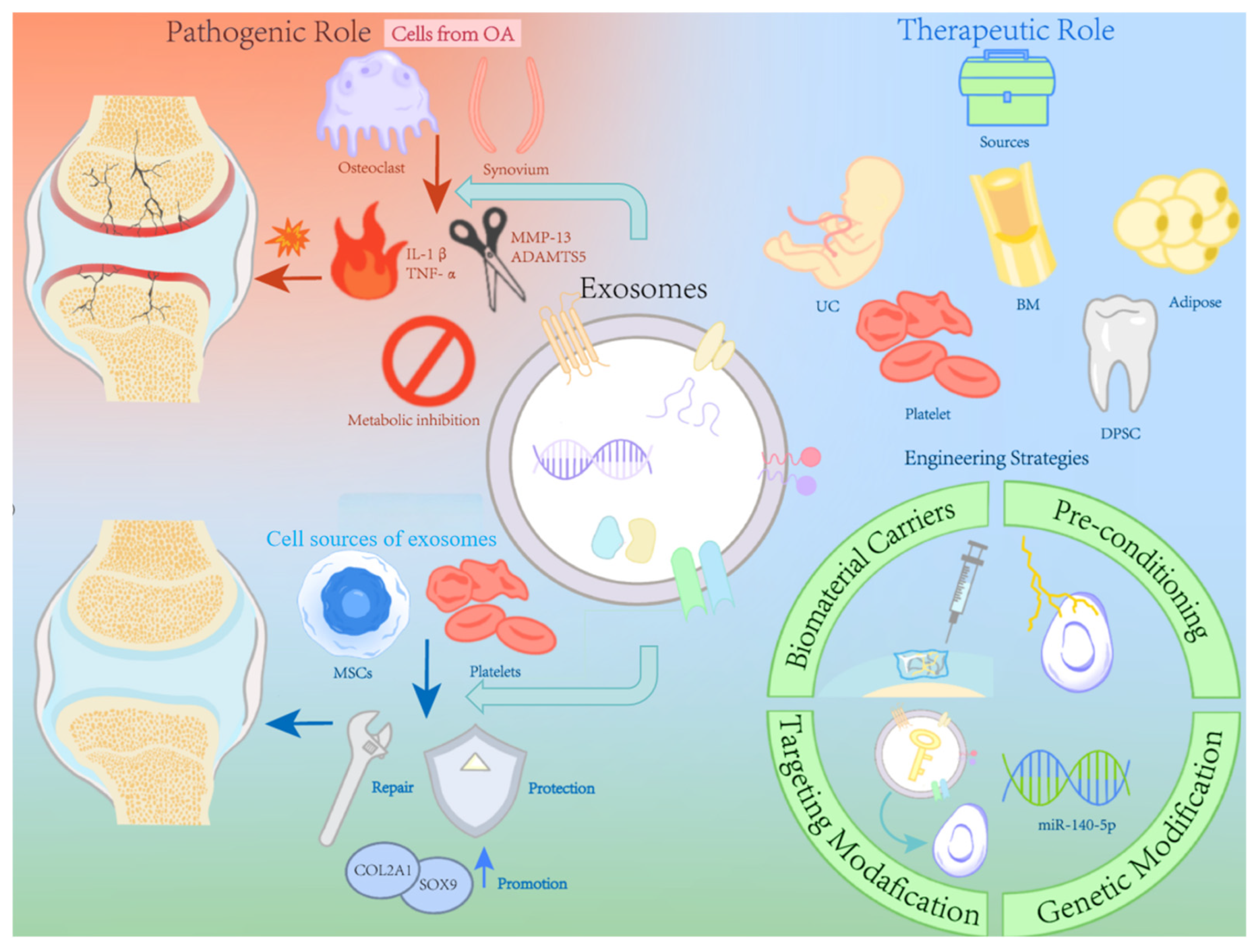
1. Introduction
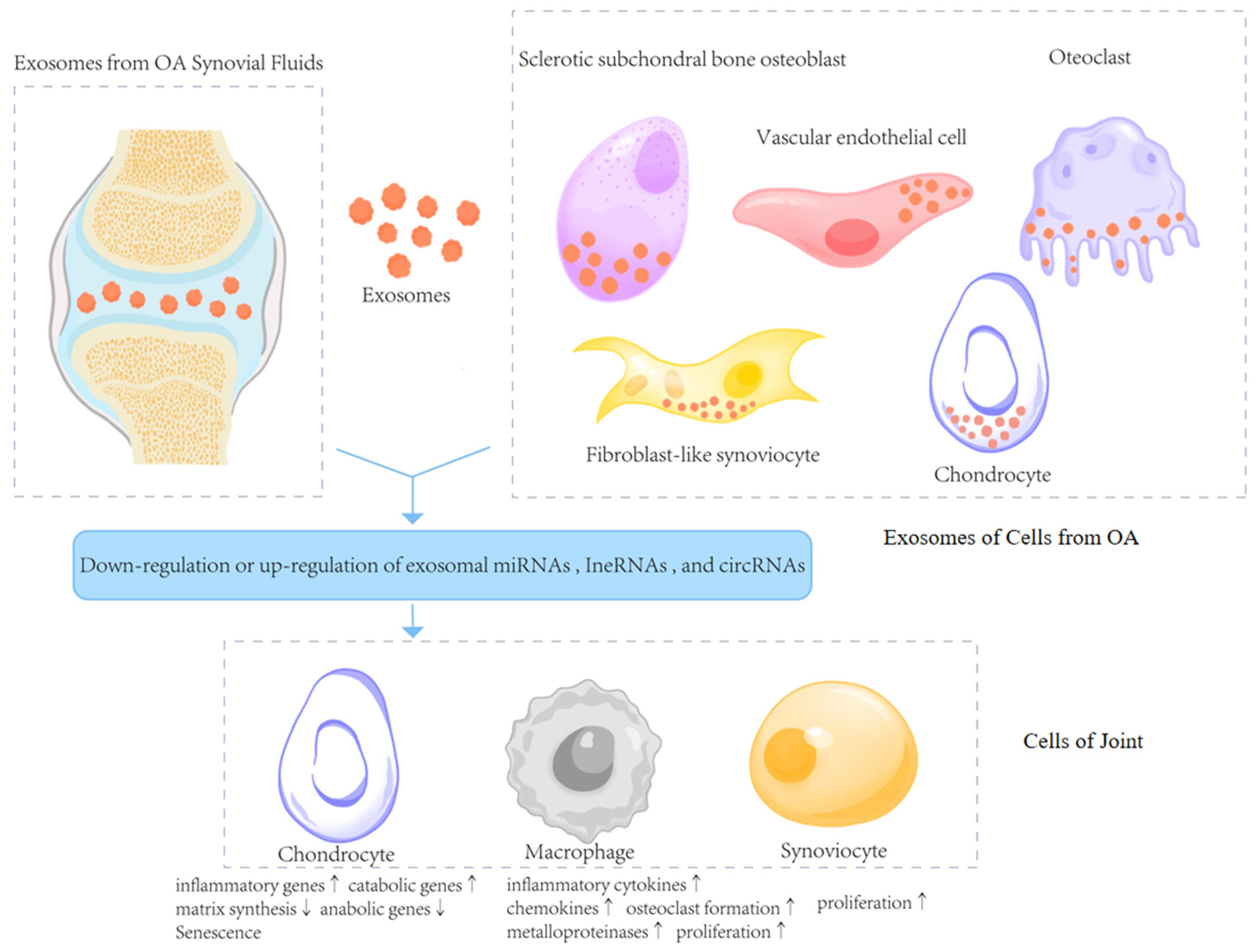
2. The Biological Characteristics of Exosomes in the Deterioration of OA
2.1. Exosomes in Synovial Fluids
2.2. Exosomes in the Tissues of OA
2.3. Exosomes as Messengers in Cartilage Diseases
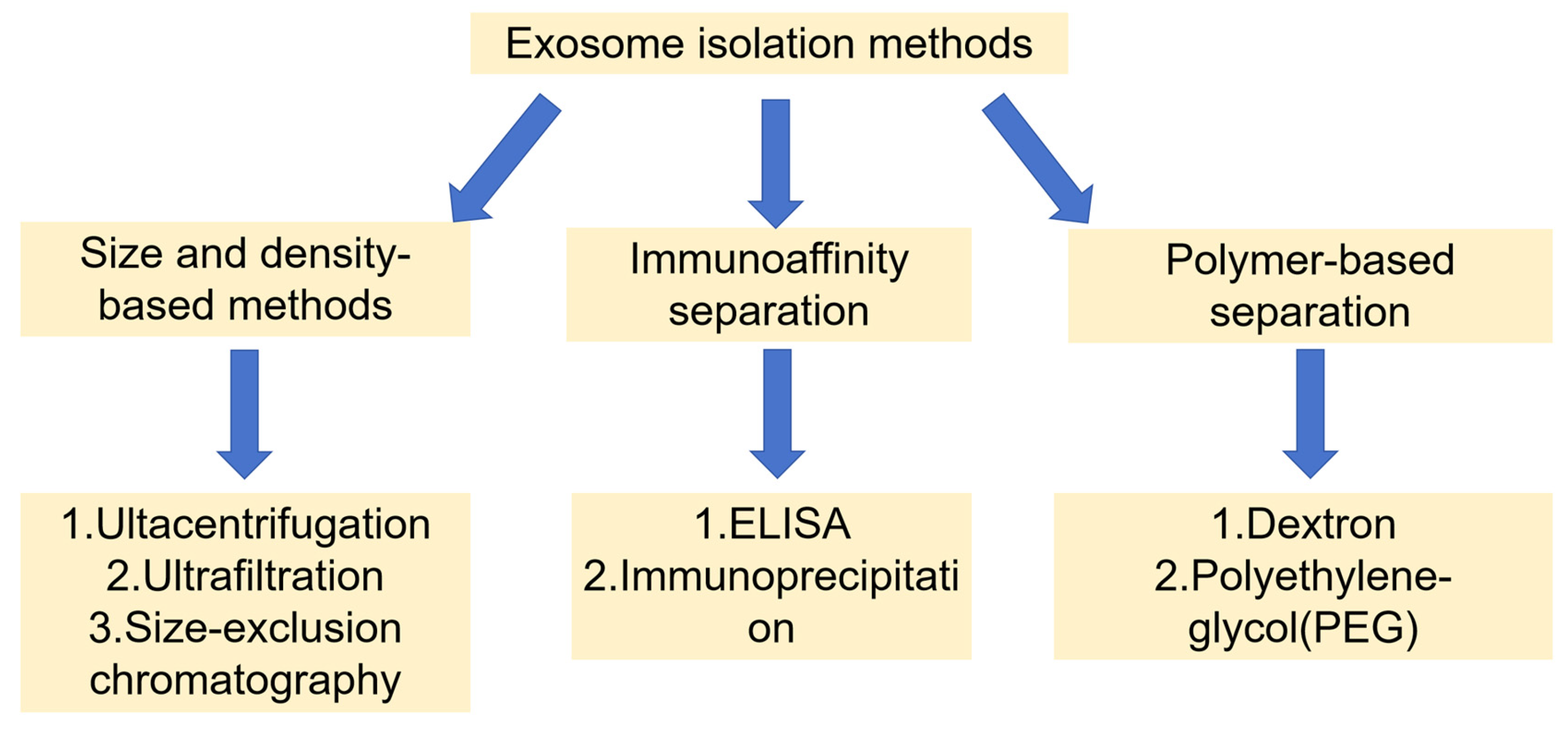
3. Exosome Isolation Methods
4. Exosomes as a Tool for Diagnosis of OA
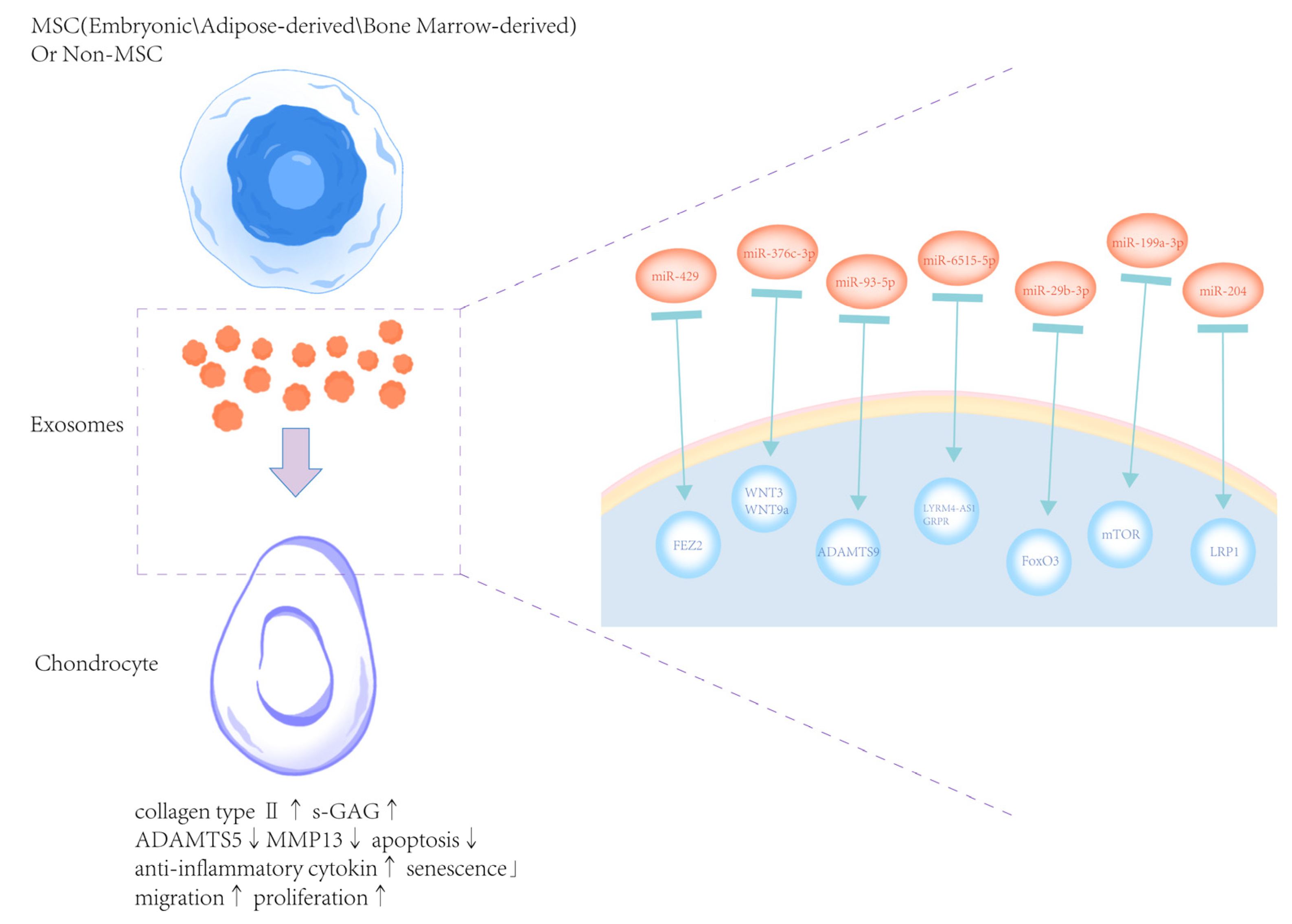
5. The Therapeutic Potential of Exosomes Derived from Natural Cells for OA
5.1. Exosomes from Embryonic MSCs
5.2. Exosomes from Adipose-Derived MSCs
5.3. Bone Marrow MSC-Derived Exosomes
5.4. Exosomes from Human Umbilical Cord-Derived MSCs (hUC-MSCs-Exos)
5.5. Exosomes from Non-MSCs
5.6. Exosomes from Plant
5.7. Exosomes from Food-Derived Sources
5.8. Exosomes from Bacterial-Derived Sources
6. Comparison of Therapeutic Potential of Exosomes Between Different Sources
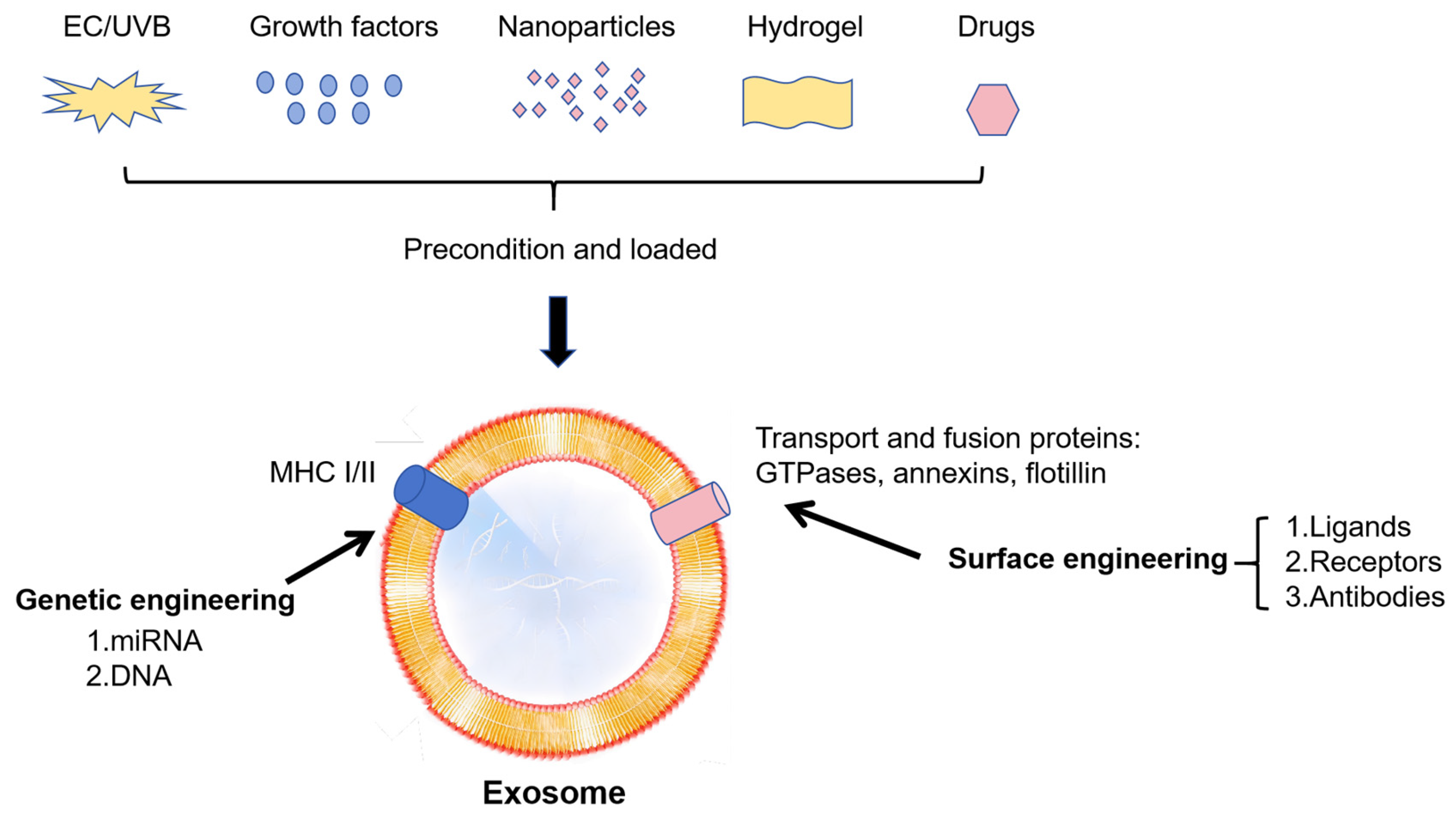
7. Exosome-Based Biochemical Engineering Strategy on OA
7.1. Advantages of Exosomes as Advanced Drug Delivery
7.2. Strategy on Cells
7.3. Strategy on 3D Culture
7.4. Strategy on Deficiencies of Exosomes
7.5. Strategy on Exosomes Carrier
7.6. Strategy on 3D Printing
8. Future Directions
8.1. Local Sustained Release System Enables the Efficacy of Agents at Small Dosages
8.2. Drug Loading Techniques for Modular Design of Contents
8.3. Future Personalized and Precision Treatment Strategies
8.4. Translational Challenges and Clinical Outlook
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hawker, G.A.; King, L.K. The Burden of Osteoarthritis in Older Adults. Clin. Geriatr. Med. 2022, 38, 181–192. [Google Scholar] [CrossRef]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xiao, K.; Xiang, S.; Li, Z.; Weng, X. Emerging Role of Exosomes in the Joint Diseases. Cell Physiol. Biochem. 2018, 47, 2008–2017. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.; Cheng, X.; Liu, Y.; Yue, M.; Hu, M.; Luo, D.; Niu, Y.; Ouyang, H.; Ji, J.; et al. Platelets promote cartilage repair and chondrocyte proliferation via ADP in a rodent model of osteoarthritis. Platelets 2016, 27, 212–222. [Google Scholar] [CrossRef]
- Sinusas, K. Osteoarthritis: Diagnosis and treatment. Am. Fam. Physician 2012, 85, 49–56. [Google Scholar]
- Telang, S.; Mayfield, C.K.; Palmer, R.; Liu, K.C.; Wier, J.; Hong, K.; Lieberman, J.R.; Heckmann, N.D. Preoperative Laboratory Values Predicting Periprosthetic Joint Infection in Morbidly Obese Patients Undergoing Total Hip or Knee Arthroplasty. J. Bone Jt. Surg. Am. 2024, 106, 1317–1327. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Jung, N.; Yu, Y.; Ryu, K.H.; Kim, H.S.; Jo, I.; Choi, B.O.; Jung, S.C. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int. J. Mol. Med. 2016, 37, 1209–1220. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Tan, S.S.; Yin, Y.; Lee, T.; Lai, R.C.; Yeo, R.W.; Zhang, B.; Choo, A.; Lim, S.K. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J. Extracell. Vesicles 2013, 2, 22614. [Google Scholar] [CrossRef]
- Pashoutan Sarvar, D.; Shamsasenjan, K.; Akbarzadehlaleh, P. Mesenchymal Stem Cell-Derived Exosomes: New Opportunity in Cell-Free Therapy. Adv. Pharm. Bull. 2016, 6, 293–299. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Wood, M.J.; Miller, R.E.; Malfait, A.M. The Genesis of Pain in Osteoarthritis: Inflammation as a Mediator of Osteoarthritis Pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef]
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Y.L.; Chen, X.Y.; Li, Q.; Zhong, J.; Dai, B.Y.; Shao, X.F.; Wu, G.B. Role of MicroRNA, LncRNA, and Exosomes in the Progression of Osteoarthritis: A Review of Recent Literature. Orthop. Surg. 2020, 12, 708–716. [Google Scholar] [CrossRef]
- Tan, F.; Wang, D.; Yuan, Z. The Fibroblast-Like Synoviocyte Derived Exosomal Long Non-coding RNA H19 Alleviates Osteoarthritis Progression Through the miR-106b-5p/TIMP2 Axis. Inflammation 2020, 43, 1498–1509. [Google Scholar] [CrossRef]
- Zeng, G.; Deng, G.; Xiao, S.; Li, F. Fibroblast-like Synoviocytes-derived Exosomal PCGEM1 Accelerates IL-1β-induced Apoptosis and Cartilage Matrix Degradation by miR-142-5p/RUNX2 in Chondrocytes. Immunol. Investig. 2022, 51, 1284–1301. [Google Scholar] [CrossRef]
- Dai, J.; Dong, R.; Han, X.; Li, J.; Gong, X.; Bai, Y.; Kang, F.; Liang, M.; Zeng, F.; Hou, Z.; et al. Osteoclast-derived exosomal let-7a-5p targets Smad2 to promote the hypertrophic differentiation of chondrocytes. Am. J. Physiol. Cell Physiol. 2020, 319, C21–C33. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Zhao, F.; Liu, M.; Wang, F.; Kang, M.; He, W.; Lv, Z. Exosomal circ-BRWD1 contributes to osteoarthritis development through the modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 2021, 23, 159. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Cai, Z.; Zhou, Q.; Li, L.; Fu, P. Exosomes from human umbilical cord mesenchymal stem cells inhibit ROS production and cell apoptosis in human articular chondrocytes via the miR-100-5p/NOX4 axis. Cell Biol. Int. 2021, 45, 2096–2106. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhan, J.; Yan, Z.; Chen, D.; Xue, X.; Pan, X. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J. Cell Mol. Med. 2021, 25, 7734–7745. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef]
- Kim, M.; Shin, D.I.; Choi, B.H.; Min, B.H. Exosomes from IL-1β-Primed Mesenchymal Stem Cells Inhibited IL-1β- and TNF-α-Mediated Inflammatory Responses in Osteoarthritic SW982 Cells. Tissue Eng. Regen. Med. 2021, 18, 525–536. [Google Scholar] [CrossRef]
- Lin, T.; Wu, N.; Wang, L.; Zhang, R.; Pan, R.; Chen, Y.F. Inhibition of chondrocyte apoptosis in a rat model of osteoarthritis by exosomes derived from miR-140-5p-overexpressing human dental pulp stem cells. Int. J. Mol. Med. 2021, 47, 7. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-Targeted MicroRNA Delivery by Engineered Exosomes toward a Cell-Free Osteoarthritis Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef]
- Dong, J.; Li, L.; Fang, X.; Zang, M. Exosome-Encapsulated microRNA-127-3p Released from Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Osteoarthritis Through Regulating CDH11-Mediated Wnt/β-Catenin Pathway. J. Pain Res. 2021, 14, 297–310. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H.; Guo, J.; Yuan, Y.; Ni, G. Effects of BMSC-Derived EVs on Bone Metabolism. Pharmaceutics 2022, 14, 1012. [Google Scholar] [CrossRef]
- Bin-Bin, Z.; Da-Wa, Z.X.; Chao, L.; Lan-Tao, Z.; Tao, W.; Chuan, L.; Chao-Zheng, L.; De-Chun, L.; Chang, F.; Shu-Qing, W.; et al. M2 macrophagy-derived exosomal miRNA-26a-5p induces osteogenic differentiation of bone mesenchymal stem cells. J. Orthop. Surg. Res. 2022, 17, 137. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, D.; Fu, Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021, 269, 118987. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, J.; Wang, Z.; Tao, H.; Bai, J.; Ge, G.; Li, W.; Zhang, W.; Hao, Y.; Yang, X.; et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg. Chem. 2021, 113, 104978. [Google Scholar] [CrossRef]
- Jin, Z.; Ren, J.; Qi, S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020, 381, 99–114. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Si, H.B.; Tang, L.; Xie, H.Q.; Shen, B. Exosomes Derived From Human Urine-Derived Stem Cells Overexpressing miR-140-5p Alleviate Knee Osteoarthritis Through Downregulation of VEGFA in a Rat Model. Am. J. Sports Med. 2022, 50, 1088–1105. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127. [Google Scholar] [CrossRef]
- Li, Y.; Tu, Q.; Xie, D.; Chen, S.; Gao, K.; Xu, X.; Zhang, Z.; Mei, X. Triamcinolone acetonide-loaded nanoparticles encapsulated by CD90(+) MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis. J. Nanobiotechnol. 2022, 20, 150. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Z. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing Long Noncoding RNA NEAT1 Relieve Osteoarthritis. Oxidative Med. Cell Longev. 2022, 2022, 5517648. [Google Scholar] [CrossRef]
- Yang, Q.; Yao, Y.; Zhao, D.; Zou, H.; Lai, C.; Xiang, G.; Wang, G.; Luo, L.; Shi, Y.; Li, Y.; et al. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am. J. Transl. Res. 2021, 13, 1245–1256. [Google Scholar]
- Meng, Y.; Qiu, S.; Sun, L.; Zuo, J. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-κB pathway via miR-93-5p. Mol. Med. Rep. 2020, 22, 5313–5325. [Google Scholar] [CrossRef]
- Tao, S.C.; Huang, J.Y.; Gao, Y.; Li, Z.X.; Wei, Z.Y.; Dawes, H.; Guo, S.C. Small extracellular vesicles in combination with sleep-related circRNA3503: A targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021, 6, 4455–4469. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, K.; Zhou, W.; Wu, H.; Lu, Y. Exosome-mediated circ_0001846 participates in IL-1β-induced chondrocyte cell damage by miR-149-5p-dependent regulation of WNT5B. Clin. Immunol. 2021, 232, 108856. [Google Scholar] [CrossRef]
- Mao, G.; Xu, Y.; Long, D.; Sun, H.; Li, H.; Xin, R.; Zhang, Z.; Li, Z.; Yang, Z.; Kang, Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 2021, 12, 389. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef]
- Ji, Y.; Xiong, L.; Zhang, G.; Xu, M.; Qiu, W.; Xiu, C.; Kuang, G.; Rui, Y. Synovial fluid exosome-derived miR-182-5p alleviates osteoarthritis by downregulating TNFAIP8 and promoting autophagy through LC3 signaling. Int. Immunopharmacol. 2023, 125, 111177. [Google Scholar] [CrossRef]
- Semerci Sevimli, T.; Sevimli, M.; Qomi Ekenel, E.; Altuğ Tasa, B.; Nur Soykan, M.; Demir Güçlüer, Z.; İnan, U.; Uysal, O.; Güneş Bağış, S.; Çemrek, F.; et al. Comparison of exosomes secreted by synovial fluid-derived mesenchymal stem cells and adipose tissue-derived mesenchymal stem cells in culture for microRNA-127-5p expression during chondrogenesis. Gene 2023, 865, 147337. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Huang, Q.; Sun, S.; Ji, Z.; Huang, L.; Li, Z.; Huang, X.; Deng, W.; Li, T. TMT-Based Quantitative Proteomics Analysis of Synovial Fluid-Derived Exosomes in Inflammatory Arthritis. Front. Immunol. 2022, 13, 800902. [Google Scholar] [CrossRef]
- Lai, C.; Liao, B.; Peng, S.; Fang, P.; Bao, N.; Zhang, L. Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats. Mol. Cell Biochem. 2023, 478, 637–649. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Zheng, M.; Hou, M.; Zhou, M.; Su, N.; Lang, H.; Zhao, L.; Gu, M.; Tang, N.; et al. The role of exosomal lncRNAs in acetaminophen-induced induced liver injury in SD rats. Noncoding RNA Res. 2024, 9, 1190–1202. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, W.; Li, H.; Ma, D.; Liu, W.; Yu, W.; Wang, L.; Cao, Y.; Jiang, Y. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol. 2020, 30, 758–764. [Google Scholar] [CrossRef]
- Wu, X.; Bian, B.; Lin, Z.; Wu, C.; Sun, Y.; Pan, Y.; Dai, Y.; Lui, T.H.; Zhuang, T.; Pan, X. Identification of exosomal mRNA, lncRNA and circRNA signatures in an osteoarthritis synovial fluid-exosomal study. Exp. Cell Res. 2022, 410, 112881. [Google Scholar] [CrossRef]
- Wu, S.; Luo, J.; Zhang, X.; Wang, L.; Cai, L.; Xu, J. Synovia tissue-specific exosomes participate in the dual variation of the osteoarthritis microenvironment via miR-182. Exp. Cell Res. 2024, 436, 113981. [Google Scholar] [CrossRef]
- Wu, X.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Osteoarthritic Subchondral Bone Release Exosomes That Promote Cartilage Degeneration. Cells 2021, 10, 251. [Google Scholar] [CrossRef]
- Yang, R.Z.; Zheng, H.L.; Xu, W.N.; Zheng, X.F.; Li, B.; Jiang, L.S.; Jiang, S.D. Vascular endothelial cell-secreted exosomes facilitate osteoarthritis pathogenesis by promoting chondrocyte apoptosis. Aging 2021, 13, 4647–4662. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, X.; Lu, J.; Huang, G.; Dang, L.; Zhang, H.; Zhong, C.; Zhang, Z.; Li, D.; Li, F.; et al. Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nat. Aging 2021, 1, 368–384. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, B.; Li, L.; Li, Y.; Li, X.; He, H.; Kuang, L. Exosomes from dysfunctional chondrocytes affect osteoarthritis in Sprague-Dawley rats through FTO-dependent regulation of PIK3R5 mRNA stability. Bone Jt. Res. 2022, 11, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xian, Y.; Chen, X.; Shi, Y.; Dong, J.; Yang, L.; An, X.; Shen, T.; Wu, W.; Ma, Y.; et al. Inflammatory Fibroblast-Like Synoviocyte-Derived Exosomes Aggravate Osteoarthritis via Enhancing Macrophage Glycolysis. Adv. Sci. 2024, 11, e2307338. [Google Scholar] [CrossRef]
- Kong, R.; Ji, L.; Pang, Y.; Zhao, D.; Gao, J. Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microRNA-19b-3p to target SLC7A11 in osteoarthritis. Front. Immunol. 2023, 14, 1181156. [Google Scholar] [CrossRef]
- Wang, C.; Hu, M.; Yuan, Y.; Lv, X.; Li, S.; Chen, S.; Zhang, F.; Wu, Y.; Zhang, Y.; Liu, Y.; et al. Modulation of Ras signaling pathway by exosome miRNAs in T-2 toxin-induced chondrocyte injury. Toxicology 2024, 506, 153858. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Huang, Y.; Li, S.; Zhang, X.; Wu, Z.; Zhao, W.; Zhu, J.; Zhang, J.; Huang, G.; et al. Investigating Novel Therapeutic Approaches for Idiopathic Short Stature: Targeting siRNA and Growth Hormone Delivery to the Growth Plate Using Exosome Nanoparticles. Adv. Sci. 2024, 11, e2309559. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Y.; Lin, S.; Li, L.; Guo, X.; Yumiseba, S.; Yang, J.D.; Hariri, R.; Ye, Q.; He, S.; et al. Characterization of human placenta-derived exosome (pExo) as a potential osteoarthritis disease modifying therapeutic. Arthritis Res. Ther. 2023, 25, 229. [Google Scholar] [CrossRef]
- Shu, S.; Yang, Y.; Allen, C.L.; Hurley, E.; Tung, K.H.; Minderman, H.; Wu, Y.; Ernstoff, M.S. Purity and yield of melanoma exosomes are dependent on isolation method. J. Extracell. Vesicles 2020, 9, 1692401. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Diaz, G.; Bridges, C.; Lucas, M.; Cheng, Y.; Schorey, J.S.; Dobos, K.M.; Kruh-Garcia, N.A. Protein Digestion, Ultrafiltration, and Size Exclusion Chromatography to Optimize the Isolation of Exosomes from Human Blood Plasma and Serum. J. Vis. Exp. 2018, 134, 57467. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed. Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef]
- Zeringer, E.; Barta, T.; Li, M.; Vlassov, A.V. Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 2015, 319–323. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Sall, I.M.; Flaviu, T.A. Plant and mammalian-derived extracellular vesicles: A new therapeutic approach for the future. Front. Bioeng. Biotechnol. 2023, 11, 1215650. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Whiteside, T.L. Immunoaffinity-Based Isolation of Melanoma Cell-Derived and T Cell-Derived Exosomes from Plasma of Melanoma Patients. Methods Mol. Biol. 2021, 2265, 305–321. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Slyusarenko, M.; Nikiforova, N.; Sidina, E.; Nazarova, I.; Egorov, V.; Garmay, Y.; Merdalimova, A.; Yevlampieva, N.; Gorin, D.; Malek, A. Formation and Evaluation of a Two-Phase Polymer System in Human Plasma as a Method for Extracellular Nanovesicle Isolation. Polymers 2021, 13, 458. [Google Scholar] [CrossRef]
- Chen, A.; Chen, Y.; Rong, X.; You, X.; Wu, D.; Zhou, X.; Zeng, W.; Zhou, Z. The application of exosomes in the early diagnosis and treatment of osteoarthritis. Front. Pharmacol. 2023, 14, 1154135. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, J.S.; Shin, J.H.; Moon, K.W.; Park, J.K.; Park, K.S.; Lee, E.Y. Role of Synovial Exosomes in Osteoclast Differentiation in Inflammatory Arthritis. Cells 2021, 10, 120. [Google Scholar] [CrossRef]
- Xue, L.; Wang, B.; Li, X.; Zhu, J.; Wang, W.; Huang, F.; Wang, X.; Jin, Y.; Xiong, C.; Tao, L.; et al. Comprehensive analysis of serum exosome-derived lncRNAs and mRNAs from patients with rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 201. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Yang, L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 2022, 80, 101684. [Google Scholar] [CrossRef]
- Meng, C.; Na, Y.; Han, C.; Ren, Y.; Liu, M.; Ma, P.; Bai, R. Exosomal miR-429 derived from adipose-derived stem cells ameliorated chondral injury in osteoarthritis via autophagy by targeting FEZ2. Int. Immunopharmacol. 2023, 120, 110315. [Google Scholar] [CrossRef]
- Li, F.; Xu, Z.; Xie, Z.; Sun, X.; Li, C.; Chen, Y.; Xu, J.; Pi, G. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA-376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis 2023, 28, 362–378. [Google Scholar] [CrossRef]
- Li, Y.; Duan, J.; Lin, W.; Liu, J. Exosomal miR-93-5p regulated the progression of osteoarthritis by targeting ADAMTS9. Open Med. 2023, 18, 20230668. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Cui, Y.; Cui, X.; Chen, C.; Wang, Z. Exosomes Isolated From Bone Marrow Mesenchymal Stem Cells Exert a Protective Effect on Osteoarthritis via lncRNA LYRM4-AS1-GRPR-miR-6515-5p. Front. Cell Dev. Biol. 2021, 9, 644380. [Google Scholar] [CrossRef]
- Yan, L.; Liu, G.; Wu, X. The umbilical cord mesenchymal stem cell-derived exosomal lncRNA H19 improves osteochondral activity through miR-29b-3p/FoxO3 axis. Clin. Transl. Med. 2021, 11, e255. [Google Scholar] [CrossRef]
- Zhao, S.; Xiu, G.; Wang, J.; Wen, Y.; Lu, J.; Wu, B.; Wang, G.; Yang, D.; Ling, B.; Du, D.; et al. Engineering exosomes derived from subcutaneous fat MSCs specially promote cartilage repair as miR-199a-3p delivery vehicles in Osteoarthritis. J. Nanobiotechnol. 2023, 21, 341. [Google Scholar] [CrossRef]
- Lu, K.; Wang, Q.; Hao, L.; Wei, G.; Wang, T.; Lu, W.W.; Xiao, G.; Tong, L.; Zhao, X.; Chen, D. miR-204 ameliorates osteoarthritis pain by inhibiting SP1-LRP1 signaling and blocking neuro-cartilage interaction. Bioact. Mater. 2023, 26, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.J.; Chang, C.H.; Huang, C.F.; Chen, H.T. Potential of Using Infrapatellar-Fat-Pad-Derived Mesenchymal Stem Cells for Therapy in Degenerative Arthritis: Chondrogenesis, Exosomes, and Transcription Regulation. Biomolecules 2022, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Jiang, T.; Chen, Y.; Mao, X. Mesenchymal Stem Cell-Derived Exosomes Modulate Chondrocyte Glutamine Metabolism to Alleviate Osteoarthritis Progression. Mediat. Inflamm. 2021, 2021, 2979124. [Google Scholar] [CrossRef]
- Zhou, L.; Ye, H.; Liu, L.; Chen, Y. Human Bone Mesenchymal Stem Cell-Derived Exosomes Inhibit IL-1β-Induced Inflammation in Osteoarthritis Chondrocytes. Cell J. 2021, 23, 485–494. [Google Scholar] [CrossRef]
- Li, B.; Shen, E.; Wu, Z.; Qi, H.; Wu, C.; Liu, D.; Jiang, X. BMSC-Derived Exosomes Attenuate Rat Osteoarthritis by Regulating Macrophage Polarization through PINK1/Parkin Signaling Pathway. Cartilage, 2024; ahead-of-print. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, Q.; Lin, F.; Wang, J. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered 2021, 12, 11225–11238. [Google Scholar] [CrossRef]
- Dong, S.; Xu, G.; Li, X.; Guo, S.; Bai, J.; Zhao, J.; Chen, L. Exosomes Derived from Quercetin-Treated Bone Marrow Derived Mesenchymal Stem Cells Inhibit the Progression of Osteoarthritis Through Delivering miR-124-3p to Chondrocytes. DNA Cell Biol. 2024, 43, 85–94. [Google Scholar] [CrossRef]
- Shen, X.; Qin, J.; Wei, Z.; Liu, F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. Biomed. Pharmacother. 2023, 167, 115488. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, X.; Wang, R.; Chen, W.; Qin, K.; Yan, J. Chondroprotective effects of bone marrow mesenchymal stem cell-derived exosomes in osteoarthritis. J. Bioenerg. Biomembr. 2024, 56, 31–44. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Ying, B.; Dong, X.; Qian, Q.; Gao, S. Effects of human umbilical cord mesenchymal stem cell-derived exosomes in the rat osteoarthritis models. Stem Cells Transl. Med. 2024, 13, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, W.; Lv, S.; Sun, Z.; Si, L.; Hu, J.; Yang, Y.; Qiu, D.; Liu, X.; Zhu, S.; et al. Human umbilical cord mesenchymal stem cells-derived exosomes exert anti-inflammatory effects on osteoarthritis chondrocytes. Aging 2023, 15, 9544–9560. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lv, S.; Jiang, W.; Si, L.; Liao, B.; Zhao, G.; Xu, Z.; Wang, L.; Zhang, J.; Wu, H.; et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann. Transl. Med. 2022, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mi, Z.; Dong, Z.; Chen, X.; Ji, G.; Kang, H.; Li, K.; Zhao, B.; Wang, F. Platelet-Derived Exosomes Alleviate Knee Osteoarthritis by Attenuating Cartilage Degeneration and Subchondral Bone Loss. Am. J. Sports Med. 2023, 51, 2975–2985. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, S.; Zhou, Y.; Qiu, L. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int. J. Mol. Sci. 2023, 24, 4926. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Palviainen, M.; Säisänen, L.; Karttunen, L.; Tollis, S.; Esrafilian, A.; Reijonen, J.; Julkunen, P.; Siljander, P.R.; Kröger, H.; et al. Tetraspanin profiles of serum extracellular vesicles reflect functional limitations and pain perception in knee osteoarthritis. Arthritis Res. Ther. 2024, 26, 33. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Li, L.; Pan, L.; Lu, L.; Zhi, S.; Li, W. Osteocyte-derived exosomes regulate the DLX2/wnt pathway to alleviate osteoarthritis by mediating cartilage repair. Autoimmunity 2024, 57, 2364686. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, J.; Jin, C.; Ye, L.; Wang, L.; Gao, Y.; Lv, N.; Zhang, J.; You, F.; Qiao, H.; et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J. Nanobiotechnol. 2023, 21, 78. [Google Scholar] [CrossRef]
- Ong, S.L.; Blenkiron, C.; Haines, S.; Acevedo-Fani, A.; Leite, J.A.S.; Zempleni, J.; Anderson, R.C.; McCann, M.J. Ruminant Milk-Derived Extracellular Vesicles: A Nutritional and Therapeutic Opportunity? Nutrients 2021, 13, 2505. [Google Scholar] [CrossRef]
- Pieters, B.C.H.; Arntz, O.J.; Aarts, J.; Feitsma, A.L.; van Neerven, R.J.J.; van der Kraan, P.M.; Oliveira, M.C.; van de Loo, F.A.J. Bovine Milk-Derived Extracellular Vesicles Inhibit Catabolic and Inflammatory Processes in Cartilage from Osteoarthritis Patients. Mol. Nutr. Food Res. 2022, 66, e2100764. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, H.; Li, J.; Zheng, T.; Yao, Y.; Tian, X.; Zhang, Z.; Yi, H. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Attenuates Cartilage Degeneration via Modulating Gut Microbiota in DMM-Induced Mice. Nutrients 2023, 15, 747. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Acebo, L.; Hazas, M.L.L.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022, 40, 1173–1194. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. Bacterial extracellular vesicles: An emerging avenue to tackle diseases. Trends Microbiol. 2023, 31, 1206–1224. [Google Scholar] [CrossRef]
- Niu, L.; Chen, W.; Yin, Z.; Tan, H.; Cui, J.; Su, J. Bacterial extracellular vesicles in osteoarthritis: A new bridge of the gut-joint axis. Gut Microbes 2025, 17, 2489069. [Google Scholar] [CrossRef]
- Yin, Z.; Qin, C.; Pan, S.; Shi, C.; Wu, G.; Feng, Y.; Zhang, J.; Yu, Z.; Liang, B.; Gui, J. Injectable hyperbranched PEG crosslinked hyaluronan hydrogel microparticles containing mir-99a-3p modified subcutaneous ADSCs-derived exosomes was beneficial for long-term treatment of osteoarthritis. Mater. Today Bio 2023, 23, 100813. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, G.; Yan, Y.; Wang, C.; Wang, Z.; Jiang, C.; Jiang, Z.; Ma, T.; Zhang, C.; Yan, Z. Exosomes derived from bone marrow mesenchymal stem cells pretreated with decellularized extracellular matrix enhance the alleviation of osteoarthritis through miR-3473b/phosphatase and tensin homolog axis. J. Gene Med. 2023, 25, e3510. [Google Scholar] [CrossRef]
- Liu, J.; Dong, M.; Sun, X.; Li, W.; Xing, L.; Yu, J. Prognostic Value of 18F-FDG PET/CT in Surgical Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0146195. [Google Scholar] [CrossRef]
- Sun, H.; Hu, S.; Zhang, Z.; Lun, J.; Liao, W.; Zhang, Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J. Cell Biochem. 2019, 120, 171–181. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Han, B.; Fang, W.; Yang, Z.; Wang, Y.; Zhao, S.; Hoang, B.X.; Vangsness, C.T., Jr. Enhancement of Chondrogenic Markers by Exosomes Derived from Cultured Human Synovial Fluid-Derived Cells: A Comparative Analysis of 2D and 3D Conditions. Biomedicines 2023, 11, 3145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wen, C.S.; Kuo, Y.L.; Fu, S.L.; Lin, T.Y.; Chen, C.M.; Wu, P.K.; Chen, W.M.; Wang, J.Y. GuiLu-ErXian Glue extract promotes mesenchymal stem cells (MSC)-Induced chondrogenesis via exosomes release and delays aging in the MSC senescence process. J. Ethnopharmacol. 2023, 317, 116784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, W.; Wang, J.; Xie, S.; Tao, W.A.; Lee, C.W.; Zhang, X.; Zhang, G.; Liu, Y.; Wei, D.; et al. Surface functionalization of exosomes for chondrocyte-targeted siRNA delivery and cartilage regeneration. J. Control Release 2024, 369, 493–505. [Google Scholar] [CrossRef]
- Zhang, C.; Pathrikar, T.V.; Baby, H.M.; Li, J.; Zhang, H.; Selvadoss, A.; Ovchinnikova, A.; Ionescu, A.; Chubinskaya, S.; Miller, R.E.; et al. Charge-Reversed Exosomes for Targeted Gene Delivery to Cartilage for Osteoarthritis Treatment. Small Methods 2024, 8, e2301443. [Google Scholar] [CrossRef]
- Cao, H.; Chen, M.; Cui, X.; Liu, Y.; Liu, Y.; Deng, S.; Yuan, T.; Fan, Y.; Wang, Q.; Zhang, X. Cell-Free Osteoarthritis Treatment with Sustained-Release of Chondrocyte-Targeting Exosomes from Umbilical Cord-Derived Mesenchymal Stem Cells to Rejuvenate Aging Chondrocytes. ACS Nano 2023, 17, 13358–13376. [Google Scholar] [CrossRef]
- Wan, J.; He, Z.; Peng, R.; Wu, X.; Zhu, Z.; Cui, J.; Hao, X.; Chen, A.; Zhang, J.; Cheng, P. Injectable photocrosslinking spherical hydrogel-encapsulated targeting peptide-modified engineered exosomes for osteoarthritis therapy. J. Nanobiotechnol. 2023, 21, 284. [Google Scholar] [CrossRef]
- Chen, M.; Lu, Y.; Liu, Y.; Liu, Q.; Deng, S.; Liu, Y.; Cui, X.; Liang, J.; Zhang, X.; Fan, Y.; et al. Injectable Microgels with Hybrid Exosomes of Chondrocyte-Targeted FGF18 Gene-Editing and Self-Renewable Lubrication for Osteoarthritis Therapy. Adv. Mater. 2024, 36, e2312559. [Google Scholar] [CrossRef]
- Cao, H.; Li, W.; Zhang, H.; Hong, L.; Feng, X.; Gao, X.; Li, H.; Lv, N.; Liu, M. Bio-nanoparticles loaded with synovial-derived exosomes ameliorate osteoarthritis progression by modifying the oxidative microenvironment. J. Nanobiotechnol. 2024, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Xu, G.; Gao, T.; Zhao, G.; Huang, G.; Shi, J.; Chen, J.; Song, J.; Xia, J.; Ma, X. Engineered Exosomes with ATF5-Modified mRNA Loaded in Injectable Thermogels Alleviate Osteoarthritis by Targeting the Mitochondrial Unfolded Protein Response. ACS Appl. Mater. Interfaces 2024, 16, 21383–21399. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J. Biomed. Mater. Res. A 2022, 110, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Su, W.; Li, T.; Wang, L.; Pan, J.; Wu, X.; Shao, Y.; Chen, H.; Lin, L.; Yang, Y.; et al. A modular hydrogel bioink containing microsphere-embedded chondrocytes for 3D-printed multiscale composite scaffolds for cartilage repair. iScience 2023, 26, 107349. [Google Scholar] [CrossRef]
- Kao, C.Y.; Papoutsakis, E.T. Extracellular vesicles: Exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Y.Z.; Zhang, W.; Chen, L.; Tong, T.; Liu, W.; Mu, Q.; Liu, H.; Ji, J.; Ouyang, H.W.; et al. Neonatal desensitization supports long-term survival and functional integration of human embryonic stem cell-derived mesenchymal stem cells in rat joint cartilage without immunosuppression. Stem Cells Dev. 2013, 22, 90–101. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Wang, D.; Uhrin, P.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, Ł.; Starzyński, R.R.; Tzvetkov, N.T.; et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: Molecular targets and pathways. Biotechnol. Adv. 2018, 36, 1586–1607. [Google Scholar] [CrossRef]
- Shao, L.T.; Luo, L.; Qiu, J.H.; Deng, D.Y.B. PTH (1-34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res. Ther. 2022, 24, 96. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Wang, X.X.; Xiang, X.N.; Peng, J.L.; He, C.Q.; He, H.C. The Effect of Different Frequencies of Pulsed Electromagnetic Fields on Cartilage Repair of Adipose Mesenchymal Stem Cell-Derived Exosomes in Osteoarthritis. Cartilage 2022, 13, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cell Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Thakur, S.; Anjum, M.M.; Jaiswal, S.; Kumar, A.; Deepak, P.; Anand, S.; Singh, S.; Rajinikanth, P.S. Novel Synergistic Approach: Tazarotene-Calcipotriol-Loaded-PVA/PVP-Nanofibers Incorporated in Hydrogel Film for Management and Treatment of Psoriasis. Mol. Pharm. 2023, 20, 997–1014. [Google Scholar] [CrossRef]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Cheng, X.; Liu, P.; Feng, Q.; Wang, S.; Li, Y.; Gu, H.; Zhong, L.; Chen, M.; et al. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen. Biomater. 2023, 10, rbac085. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D Printing of Microenvironment-Specific Bioinspired and Exosome-Reinforced Hydrogel Scaffolds for Efficient Cartilage and Subchondral Bone Regeneration. Adv. Sci. 2023, 10, e2303650. [Google Scholar] [CrossRef]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.S.; Jiang, W. Engineering therapeutical extracellular vesicles for clinical translation. Trends Biotechnol. 2025, 43, 61–82. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
| Kinds of RNAs | Sample | Exosome Source | Role | Reference | |
|---|---|---|---|---|---|
| miRNAs | |||||
| miRNA-100-5p | Human | Human umbilical cord MSCs | Inhibits cyclic strain-induced ROS production and apoptosis in chondrocytes by targeting NOX4 | [19] | |
| miRNA-126-3p | Rats | Synovial fibroblasts | Promotes chondrocyte proliferation and suppresses apoptosis by constraining chondrocyte inflammation | [20] | |
| miRNA-206 | Mice | Bone marrow-derived MSCs | Promotes proliferation and differentiation of osteoblasts in OA by reducing ELF3 | [21] | |
| miR-136-5p | Human | Bone marrow-derived MSCs | Inhibits chondrocyte degeneration in OA by targeting ELF3 | [22] | |
| miRNA-147b | Human | MSCs treated with IL-1β and TNF-a | Inhibits the inflammatory response of OA SW982 cells | [23] | |
| miRNA-140-5p | Human | Dental pulp stem cells | Inhibits IL-1β-induced chondrocyte apoptosis | [24] | |
| miRNA-140 | Rats | Dendritic cells | Alleviates OA progression in a rat model | [25] | |
| miR-127-3p | Rats | Bone marrow-derived MSCs | Alleviates OA by regulating the CDH11-mediated Wnt/β-catenin pathway | [26] | |
| miRNA-1208 | Human | Umbilical cord-derived MSCs | Suppress cartilage ECM degradation via decreasing level of pro-inflammatory factors | [27] | |
| miRNA-26a-5p | Rats | Bone marrow-derived MSCs | Promotes osteogenic differentiation and inhibit adipogenic differentiation | [28] | |
| miRNA-129-5p | Human | Synovial MSCs | Relieves IL-1β-induced OA by targeting HMGB1 | [29] | |
| miRNA-361-5p | Human | Bone marrow-derived MSCs | Alleviates OA by targeting DDX20 and NF-κB signaling pathway | [30] | |
| miRNA-9-5p | Rats | Bone marrow-derived MSCs | Alleviates OA degeneration by targeting SDC1 in an OA rat model | [31] | |
| miRNA-140-5p | Human | Urine-derived stem cells | Inhibits the progression of KOA by mediating VEGFA | [32] | |
| miRNA-135b | Rats | Bone marrow-derived MSCs | Attenuates cartilage injury by promoting synovial macrophage M2 polarization by targeting MAPK6 | [33] | |
| miRNA-338-3p | Human | Adipose tissue-derived MSCs | Stimulate cell proliferation and inhibit cell apoptosis | [34] | |
| lncRNAs | |||||
| lncRNA-PCGEM1 | Human | Fibroblast-like synoviocytes | Facilitates IL-1β-induced apoptosis and cartilage matrix degradation in chondrocytes by targeting the miR-142-5p/RUNX2 axis | [16] | |
| lncRNA-NEAT1 | Human | Bone marrow-derived MSCs | Activate the proliferation and autophagy of chondrocytes | [35] | |
| lncRNA-H19 | Human | Umbilical cord blood MSCs | Improves pain and central sensitization of advanced OA via miRNA-29a-3p/FOS axis | [36] | |
| lncRNA-PVT1 | Human | C28/I2 cells | Modulates chondrocyte viability, apoptosis, and inflammation responses by miR-93-5p/HMGB1/TLR4/NF-κB pathway | [37] | |
| lncRNA-H19 | Human | Fibroblast-like synoviocytes | Promotes chondrocyte proliferation and migration and inhibits matrix degradation in OA possibly by targeting the miR-106b-5p/TIMP2 axis | [15] | |
| circRNAs | |||||
| circRNA-3503 | Human | CircRNA3503-overexpressed synovium MSCs | Alleviates chondrocyte apoptosis and ECM imbalance by acting as sponges of miR181c-3p and let-7b-3p | [38] | |
| circRNA-0001846 | Human | Chondrocyte cell line CHON-001 treated with IL-1β | Modulates IL-1β-induced chondrocyte damage by miR-149-5p/WNT5B axis | [39] | |
| circRNA-BRWD1 | Human | Chondrocyte cell line CHON-001 treated with IL-1β | Promotes OA progression by regulating the miR-1277/TRAF6 axis | [18] | |
| circRNA-0001236 | Human | MSCs | Alleviates cartilage degradation through the miR3677-3p/Sox9 axis | [40] | |
| circRNA-HIPK3 | Human | MSCs | Promotes chondrocyte proliferation and migration and suppresses apoptosis via the miR-124-3p/MYH9 axis | [41] | |
| Isolation Methods | Advantages | Disadvantages | Sample Matrix |
|---|---|---|---|
| UC | Gold standard Simplicity of operator Easily required | Time consuming Decreased in biological activity High requirements for equipment Limited mass production | Cell culture medium Serum Urine |
| Microfluidic technology | Rapid Save samples and reagents High purity and efficiency | Not suitable for mass generation Methods need to be further standardized | Cell culture medium Serum |
| Ion exchange | Simplicity of operator High purity | Unknown | Cell culture medium |
| AF4 | High purity High efficiency Identify subset | High requirements for equipment and personnel Limited mass production | Cell culture medium Serum Urine |
| SEC | High purity Commercial kits available High productivity | Co-separation of proteins with similar diameters Not satisfy the downstream application | Cell culture medium Serum Urine Cerebrospinal fluid |
| Polymer precipitation | Mass production Simplicity of operator | Decreased purity Protein contamination Expensive kit | Cell culture medium |
| Immuno-isolation | Rapid High purity and specificity | Additional separation and purification are required Not suitable for mass generation | Cell culture medium Serum |
| UF | Rapid Simplicity of operation | Protein contamination Exosomes are damaged | Cell culture medium Serum Urine |
| Differential centrifugation | Rapid Mass production | Heterogeneity Easy to drain | Cell culture medium Serum Urine |
| Strategy | Characters/Advantages | Applications for OA/Cartilage Repair |
|---|---|---|
| Modification of exosome-derived cells | Changing biological characteristics of exosomes: size, content, function, secretion, production efficiency, penetration | TGF-β1 [111] Overexpressing: miR-92a-3p [68], miR-320c [112] miR-135b [81] miR-95-5p [113] |
| 3D culture | A more favorable environment for the proliferation of human synovial cells and the secretion of exosomes [114] | |
| Deficiencies of Exosomes | GuiLu-ErXian Glue [115] Cartilage affinity peptide through lipid insertion [116] Buffer pH as a charge-reversal switch [117] | |
| Exosomes Carrier | Good exosome retention and sustained release function as working platforms; Increasing the stability of content of exosomes; | Thiolated hyaluronic acid microgels [118] Photocrosslinking spherical gelatin methacryloyl hydrogel [119] Methacrylic anhydride-modified hyaluronic hydrogel [120] S-EXO-containing hydrogel microspheres [121] Injectable thermosensitive hydrogel [122] |
| 3D printing | Designing more optimized 3D culture microenvironment; Designing scaffolds with more optimized geometric structure; | A photo-crosslinkable bioink containing different concentrations of silk methacrylate and polyethylene glycol diacrylate mixed with chondrocytes for biofabrication of 3D-bioprinted cartilage constructs [123] A modular hydrogel-based bioink containing microsphere-embedded chondrocytes for 3D printing multiscale scaffolds [124] |
| No | Title | ID | Conditions | Interventions |
|---|---|---|---|---|
| 1 | Mesenchymal Stem Cells Derived Exosomes in Osteoarthritis Patients | NCT06466850 | Osteoarthritis, Knee | Biological: Exosome |
| 2 | Intra-articular Injection of MSC-derived Exosomes in Knee Osteoarthritis (ExoOA-1) (ExoOA-1) | NCT05060107 | Osteoarthritis, Knee | Biological: Exosomes (sEVs) |
| 3 | Phase 1b Clinical Trial to Evaluate PEP and EUFLEXXA for Knee Osteoarthritis | NCT06463132 | Osteoarthritis, Knee | Combination Product: PEP/Euflexxa Drug: Purified Exosome Product(PEP) |
| 4 | Intra-articular Injection of UC-MSC Exosome in Knee Osteoarthritis (EXO-OA01) | NCT06431152 | Osteoarthritis, Knee | Biological: UC-MSC Exosomes (sEVs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-H.; Chen, S.-Y.; Zhou, Q.-F.; Cai, Y.-Z. Exosomes in Osteoarthritis: Breakthrough Innovations and Advanced Tissue Engineering for Cartilage Regeneration Since 2020. Biomedicines 2025, 13, 2486. https://doi.org/10.3390/biomedicines13102486
Yang X-H, Chen S-Y, Zhou Q-F, Cai Y-Z. Exosomes in Osteoarthritis: Breakthrough Innovations and Advanced Tissue Engineering for Cartilage Regeneration Since 2020. Biomedicines. 2025; 13(10):2486. https://doi.org/10.3390/biomedicines13102486
Chicago/Turabian StyleYang, Xiao-He, Shu-Yin Chen, Quan-Fa Zhou, and You-Zhi Cai. 2025. "Exosomes in Osteoarthritis: Breakthrough Innovations and Advanced Tissue Engineering for Cartilage Regeneration Since 2020" Biomedicines 13, no. 10: 2486. https://doi.org/10.3390/biomedicines13102486
APA StyleYang, X.-H., Chen, S.-Y., Zhou, Q.-F., & Cai, Y.-Z. (2025). Exosomes in Osteoarthritis: Breakthrough Innovations and Advanced Tissue Engineering for Cartilage Regeneration Since 2020. Biomedicines, 13(10), 2486. https://doi.org/10.3390/biomedicines13102486





