Mechanisms of Resistance to Chemotherapy in Hypopharyngeal Carcinoma
Abstract
1. Introduction
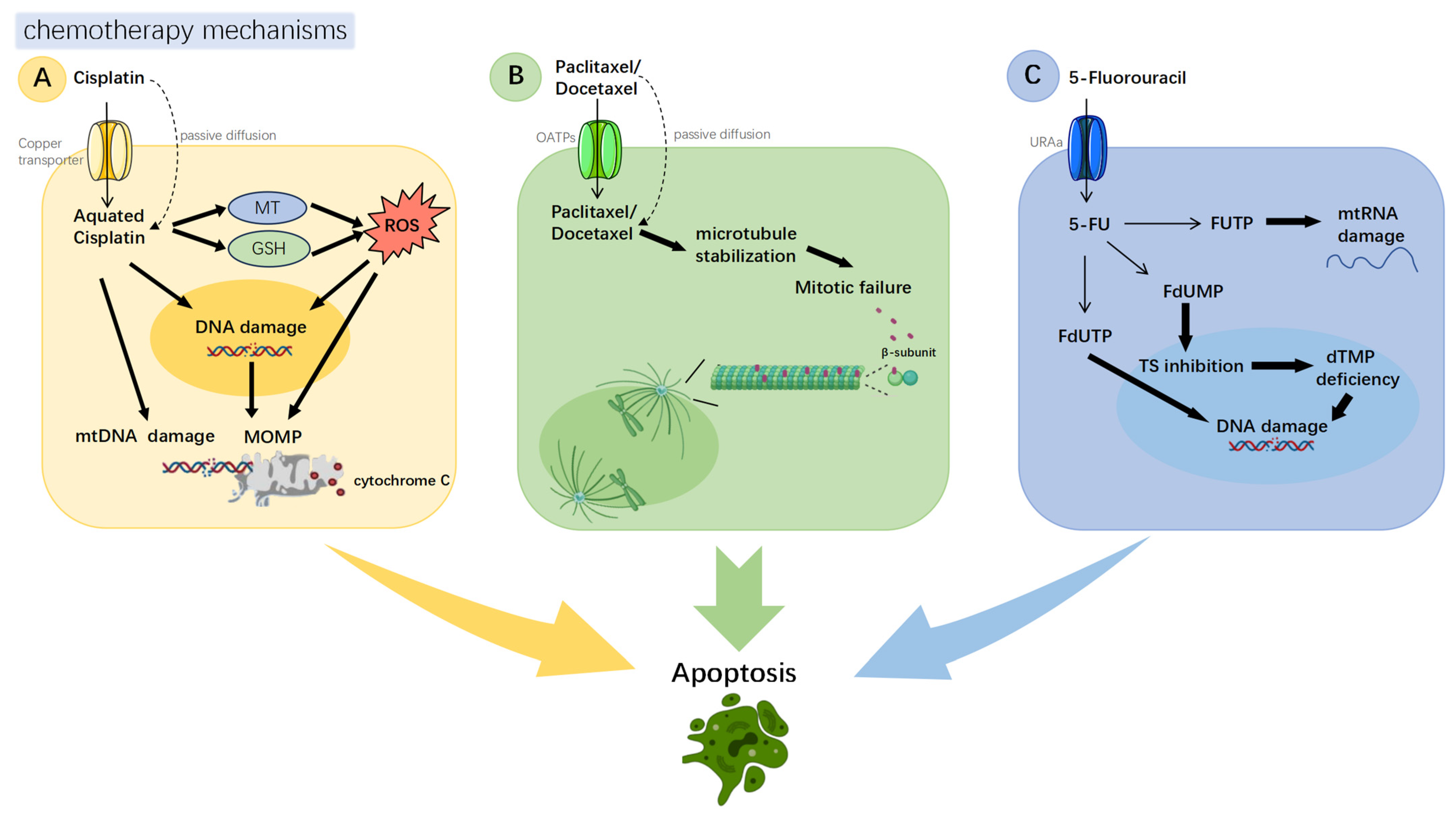
2. Common Chemotherapeutic Agents for Hypopharyngeal Carcinoma and Their Mechanisms of Action
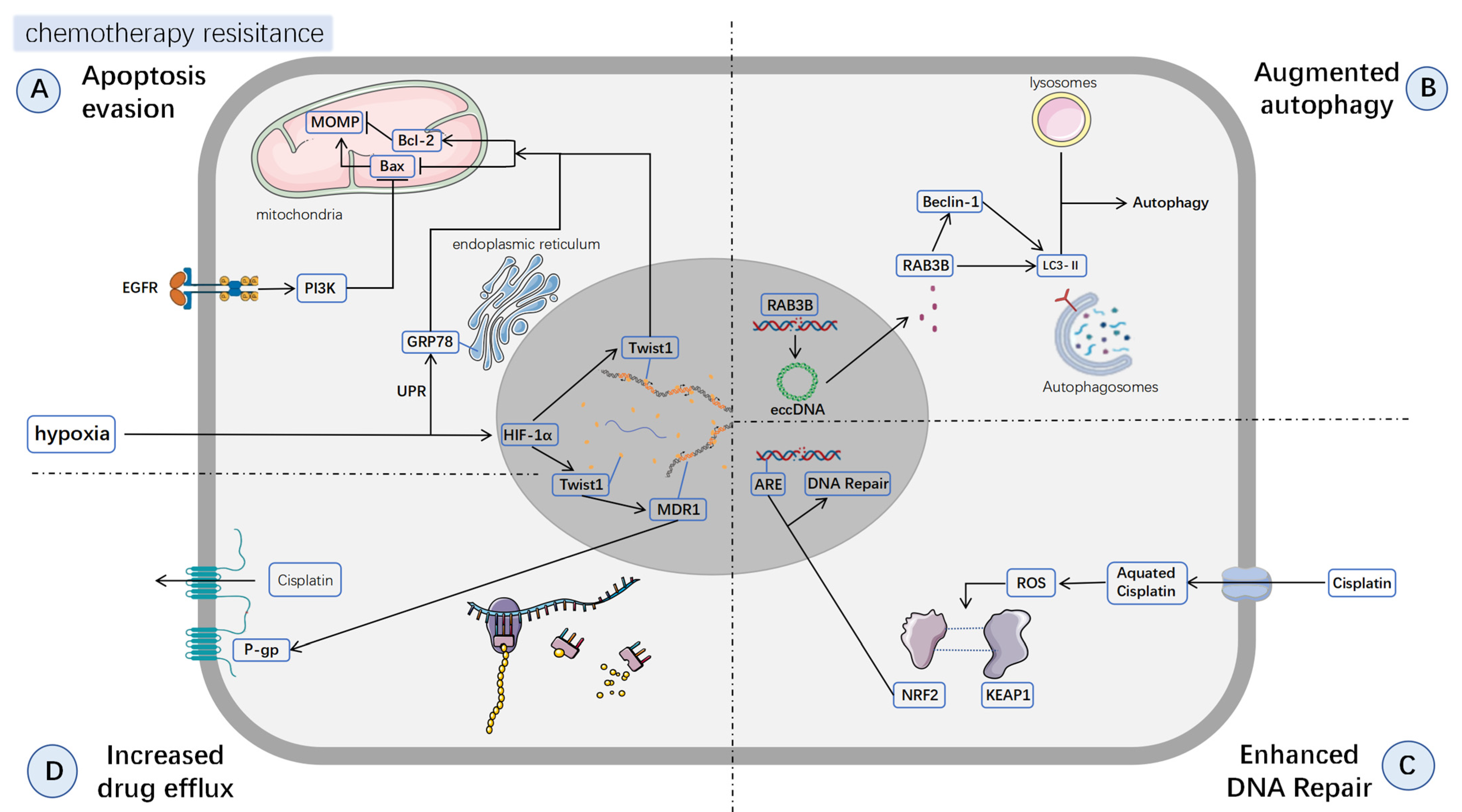
3. Mechanisms of Chemoresistance in Hypopharyngeal Cancer
3.1. Apoptosis Inhibition
3.2. Augmented Autophagy
3.3. Enhanced DNA Repair
3.4. Increased Drug Efflux
4. Cancer Stem Cells, EMT, and CAFs in Chemoresistance
5. Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Cooper, J.S.; Porter, K.; Mallin, K.; Hoffman, H.T.; Weber, R.S.; Ang, K.K.; Gay, E.G.; Langer, C.J. National Cancer Database Report on Cancer of the Head and Neck: 10-Year Update. Head. Neck 2009, 31, 748–758. [Google Scholar] [CrossRef]
- Marzouki, H.; Addas, M.A.; Nujoom, M.; Zawawi, F.; Almarzouki, H.Z.; Merdad, M. Hypopharyngeal Reconstruction: Possibilities, Outcomes, and Updates for Improving the Human Health for Quality of Life. Comput. Intell. Neurosci. 2022, 2022, 6132481. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.I.; Miles, B.A.; Education Committee of the American Head and Neck Society (AHNS). Hypopharyngeal Carcinoma: Do You Know Your Guidelines? Head. Neck 2019, 41, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology in: Journal of the National Comprehensive Cancer Network; Volume 18, Issue 7. 2020. Available online: https://jnccn.org/view/journals/jnccn/18/7/article-p873.xml (accessed on 12 March 2025).
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Maruyama, R.; Okamoto, I.; Sato, H.; Katsube, Y.; Kondo, T.; Tsukahara, K. Prediction of Therapeutic Effects from One Course of TPF Chemotherapy for Advanced Hypopharyngeal Laryngeal Cancer. Vivo 2020, 34, 2891–2896. [Google Scholar] [CrossRef]
- Risk Factors of Secondary Cancer in Laryngeal, Oropharyngeal, or Hypopharyngeal Cancer after Definitive Therapy. Int. J. Clin. Oncol. 2023, 29, 103–114. Available online: https://link.springer.com/article/10.1007/s10147-023-02433-8 (accessed on 23 May 2025). [CrossRef]
- Newman, J.R.; Connolly, T.M.; Illing, E.A.; Kilgore, M.L.; Locher, J.L.; Carroll, W.R. Survival Trends in Hypopharyngeal Cancer: A Population-Based Review. Laryngoscope 2015, 125, 624–629. [Google Scholar] [CrossRef]
- Pala, M.; Novakova, P.; Tesar, A.; Vesela, L.; Vrana, A.; Sukova, J.; Pechacova, Z.; Holeckova, P.; Drbohlavova, T.; Podlesak, T.; et al. Prognostic Factors and Long-Term Outcome Prediction in Patients with Hypopharyngeal Carcinoma Treated with (Chemo) Radiotherapy: Development of a Prognostic Model. Biomedicines 2025, 13, 417. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular Mechanisms of Cisplatin Resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E.; Wolf, S.G.; Khan, I.A.; Ludueña, R.F.; Downing, K.H. Structure of Tubulin at 6.5 A and Location of the Taxol-Binding Site. Nature 1995, 375, 424–427. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Schwarz, S.; Hägg, M.; Havelka, A.M.; Linder, S. Docetaxel Induces Apoptosis in Hormone Refractory Prostate Carcinomas during Multiple Treatment Cycles. Br. J. Cancer 2006, 94, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Amadori, D.; Carloni, S.; Brigliadori, G.; Tesei, A.; Ulivi, P.; Rosetti, M.; Vannini, I.; Arienti, C.; Zoli, W.; et al. Mitotic Catastrophe and Apoptosis Induced by Docetaxel in Hormone-Refractory Prostate Cancer Cells. J. Cell Physiol. 2008, 217, 494–501. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Miller, M.S.; Cowan, A.D.; Brouwer, J.M.; Smyth, S.T.; Peng, L.; Wardak, A.Z.; Uren, R.T.; Luo, C.; Roy, M.J.; Shah, S.; et al. Sequence Differences between BAX and BAK Core Domains Manifest as Differences in Their Interactions with Lipids. FEBS J. 2024, 291, 2335–2353. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Lindahl, T. An N-Glycosidase from Escherichia Coli That Releases Free Uracil from DNA Containing Deaminated Cytosine Residues. Proc. Natl. Acad. Sci. USA 1974, 71, 3649–3653. [Google Scholar] [CrossRef]
- Ma, J.; Lu, S.; Yu, L.; Tian, J.; Li, J.; Wang, H.; Xu, W. FaDu Cell Characteristics Induced by Multidrug Resistance. Oncol. Rep. 2011, 26, 1189–1195. [Google Scholar] [CrossRef]
- Vlahopoulos, S.; Zoumpourlis, V.C. JNK: A Key Modulator of Intracellular Signaling. Biochemistry 2004, 69, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, C.; Wan, C.; Li, G.; Peng, L.; Peng, Y.; Fang, R. The Roles of C-Jun N-Terminal Kinase (JNK) in Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 9640. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Liu, S.; Lian, M.; Fang, J.; Zhai, J.; Shen, X.; Wang, R. C-Jun and Camk2a Contribute to the Drug Resistance of Induction Docetaxel/Cisplatin/5-Fluorouracil in Hypopharyngeal Carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 4605–4613. [Google Scholar]
- Xie, P.; Yang, J.-P.; Cao, Y.; Peng, L.-X.; Zheng, L.-S.; Sun, R.; Meng, D.-F.; Wang, M.-Y.; Mei, Y.; Qiang, Y.-Y.; et al. Promoting Tumorigenesis in Nasopharyngeal Carcinoma, NEDD8 Serves as a Potential Theranostic Target. Cell Death Dis. 2017, 8, e2834. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Yang, M.-H.; Wu, M.-Z.; Chiou, S.-H.; Chen, P.-M.; Chang, S.-Y.; Liu, C.-J.; Teng, S.-C.; Wu, K.-J. Direct Regulation of TWIST by HIF-1alpha Promotes Metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Lu, S.; Yu, L.; Mu, Y.; Ma, J.; Tian, J.; Xu, W.; Wang, H. Role and Mechanism of Twist1 in Modulating the Chemosensitivity of FaDu Cells. Mol. Med. Rep. 2014, 10, 53–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knockdown of Glucose-Regulated Protein 78 Abrogates Chemoresistance of Hypopharyngeal Carcinoma Cells to Cisplatin Induced by Unfolded Protein in Response to Severe Hypoxia. Available online: https://www.spandidos-publications.com/10.3892/ol.2013.1753 (accessed on 31 March 2025).[Green Version]
- Kanno, Y.; Chen, C.-Y.; Lee, H.-L.; Chiou, J.-F.; Chen, Y.-J. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front. Oncol. 2021, 11, 640392. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Tan, G.; Wang, T.; Wang, X.; Zhou, Z.; Xiao, J.; Li, T.; Zhang, G.; Li, W. Downregulation of S100A9 Reverses Cisplatin-Resistance and Inhibits Proliferation and Migration in Hypopharyngeal Carcinoma. J. Oncol. 2022. Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1155/2022/9341731 (accessed on 1 April 2025).
- Xu, T.; Yang, Y.; Chen, Z.; Wang, J.; Wang, X.; Zheng, Y.; Wang, C.; Wang, Y.; Zhu, Z.; Ding, X.; et al. TNFAIP2 Confers Cisplatin Resistance in Head and Neck Squamous Cell Carcinoma via KEAP1/NRF2 Signaling. J. Exp. Clin. Cancer Res. 2023, 42, 190. [Google Scholar] [CrossRef]
- Aird, K.M.; Ghanayem, R.B.; Peplinski, S.; Lyerly, H.K.; Devi, G.R. X-Linked Inhibitor of Apoptosis Protein Inhibits Apoptosis in Inflammatory Breast Cancer Cells with Acquired Resistance to an ErbB1/2 Tyrosine Kinase Inhibitor. Mol. Cancer Ther. 2010, 9, 1432–1442. [Google Scholar] [CrossRef]
- Martin, A.P.; Miller, A.; Emad, L.; Rahmani, M.; Walker, T.; Mitchell, C.; Hagan, M.P.; Park, M.A.; Yacoub, A.; Fisher, P.B.; et al. Lapatinib Resistance in HCT116 Cells Is Mediated by Elevated MCL-1 Expression and Decreased BAK Activation and Not by ERBB Receptor Kinase Mutation. Mol. Pharmacol. 2008, 74, 807–822. [Google Scholar] [CrossRef]
- Song, H.; Song, Q.; Zhao, X.; Yang, Y.; Mou, Y.; Li, Y.; Song, X. Anlotinib Suppressed Tumor Cell Proliferation and Migration in Hypopharyngeal Carcinoma. Braz. J. Otorhinolaryngol. 2024, 90, 101397. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kwon, S.-B.; Ham, S.H.; Jeong, E.-S.; Choi, Y.-K.; Choi, K.D.; Hong, J.T.; Jung, S.H.; Yoon, D.-Y. H9 Inhibits Tumor Growth and Induces Apoptosis via Intrinsic and Extrinsic Signaling Pathway in Human Non-Small Cell Lung Cancer Xenografts. J. Microbiol. Biotechnol. 2015, 25, 648–657. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Xie, Z.; Rao, J.; Xu, G.; Huang, K.; Li, W.; Yin, Z. Chlorogenic Acid Inhibits Proliferation and Induces Apoptosis in A498 Human Kidney Cancer Cells via Inactivating PI3K/Akt/mTOR Signalling Pathway. J. Pharm. Pharmacol. 2019, 71, 1100–1109. [Google Scholar] [CrossRef]
- Kleszcz, R.; Frąckowiak, M.; Dorna, D.; Paluszczak, J. Combinations of PRI-724 Wnt/β-Catenin Pathway Inhibitor with Vismodegib, Erlotinib, or HS-173 Synergistically Inhibit Head and Neck Squamous Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10448. [Google Scholar] [CrossRef]
- Sohel, M. Comprehensive Exploration of Biochanin A as an Oncotherapeutics Potential in the Treatment of Multivarious Cancers with Molecular Insights. Phytother. Res. 2024, 38, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, H.; Lai, H.; Liang, G.; Huang, J.; Zhao, F.; Xie, X.; Peng, C. Erianin: A Phytoestrogen with Therapeutic Potential. Front. Pharmacol. 2023, 14, 1197056. [Google Scholar] [CrossRef]
- Cho, I.-A.; You, S.-J.; Kang, K.-R.; Kim, S.-G.; Oh, J.-S.; You, J.-S.; Lee, G.-J.; Seo, Y.-S.; Kim, D.K.; Kim, C.S.; et al. Biochanin-A Induces Apoptosis and Suppresses Migration in FaDu Human Pharynx Squamous Carcinoma Cells. Oncol. Rep. 2017, 38, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Chemical Modification of Curcumin Increases Its Potency against Hypopharyngeal Carcinoma. J. Drug Targeting. 2023, 31, 867–877. Available online: https://www.tandfonline.com/doi/full/10.1080/1061186X.2023.2247581 (accessed on 28 March 2025). [CrossRef]
- Lee, S.H.; Cho, Y.-C.; Lim, J.S. Costunolide, a Sesquiterpene Lactone, Suppresses Skin Cancer via Induction of Apoptosis and Blockage of Cell Proliferation. Int. J. Mol. Sci. 2021, 22, 2075. [Google Scholar] [CrossRef]
- Li, J.; Duan, B.; Cheng, Z.; Kou, M. Costunolide Enhances Cisplatin-Induced Cytotoxicity in Hypopharyngeal SCC FaDu Cells by Increasing the Production of Reactive Oxygen Species. Pathol.-Res. Pract. 2022, 236, 153966. [Google Scholar] [CrossRef]
- Levine, A.J. P53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Co-Expression of ING4 and P53 Enhances Hypopharyngeal Cancer Chemosensitivity to Cisplatin In Vivo. Available online: https://www.spandidos-publications.com/10.3892/mmr.2016.5552 (accessed on 11 March 2025).
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Nakatogawa, H. Mechanisms Governing Autophagosome Biogenesis. Nat. Rev. Mol. Cell Biol. 2020, 21, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Singh, M.P.; Amaravadi, R.K. Recent Advances in Targeting Autophagy in Cancer. Trends Pharmacol. Sci. 2023, 44, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Y.; Zhang, F.; Liu, B.; Xie, C.; Song, Y. Encoding Gene RAB3B Exists in Linear Chromosomal and Circular Extrachromosomal DNA and Contributes to Cisplatin Resistance of Hypopharyngeal Squamous Cell Carcinoma via Inducing Autophagy. Cell Death Dis. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and Chemotherapy Resistance: A Promising Therapeutic Target for Cancer Treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zou, Z. Targeting Autophagy to Overcome Drug Resistance: Further Developments. J. Hematol. Oncol. 2020, 13, 159. [Google Scholar] [CrossRef]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in Cancer: Moving from Understanding Mechanism to Improving Therapy Responses in Patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Liu, Y.; Ding, J.; Wang, J.; Yu, Z.; Huang, R.; Yang, S.; Sun, Y.; Dong, P. 3-MA Enhanced Chemosensitivity in Cisplatin Resistant Hypopharyngeal Squamous Carcinoma Cells via Inhibiting Beclin -1 Mediated Autophagy. Curr. Pharm. Des. 2021, 27, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA Damage Response and Cancer Therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA Repair Pathways as Targets for Cancer Therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Osman, A.A.; Arslan, E.; Bartels, M.; Michikawa, C.; Lindemann, A.; Tomczak, K.; Yu, W.; Sandulache, V.; Ma, W.; Shen, L.; et al. Dysregulation and Epigenetic Reprogramming of NRF2 Signaling Axis Promote Acquisition of Cisplatin Resistance and Metastasis in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2023, 29, 1344–1359. [Google Scholar] [CrossRef]
- Jun, H.J.; Ahn, M.J.; Kim, H.S.; Yi, S.Y.; Han, J.; Lee, S.K.; Ahn, Y.C.; Jeong, H.-S.; Son, Y.-I.; Baek, J.-H.; et al. ERCC1 Expression as a Predictive Marker of Squamous Cell Carcinoma of the Head and Neck Treated with Cisplatin-Based Concurrent Chemoradiation. Br. J. Cancer 2008, 99, 167–172. [Google Scholar] [CrossRef]
- Chiu, T.-J.; Chen, C.-H.; Chien, C.-Y.; Li, S.-H.; Tsai, H.-T.; Chen, Y.-J. High ERCC1 Expression Predicts Cisplatin-Based Chemotherapy Resistance and Poor Outcome in Unresectable Squamous Cell Carcinoma of Head and Neck in a Betel-Chewing Area. J. Transl. Med. 2011, 9, 31. [Google Scholar] [CrossRef]
- Xuelei, M.; Jingwen, H.; Wei, D.; Hongyu, Z.; Jing, Z.; Changle, S.; Lei, L. ERCC1 Plays an Important Role in Predicting Survival Outcomes and Treatment Response for Patients with HNSCC: A Meta-Analysis. Oral. Oncol. 2015, 51, 483–492. [Google Scholar] [CrossRef]
- Kelley, M.R.; Logsdon, D.; Fishel, M.L. Targeting DNA Repair Pathways for Cancer Treatment: What’s New? Future Oncol. 2014, 10, 1215–1237. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC Transporters in Cancer: More than Just Drug Efflux Pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Yano, K.; Tomono, T.; Ogihara, T. Advances in Studies of P-Glycoprotein and Its Expression Regulators. Biol. Pharm. Bull. 2018, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Safaei, R.; Holzer, A.K.; Katano, K.; Samimi, G.; Howell, S.B. The Role of Copper Transporters in the Development of Resistance to Pt Drugs. J. Inorg. Biochem. 2004, 98, 1607–1613. [Google Scholar] [CrossRef]
- Warta, R.; Theile, D.; Mogler, C.; Herpel, E.; Grabe, N.; Lahrmann, B.; Plinkert, P.K.; Herold-Mende, C.; Weiss, J.; Dyckhoff, G. Association of Drug Transporter Expression with Mortality and Progression-Free Survival in Stage IV Head and Neck Squamous Cell Carcinoma. PLoS ONE 2014, 9, e108908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, D.; Zhang, J. Research Advances in Resistance to Platinum-Based Chemotherapy in Lung Cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017, 39, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lv, Z.; Liu, X.; Liu, X.; Xu, W. MG-132 Reverses Multidrug Resistance by Activating the JNK Signaling Pathway in FaDu/T Cells. Mol. Med. Rep. 2018, 18, 1820–1825. [Google Scholar] [CrossRef]
- Cirillo, N.; Wu, C.; Prime, S.S. Heterogeneity of Cancer Stem Cells in Tumorigenesis, Metastasis, and Resistance to Antineoplastic Treatment of Head and Neck Tumours. Cells 2021, 10, 3068. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer Stem Cells as Key Drivers of Tumour Progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-M.; Zhang, J.-G.; Zhang, X.; Li, Q. Targeting Cancer Stem Cells for Reversing Therapy Resistance: Mechanism, Signaling, and Prospective Agents. Signal Transduct. Target. Ther. 2021, 6, 62. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug Resistance and Cancer Stem Cells. Cell Commun. Signal 2021, 19, 19. [Google Scholar] [CrossRef]
- Makena, M.R.; Ranjan, A.; Thirumala, V.; Reddy, A.P. Cancer Stem Cells: Road to Therapeutic Resistance and Strategies to Overcome Resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165339. [Google Scholar] [CrossRef]
- Sagar, J.; Chaib, B.; Sales, K.; Winslet, M.; Seifalian, A. Role of Stem Cells in Cancer Therapy and Cancer Stem Cells: A Review. Cancer Cell Int. 2007, 7, 9. [Google Scholar] [CrossRef]
- Vidal, S.J.; Rodriguez-Bravo, V.; Galsky, M.; Cordon-Cardo, C.; Domingo-Domenech, J. Targeting Cancer Stem Cells to Suppress Acquired Chemotherapy Resistance. Oncogene 2014, 33, 4451–4463. [Google Scholar] [CrossRef]
- Chen, L.; Fang, R.; Cai, Z.; Huang, B.; Zhang, J.; Li, Y.; Chen, Y.; Xu, Z.; Lei, W.; Zhang, M. CD271high Cancer Stem Cells Regulate Macrophage Polarization in Head and Neck Squamous Cell Carcinoma. Oral. Oncol. 2025, 162, 107181. [Google Scholar] [CrossRef]
- Imai, T.; Tamai, K.; Oizumi, S.; Oyama, K.; Yamaguchi, K.; Sato, I.; Satoh, K.; Matsuura, K.; Saijo, S.; Sugamura, K.; et al. CD271 Defines a Stem Cell-Like Population in Hypopharyngeal Cancer. PLoS ONE 2013, 8, e62002. [Google Scholar] [CrossRef]
- Saito, S.; Ozawa, H.; Imanishi, Y.; Sekimizu, M.; Watanabe, Y.; Ito, F.; Ikari, Y.; Nakahara, N.; Kameyama, K.; Ogawa, K. Cyclooxygenase-2 Expression Is Associated with Chemoresistance through Cancer Stemness Property in Hypopharyngeal Carcinoma. Oncol. Lett. 2021, 22, 533. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, Y.; Chen, D.; Liu, H.; Mi, K. Tunicamycin-Induced Endoplasmic Reticulum Stress Inhibits Chemoresistance of FaDu Hypopharyngeal Carcinoma Cells in 3D Collagen I Cultures and in Vivo. Exp. Cell Res. 2021, 405, 112725. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and Drug Resistance: The Mechanistic Link and Clinical Implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, L.; Chen, S.; Fang, R.; Chen, X.; Lei, W. Single-Cell RNA Sequencing Reveals pro-Invasive Cancer-Associated Fibroblasts in Hypopharyngeal Squamous Cell Carcinoma. Cell Commun. Signal. 2023, 21, 292. [Google Scholar] [CrossRef]
- Vitali, R.; Mancini, C.; Cesi, V.; Tanno, B.; Mancuso, M.; Bossi, G.; Zhang, Y.; Martinez, R.V.; Calabretta, B.; Dominici, C.; et al. Slug (SNAI2) down-Regulation by RNA Interference Facilitates Apoptosis and Inhibits Invasive Growth in Neuroblastoma Preclinical Models. Clin. Cancer Res. 2008, 14, 4622–4630. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, S.; Ishikawa, K. Cyclophilin A-EMMPRIN Interaction Induces Invasion of Head and Neck Squamous Cell Carcinoma. Oncol. Rep. 2012, 27, 198–203. [Google Scholar] [CrossRef]
- Downregulation of RBM17 Enhances Cisplatin Sensitivity and Inhibits Cell Invasion in Human Hypopharyngeal Cancer Cells. Available online: https://www.degruyter.com/document/doi/10.1515/med-2023-0669/html (accessed on 30 March 2025).
- Xu, Z.; Zhang, Y.; Dai, H.; Han, B. Epithelial-Mesenchymal Transition-Mediated Tumor Therapeutic Resistance. Molecules 2022, 27, 4750. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E.; Shi, H.; Lin, F.; Dasari, S.; Bednash, J.; Thorne, S.; Watkins, S.; Joshi, R.; Thomas, S.M. Enhancement of Head and Neck Squamous Cell Carcinoma Proliferation, Invasion, and Metastasis by Tumor-Associated Fibroblasts in Preclinical Models. Head. Neck 2014, 36, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hou, J.; Yu, S.; Li, W.; Wang, X.; Sun, H.; Qin, T.; Claret, F.X.; Guo, H.; Liu, Z. Role of Cancer-Associated Fibroblasts in the Resistance to Antitumor Therapy, and Their Potential Therapeutic Mechanisms in Non-Small Cell Lung Cancer. Oncol. Lett. 2021, 21, 413. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance. OMICS A J. Integr. Biol. 2020, 24, 314–339. [Google Scholar] [CrossRef]
- Kinugasa, Y.; Matsui, T.; Takakura, N. CD44 Expressed on Cancer-Associated Fibroblasts Is a Functional Molecule Supporting the Stemness and Drug Resistance of Malignant Cancer Cells in the Tumor Microenvironment. Stem Cells 2014, 32, 145–156. [Google Scholar] [CrossRef]
- Raudenska, M.; Balvan, J.; Hanelova, K.; Bugajova, M.; Masarik, M. Cancer-Associated Fibroblasts: Mediators of Head and Neck Tumor Microenvironment Remodeling. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188940. [Google Scholar] [CrossRef]
- Huang, Q.; Hsueh, C.-Y.; Shen, Y.-J.; Guo, Y.; Huang, J.-M.; Zhang, Y.-F.; Li, J.-Y.; Gong, H.-L.; Zhou, L. Small Extracellular Vesicle-Packaged TGFβ1 Promotes the Reprogramming of Normal Fibroblasts into Cancer-Associated Fibroblasts by Regulating Fibronectin in Head and Neck Squamous Cell Carcinoma. Cancer Lett. 2021, 517, 1–13. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved Survival of Patients with Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Tian, S.; Switchenko, J.M.; Jhaveri, J.; Cassidy, R.J.; Ferris, M.J.; Press, R.H.; Pfister, N.T.; Patel, M.R.; Saba, N.F.; McDonald, M.W.; et al. Survival Outcomes by High-Risk Human Papillomavirus Status in Nonoropharyngeal Head and Neck Squamous Cell Carcinomas: A Propensity-Scored Analysis of the National Cancer Data Base. Cancer 2019, 125, 2782–2793. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Prognostic Value of HPV Status among Patients with Hypopharyngeal Carcinoma: A Population-Based Study. Clin. Transl. Oncol. 2020, 22, 1645–1650. [Google Scholar] [CrossRef]
- Mehanna, H.; Taberna, M.; von Buchwald, C.; Tous, S.; Brooks, J.; Mena, M.; Morey, F.; Grønhøj, C.; Rasmussen, J.H.; Garset-Zamani, M.; et al. Prognostic Implications of P16 and HPV Discordance in Oropharyngeal Cancer (HNCIG-EPIC-OPC): A Multicentre, Multinational, Individual Patient Data Analysis. Lancet Oncol. 2023, 24, 239–251. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Su, W.; Dou, Z.; Zhao, D.; Jin, X.; Lei, H.; Wang, J.; Xie, X.; Cheng, B.; et al. Mutant P53 in Cancer: From Molecular Mechanism to Therapeutic Modulation. Cell Death Dis. 2022, 13, 974. [Google Scholar] [CrossRef]
- Kawata-Shimamura, Y.; Eguchi, H.; Kawabata-Iwakawa, R.; Nakahira, M.; Okazaki, Y.; Yoda, T.; Grénman, R.; Sugasawa, M.; Nishiyama, M. Biomarker Discovery for Practice of Precision Medicine in Hypopharyngeal Cancer: A Theranostic Study on Response Prediction of the Key Therapeutic Agents. BMC Cancer 2022, 22, 779. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, L.; Yang, Y.; He, S.; Chen, G.G.; Chan, J.Y.; Tong, M.C.; van Hasselt, C.A.; Xu, W.; Feng, L.; et al. Construction of a Novel Six-Gene Signature to Predict Tumour Response to Induction Chemotherapy and Overall Survival in Locoregionally Advanced Laryngeal and Hypopharyngeal Carcinoma. Genes. Dis. 2024, 11, 101022. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.H.; Goloubeva, O.; Haddad, R.I.; Cullen, K.; Sarlis, N.; Tishler, R.; Tan, M.; Fasciano, J.; Sammartino, D.E.; Posner, M.R.; et al. Induction Chemotherapy with Cisplatin and Fluorouracil Alone or in Combination with Docetaxel in Locally Advanced Squamous-Cell Cancer of the Head and Neck: Long-Term Results of the TAX 324 Randomised Phase 3 Trial. Lancet Oncol. 2011, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, Y.; Ma, R.; Sun, W.; Chen, L.; Cai, Z.; Wen, W.; Lei, W. Nomogram Prediction of Response to Neoadjuvant Chemotherapy Plus Pembrolizumab in Locally Advanced Hypopharyngeal Squamous Cell Carcinoma. J. Otolaryngol. Head. Neck Surg. 2025, 54, 19160216251318255. [Google Scholar] [CrossRef]
- Li, P.; Xie, Y.; Wang, J.; Bao, C.; Duan, J.; Liu, Y.; Luo, Q.; Xu, J.; Ren, Y.; Jiang, M.; et al. Gene Engineered Exosome Reverses T Cell Exhaustion in Cancer Immunotherapy. Bioact. Mater. 2024, 34, 466–481. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Desilets, A.; Pfister, D.G.; Stein, S.; Wong, W.; Sherman, E.J.; Fetten, J.; Hung, T.K.W.; Kriplani, A.; Dunn, L.A.; Ho, A.L.; et al. A Phase 1 Study of Concurrent Cabozantinib and Cetuximab in Recurrent or Metastatic Head and Neck Squamous Cell Cancer. Oral. Oncol. 2024, 154, 106861. [Google Scholar] [CrossRef]
- Khattri, A.; Sheikh, N.; Agrawal, N.; Kaushik, S.; Kochanny, S.; Ginat, D.; Lingen, M.W.; Blair, E.; Seiwert, T.Y. Switching Anti-EGFR Antibody Re-Sensitizes Head and Neck Cancer Patient Following Acquired Resistance to Cetuximab. Cancer Gene Ther. 2024, 31, 1477–1485. [Google Scholar] [CrossRef]
- Van den Bossche, V.; Vignau, J.; Vigneron, E.; Rizzi, I.; Zaryouh, H.; Wouters, A.; Ambroise, J.; Van Laere, S.; Beyaert, S.; Helaers, R.; et al. PPARα-Mediated Lipid Metabolism Reprogramming Supports Anti-EGFR Therapy Resistance in Head and Neck Squamous Cell Carcinoma. Nat. Commun. 2025, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
| Type | Sort | Cancer | Marker/Pathway | Drug | Mechanisms | Action | Ref. |
|---|---|---|---|---|---|---|---|
| Apoptosis evasion | O | HPC | C-Jun | Docetaxel Cisplatin 5-Fu | TPF induces c-Jun upregulation SAG-SCF E3 ligase inhibits c-Jun degradation c-Jun resists apoptosis and regulates cell cycle (in NPC) | - | [28,29] |
| HPC | Twist1 | paclitaxel | Microenvironment hypoxia leads to Twist1 upregulation. Twist1 regulates Bax and Bcl-2 to resist apoptosis | - | [32] | ||
| HNSC | GRP78 CHOP HIF-1α | Cisplatin | Severe hypoxia induces UPR, leading to GRP78 upregulation GRP78 regulates Bax and Bcl-2 to resist apoptosis | - | [33] | ||
| HNSC (sample includes HPC) | TNFAIP2 KEAP1 NRF2 JNK pathway | Cisplatin | High TNFAIP2 stabilizes NRF2 by competitively binding KEAP1, enhances antioxidant defense, reduces ROS-induced JNK activation, and suppresses apoptosis, thereby promoting chemoresistance. | [36] | |||
| T | HPC | EGFR | Cisplatin | EGFR activates the PI3K pathway PI3K pathway inactivates BAD, blocking apoptosis EGFR activates PI3K pathway to downregulate BAD, blocking apoptosis | Anlotinib | [39] | |
| HNSC (The sample was taken from HPC) | Wnt pathway EGFR PI3K pathway | Wnt pathway/β-catenin upregulates anti-apoptotic gene expression | PRI-724 and Erlotinib | [42] | |||
| HPC | Bcl-2 FasL | Biochanin-A regulates FasL, reducing Bcl-2 | Biochanin-A | [45] | |||
| HPC | Bcl-2 Bax | L42H17 reduces Bcl-2 and increases Bax | L42H17 | [46] | |||
| HPC | ROS AKT pathway NFκB pathway | Cisplatin | CTL induces reactive oxygen species (ROS) production CTL inhibits AKT and NFκB pathways to induce apoptosis CTL regulates Bcl-2 and Bax to induce apoptosis | CTL | [48] | ||
| Augmentedautophagy | O | HPC | RAB3B eccDNA | Cisplatin | RAB3B induces cellular autophagy eccDNA amplification increases RAB3B gene copy, enhancing autophagy | - | [56] |
| T | HPC | Beclin-1 | Cisplatin | 3-MA disrupts the formation of the Beclin-1 complex | 3—MA | [60] | |
| Enhanced DNA Repair | O | HNSC (mention HPC) | NRF2 KEAP1 | Cisplatin | Cisplatin induces ROS production ROS disrupts the binding of NRF2 to KEAP1 NRF2 binds to ARE, initiating DNA repair | - | [63] |
| Increased drug efflux | T | HPC | Twist1 P-gp | Taxel | Microenvironment hypoxia leads to Twist1 upregulation. Twist1 regulates P-gp to increase drug efflux | - | [33] |
| HPC | MG-132 P-gp JNK pathway | Taxel | MG-132 downregulates P-gp expression by activating the JNK pathway | MG-132 | [73] |
| Type | Sort | Cancer | Markers | Mechanisms | Ref. |
|---|---|---|---|---|---|
| CSCs | O | HPC | CD271 ATP-Binding Cassette Transporter IL-6 | Increased expression of ABC transporter proteins and IL-6 in CD271+ cells. | [82,83] |
| HPC | COX-2 | COX-2 suppresses the expression of CSC-related genes while reducing cellular drug tolerance | [84] | ||
| T | HPC | TGF-β1/Smad | TM inhibits the maturation of integrin β1 (130-kD), disrupts the integrin β1/TGF-β1/Smad signaling pathway, reduces TGF-β1 secretion, and undermines the microenvironmental support for CSCs. | [87] | |
| HPC | Wnt/Hh pathway | The Wnt/Hh pathway promotes the expression of CSC-related genes. | [42] | ||
| EMT | O | HPC | CD147 | CD147 significantly upregulates MMP-9 through interaction with cyclosporin A, enhancing chemoresistance. | [94] |
| HNSC (mention HPC) | KEAP1 NRF2 | Hyperactivation of the KEAP1/NRF2 signaling pathway upon CDDP exposure not only induces CDDP resistance but also enhances the metastatic phenotype in HNSCC. | [63] | ||
| HPC | S100A9 | S100A9 promotes EMT and contributes to cellular chemoresistance. | [35] | ||
| T | HPC | RBM17 | Knockdown of RBM17 reduces Vimentin and ZEB1 expression, increases E-cadherin expression, significantly enhances cisplatin’s inhibitory effect on FaDu cells, and reduces cell viability. | [91] | |
| CAFs | O | HPC | Notch pathway Wnt pathway TGF-βpathway | Interactions between metastatic cancer-2 cells and mCAFs are the most frequent, with ligand-receptor pairs enriched in various cancer-related pathways, including Notch, Wnt, and TGF-β pathways. | [88] |
| HNSC (The sample was taken from larynx and hypopharynx) | sEV TGF-β1 | Fibronectin is significantly upregulated in NFs following sEV-TGFβ1 treatment. Fibronectin promotes ECM assembly by CAFs, enhances tumor cell invasion, and is associated with poor prognosis in HNSCC patients. | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Qiang, Z.; Lei, W.; Cai, Z. Mechanisms of Resistance to Chemotherapy in Hypopharyngeal Carcinoma. Biomedicines 2025, 13, 2485. https://doi.org/10.3390/biomedicines13102485
Lu Z, Qiang Z, Lei W, Cai Z. Mechanisms of Resistance to Chemotherapy in Hypopharyngeal Carcinoma. Biomedicines. 2025; 13(10):2485. https://doi.org/10.3390/biomedicines13102485
Chicago/Turabian StyleLu, Zhaoyue, Zhiwei Qiang, Wenbin Lei, and Zhimou Cai. 2025. "Mechanisms of Resistance to Chemotherapy in Hypopharyngeal Carcinoma" Biomedicines 13, no. 10: 2485. https://doi.org/10.3390/biomedicines13102485
APA StyleLu, Z., Qiang, Z., Lei, W., & Cai, Z. (2025). Mechanisms of Resistance to Chemotherapy in Hypopharyngeal Carcinoma. Biomedicines, 13(10), 2485. https://doi.org/10.3390/biomedicines13102485






