1. Introduction
The intestinal mucosa is composed of single-layered columnar epithelia that form the body’s largest interface between the body interior and the external environment in the form of luminal contents [
1]. The intestinal epithelium maintains its integrity and function by coordinating processes like proliferation, differentiation, migration, and apoptosis [
2]. Intestinal epithelial cells are connected at the most apical region of their lateral membranes by specialized structures known as the apical junctional complex, which includes tight junctions (TJs) and adherens junctions. This complex connects the cells and restricts the movement of luminal contents, such as toxins and microbiota, while permitting the passage of ions, small molecules, and fluids through the paracellular space [
3,
4]. Myosin light chain II (MLC-2) is a crucial regulatory protein that influences paracellular permeability when the intestinal barrier is compromised. Research has indicated that the phosphorylation of MLC-2 leads to the contraction of the perijunctional actomyosin ring, thereby enhancing paracellular permeability by modulating the apical junctional complex. The myosin light chain kinase (MLCK) and Rho-associated coiled-coil containing protein kinase (ROCK) pathways play a crucial role in regulating the phosphorylation of MLC-2, which is vital for controlling intestinal permeability during intestinal disorders [
5]. Intestinal mucosal permeability is elevated in gastrointestinal disorders, including inflammatory bowel diseases, [
6] celiac disease, [
7] and others, and this pathogenesis is associated with profound tissue anoxia [
8]. In our previous study, anoxia/reoxygenation (A/R) injury elevated the paracellular permeability via TJ destabilization [
9]. The disruption of TJ proteins was induced by increased MLC phosphorylation [
9].
Larazotide acetate (LA) is a synthetic, eight amino acid peptide (H-Gly-Gly-Val-Leu-Val-Gln-Pro-Gly-OH) that is known to act as a TJ regulator capable of closing “leaky” interepithelial junctions [
10,
11,
12]. Several clinical trials have confirmed the safety of this agent and suggest a potential beneficial effect of LA on gastrointestinal symptoms of patients with celiac disease. The epithelial monolayers pre-treated with LA showed protected paracellular permeability measured by transepithelial electrical resistance (TEER) and Lucifer Yellow flux assays in various barrier dysfunction models [
11,
12]. Additionally, the LA promoted actin rearrangement and redistribution of TJ proteins, including ZO-1, occludin, and claudins [
11,
12]. LA is developed and recognized as a zonulin antagonist that promotes the tightening of intestinal TJs [
11,
12]. Nevertheless, some debate remains regarding LA’s mechanism of action, including uncertainties surrounding zonulin’s identity, insufficient direct evidence, questions about LA’s specificity, and the potential for alternative cellular functions [
13,
14].
In this study, we investigated the mechanistic and functional role of LA in regulating epithelial integrity during cellular injury. Our findings indicate that apically administrated LA on A/R inured Caco-2BBe1 monolayer and leaky IPEC-J2 cells protected epithelial barrier functions via regulating TJ proteins and actin stabilization. Transcriptomic studies and subsequent validation assays suggest that LA regulates ROCK-mediated MLC-2 phosphorylation and enhances proliferation pathways, such as Wnt and Notch signaling. This study presents novel functions and cellular mechanisms of LA in the intestinal epithelium.
2. Materials and Methods
2.1. Cell Culture and Reagents
A human intestine Caco-2BBe1 (C2BBe1) cell line derived from Caco-2 cells was obtained from ATCC (Manassas, VA, USA) and grown in standard DMEM with 10% FBS and 0.01 mg/mL human transferrin (Invitrogen, Carlsbad, CA, USA) [
15]. Intestinal porcine epithelial cell-jejunum 2 (IPEC-J2) cell lines were obtained from Dr. Anthony Blikslager at North Carolina State University. These cells were grown in standard DMEM media with 5% FBS, 1% insulin/selenium/transferrin, 0.5% epidermal growth factor, and 0.125% penicillin/streptomycin. The cells were maintained in a humidified 37 °C, 5% CO
2 incubator.
Larazotide acetate (also known as AT1001) [
16] was obtained from Innovative Biopharmaceuticals, Inc. (Raleigh, NC, USA). The final product was isolated as an acetate salt in a lyophilized form (>99% purity by HPLC/MS). The MLCK inhibitor peptide 18 and ROCK inhibitor Fasudil were purchased from Tocris Bioscience, Inc. (Bristol, UK) and STEMCELL Technologies (Vancouver, BC, Canada), respectively.
2.2. Anoxia/Reoxygenation Injury and Treatment of Reagents
To prepare cellular monolayers, C2BBe1 cells were plated at 100,000 cells per 24-well 0.4 µm pore-sized permeable supports (Corning, Tewksbury, MA, USA). Experiments were conducted 10–14 days post-plating. Larazotide acetate (0.01, 1, and 10 mM), peptide 18 (400 μM), or fasudil (100 μM) were pre-treated apically on the C2BBe1 monolayer before A/R injury.
To generate A/R injury, Caco-2BBe1 cells were placed in a modular incubator chamber (Billups-Rothenberg Inc., San Diego, CA, USA) and flushed with 95% N
2/5% CO
2 for 5 min. The modular chamber was then sealed airtight and placed in an incubator for 2 h at 37 °C. After 2 h, the cells were removed from the modular incubator chamber and placed in a 21% O
2 normal environment. The anoxic injury was confirmed by detecting increased hypoxia-inducible factor-1α using a Western blot assay [
9]. The cells were then incubated under normal environmental conditions for up to 4 h.
2.3. Leaky IPEC-J2 Model
For the leaky IPEC-J2 cells model, the media was composed of components enriched in the crypt, including 50% L-WRN (Wnt-3a, R-Spondin, Noggin) with advanced DMEM, 2 mM Glutamax, 10 mM HEPES (Thermo Fisher Scientific Inc., Waltham, MA, USA), 50 μg/mL Primocin (InvivoGen Corp., San Diego, CA, USA), 1 mM N-Acetyl-L-cysteine (MP Biomedicals., Irvine, CA, USA), and 250 μg/mL EGF (Thermo Fisher Scientific Inc., Waltham, MA, USA). The cells were passaged at least 5 times, considered a leaky IPEC-J2 model. The cells were plated at 100,000 cells per 24-well 0.4 µm pore-sized permeable supports (Corning, Tewksbury, MA, USA).
2.4. Measurement of Transepithelial Electrical Resistance
Cells on permeable support were used in this experiment once a TEER value of greater than 400 Ω cm2 for C2BBe1 or 30 Ω cm2 for IPEC-J2 cells was achieved. TEER was determined by a Chopstick Electrode Set (WPI, Sarasota, FL, USA) positioned on the apical and basal sides of the monolayers and attached to an Epithelial Volt Ohm Meter 2 (WPI, Sarasota, FL, USA). For all TEER measurements, the inserts were plated at an equal density; the readings were taken in triplicate per monolayer and averaged. The TEER recorded on blank inserts was subtracted from the TEER of inserts with cells.
2.5. Gel Electrophoresis and Western Blotting
The cell monolayers were washed with cold PBS and scraped in M-PER mammalian protein extraction reagent, including a protease inhibitor cocktail, 1 mM NaF, and 1 mM NaVO4 (Thermo Scientific, Rockford, IL, USA). Mem-PER® Eukaryotic Membrane Protein Extraction Reagent Kit (Thermo Scientific, Rockford, IL, USA) was used to fractionate the membrane and cytosolic compartments according to the manufacturer’s protocol. Briefly, cells were sequentially treated with the provided permeabilization and solubilization buffers, with centrifugation steps used to separate the soluble cytosolic fraction from the solubilized membrane fraction. Protein analysis of extract aliquots was performed using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Tissue extracts, with amounts equalized by protein concentration, were mixed with XT sample buffer and a reducing agent (both Bio-Rad Laboratories Inc., Hercules, CA, USA) and boiled for 5 min. Lysates were loaded on a 4–10% SDS polyacrylamide gel, and electrophoresis was carried out according to standard protocols. Proteins were transferred to a PVDF membrane (Bio-Rad Laboratories Inc., Hercules, CA, USA) by using an electroblotting transfer apparatus. Membranes were blocked in Tris-buffered saline plus 0.1% Tween20 (TBST) with 5% BSA or skim milk and then incubated overnight with primary antibodies at 4 °C. The primary antibodies used were occludin, ZO-1 (Invitrogen, Carlsbad, CA, USA), pMLC-2, and MLC-2 (Cell signaling, Danvers, MA, USA). After washing in TBST, membranes were incubated with horseradish peroxidase conjugated secondary antibodies and developed for protein visualization with chemiluminescence (Thermo Scientific, Rockford, IL, USA).
2.6. Immunofluorescence Microscopy Analysis
The cells grown on permeable supports were fixed with cold (−20 °C) absolute methanol and stored at −80 °C until used. The cells were thawed, rinsed in PBS, blocked with protein block serum-free (DakoCytomation, Via Real Carpinteria, CA, USA), and incubated overnight at 4 °C with primary antibodies in antibody diluent (DakoCytomation, Via Real Carpinteria, CA, USA). The cells were washed thoroughly with PBS and incubated in secondary antibodies conjugated with fluorescent dyes, and nuclei were stained with DAPI (Thermo Scientific, Rockford, IL, USA). Following rinsing in PBS, the cells were mounted on fluorescent mounting media (DakoCytomation, Via Real Carpinteria, CA, USA) and examined with an Olympus IX83 Inverted Motorized Microscope with cellSens software (Olympus corporation, Version 4.2.1, Shinjuku, Tokyo, Japan).
2.7. Total RNA Isolation and RNA Sequencing
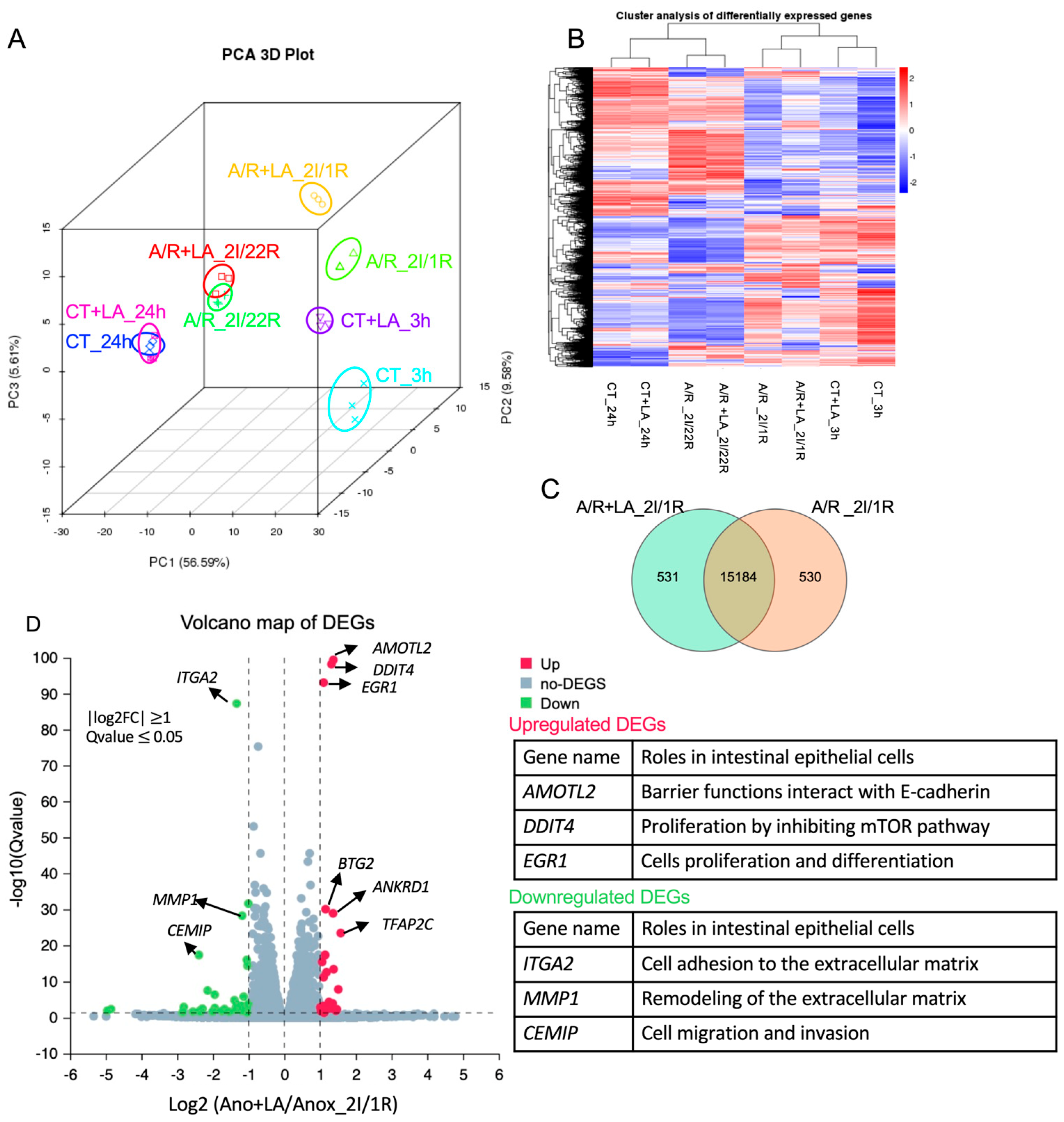
The confluent C2BBe1 monolayers were subjected to be administrated with or without 10 mM LA before undergoing A/R injury. After 2 h of A/R injury, as previously described, we collected the cell lysate at either 1 or 22 h of reoxygenation. Control cells, both treated and untreated with 10 mM LA, were collected at 3 or 24 h. The total RNAs were isolated using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) and submitted for next-generation RNA sequencing performed by Novogenes, Inc. (Sacramento, CA, USA) (
n = 3/group). Briefly, the process followed mRNA enrichment and purification, fragmentation and reverse transcription, adaptor litigation, and PCR amplification to prepare the cDNA library. The library preparations were sequenced on an Illumina Hiseq 2500 platform. The QC analysis (
Table S1) and differential expression analysis of each group were performed using the DESeq2 R package (2_1.6.3). The resulting
p-values were adjusted using Benjamini and Hochberg’s approach for controlling the False Discovery Rate (FDR). Genes with an adjusted
p-value < 0.05 found by DESeq2 were assigned as differentially expressed.
2.8. GO, KEGG, and GSEA Enrichment Analysis of Differentially Expressed Genes
Gene Ontology (GO) enrichment analysis of differentially expressed genes was implemented by the clusterProfiler R package, in which gene length bias was corrected. GO terms with corrected p-value less than 0.05 were considered significantly enriched by differentially expressed genes.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database resource for understanding the high-level functions and utilities of biological systems, such as cells, organisms, and ecosystems, from molecular-level information, particularly large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies (
http://www.genome.jp/kegg/, URL accessed on 8 July 2024). We used clusterProfiler R package to test the statistical enrichment of differential expression genes in KEGG pathways. GSEA was performed with Dr. Tom’s online platform (
https://biosys.innomics.com, URL accessed on 15 September 2024).
2.9. Proliferation and Migration Assays
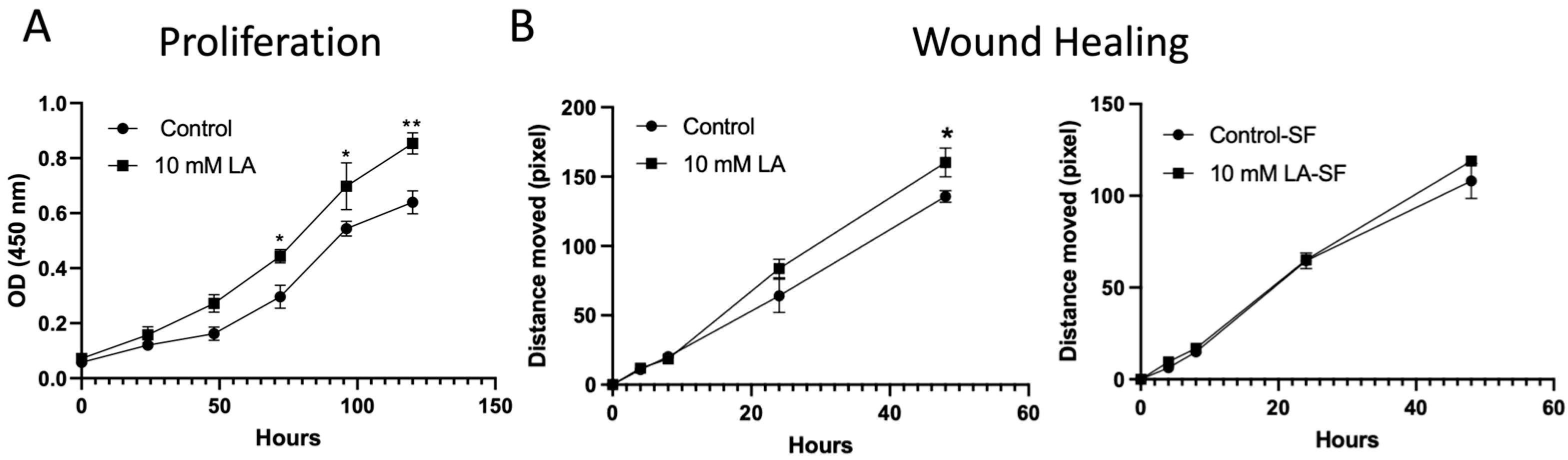
The proliferation of non-treated or LA-treated (10 mM) C2BBe1 cells was evaluated by the CCK-8 assay kit (Dojindo Molecular Technologies, Rockville, MD, USA) according to the manufacturer’s instructions. 10 mM LA was administered to cells seeded in 96-well culture plates (3000 cells/well) every other day. Viable cells were evaluated with the CCK-8 Assay Kit for five consecutive days.
Migration was assessed by seeding C2BBe1 cells in 12-well culture-inserts (100,000 cells/well) (ibidi GmbH, Gräfelfing, Germany). Cells were grown to 100% confluence before the inserts were removed. For the serum-free condition, 16 h before removing the insert, the cells will be cultured with serum-free media to prevent cell proliferation. After removing the inserts, the 10 mM LA was added to the serum-free medium to assess migration. Images were captured at 0, 4, 8, 24, and 48 h with an Axio Vert A1 microscope (Carl Zeiss AG, Oberkochen, Germany), and the closing of the wound was analyzed by measuring migration distance with ImageJ software (Version 1.54i,
https://imagej.net/ij. URL accessed on 11 September 2024).
2.10. Statistical Analysis
Data are reported as mean ± standard error. Differences between groups were tested with two- or one-way ANOVA with Tukey or LSD post hoc tests (GraphPad Prism 10). Where appropriate, the difference between the two groups was assessed with a T-test (GraphPad Prism 10). A p-value of <0.05 was considered significant for all statistical analyses.
4. Discussion
In this study, administration of LA in A/R-injured Caco-2BBe1 or leaky IPEC-J2 cell models significantly elevated TEER compared to non-treated cells. Additionally, the TJ protein actin structure was intensely disrupted during A/R injury of C2BBe1 cells, and the monolayer pretreated with LA was protected from these disruptions. The RNAseq analysis and cell functional validation studies also uncovered novel potential mechanistic pathways and cellular functions through which LA regulates the TJ barrier and proliferation.
The mechanism of action of LA remains under study. It demonstrates a protective effect against the increased permeability induced by AT-1002, a hexamer of zonula occludens toxin (Zot) derived from Vibrio cholerae, or zonulin, the human counterpart of Zot [
11,
16,
17]. LA was initially identified as an octapeptide matching the N-terminal sequence of zonulin, which is the human equivalent of Zot [
11]. Thus, it was proposed as a zonulin antagonist that stabilizes TJs and maintains intestinal barrier integrity by inhibiting the zonulin binding to its receptor. However, evidence suggests LA derives from an immunoglobulin sequence, questioning its relevance to zonulin biology [
14]. Moreover, the belief that zonulin is a reliable biomarker of intestinal permeability is challenged by findings that ELISA kits do not accurately measure zonulin (pre-haptoglobin 2) but detect unrelated proteins like properdin [
18]. Despite these challenges, Larazotide has demonstrated barrier-protective and therapeutic effects in various in vitro and in vivo studies [
11,
12,
15,
19]. In terms of safety, LA has been extensively studied in Phase 1, 2, and 3 clinical trials for celiac disease. Across these studies, the compound has demonstrated a favorable safety profile, showing no significant difference in adverse events compared to placebo and no evidence of systemic toxicity, reinforcing its potential as a locally acting, non-systemic therapeutic agent [
20,
21]. Therefore, understanding the mechanism of action of Larazotide is crucial for future approaches.
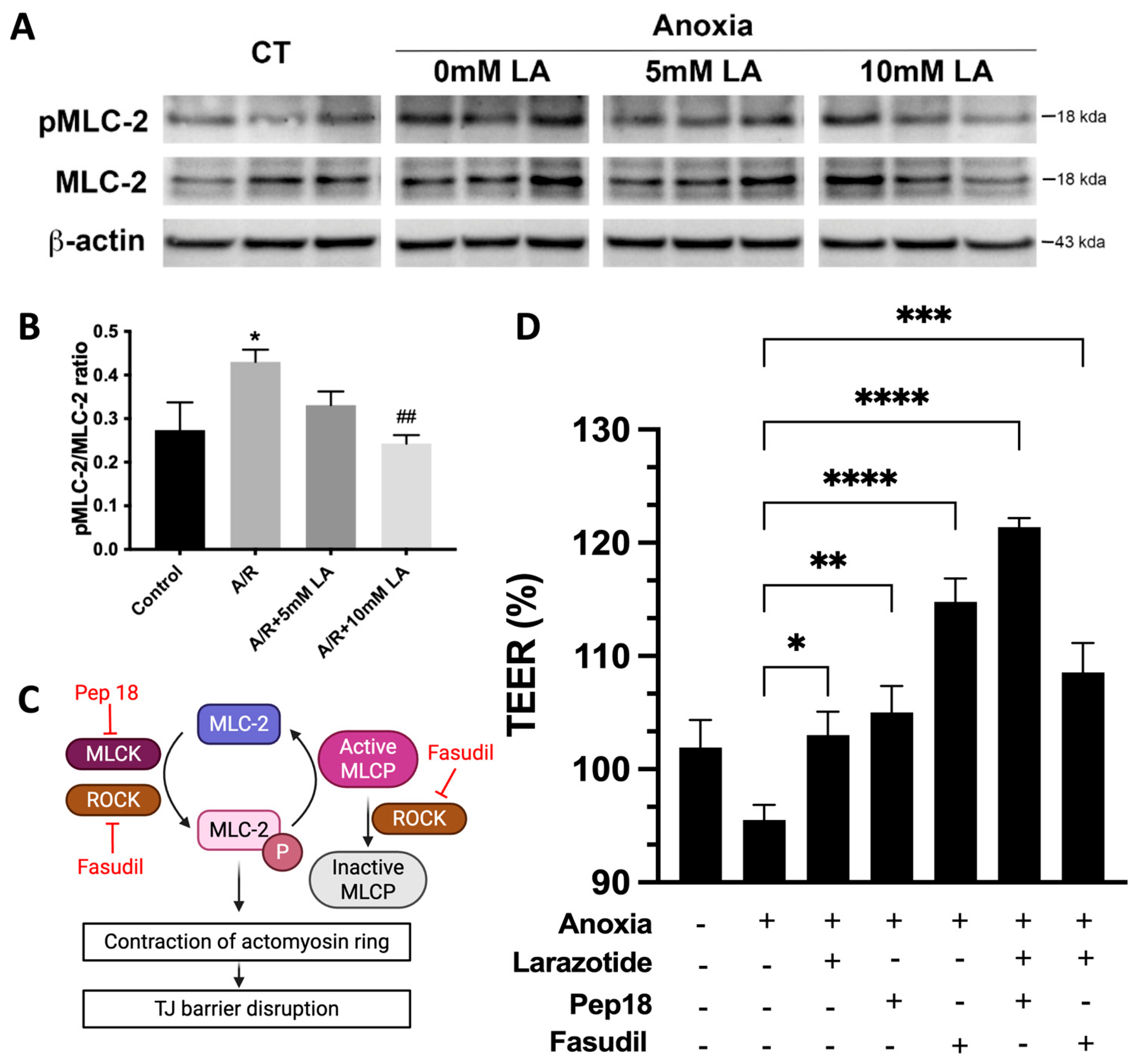
Our study highlights a critical mechanism through which LA preserves intestinal barrier integrity by modulating the phosphorylation status of MLC-2, a key regulator of TJ dynamics. A/R injury significantly increased MLC-2 phosphorylation, contributing to cytoskeletal contraction and destabilization of TJ proteins such as occludin and ZO-1—a finding consistent with prior studies on MLC-2–mediated epithelial barrier dysfunction [
9]. Pretreatment with LA markedly reduced this phosphorylation, thereby mitigating disruption of the TJs (
Figure 7). This protective effect appears to be mediated via the ROCK pathway, as inhibition of ROCK with fasudil mirrored the effects of LA and abolished any synergistic response when combined (
Figure 7), suggesting overlapping mechanisms. Conversely, combined inhibition of MLCK and LA exhibited a synergistic effect on barrier restoration, implying that MLCK and LA act through distinct but complementary signaling cascades. Taken together, these findings suggest that LA exerts its barrier-stabilizing effect by attenuating MLC-2 phosphorylation through modulation of the ROCK pathway. Additionally, it sheds light on possible combination treatment options between LA and MLCK inhibitors for treating gastrointestinal disorders.
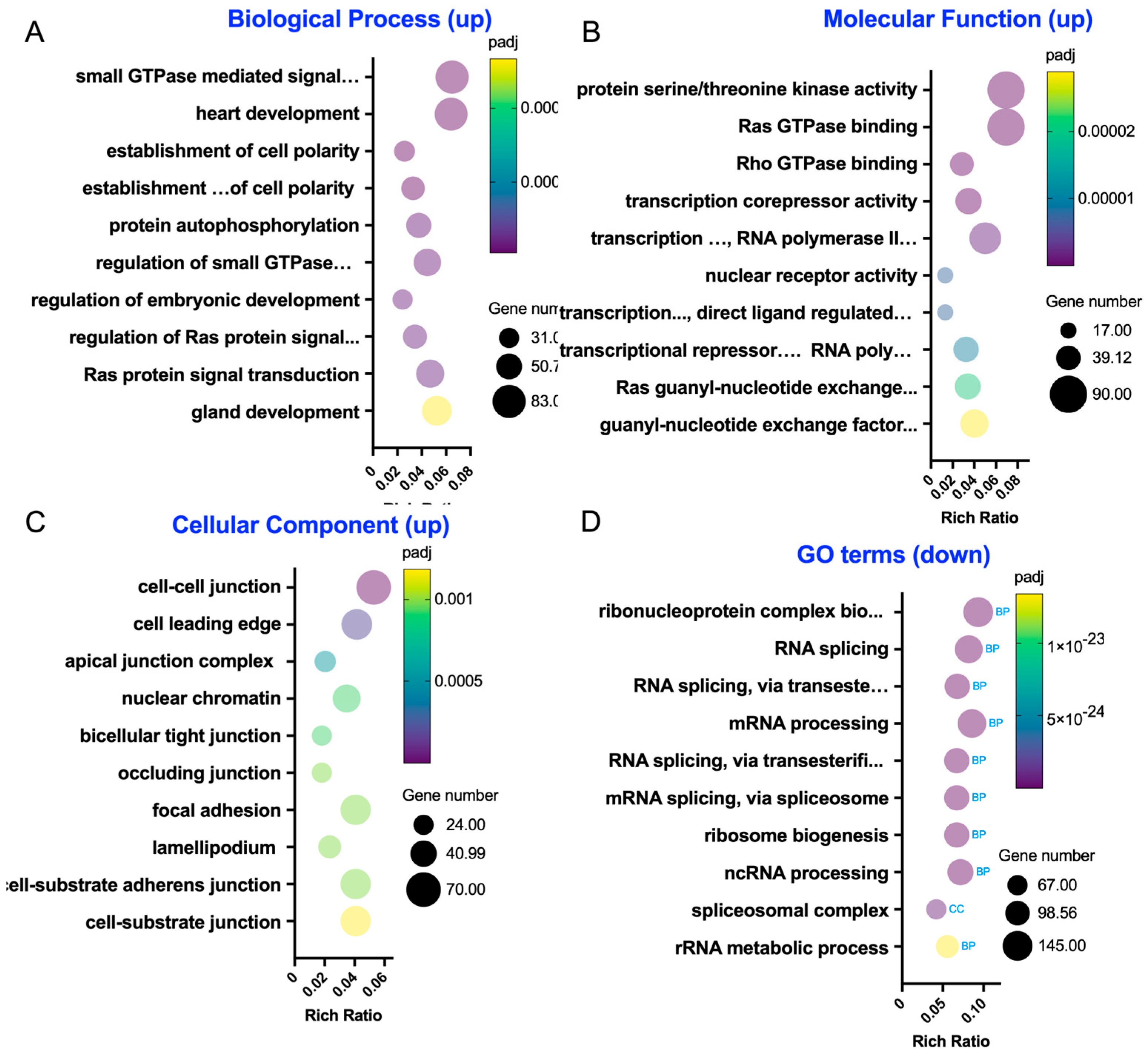
The RNA sequencing data further supported this mechanistic model, showing enrichment in genes associated with small GTPase signaling and cytoskeletal regulation following LA administration (
Figure 5). Specifically, enriched GO terms included Rho GTPase binding, Ras signaling, and serine/threonine kinase activity—molecular functions closely associated with cytoskeletal regulation and TJ remodeling. These findings reinforce the hypothesis that LA exerts its barrier-protective effects by modulating upstream regulators of ROCK, such as Rho and Ras family GTPases. The enrichment of apical junction and cell–cell adhesion genes with upregulated DEGs, alongside decreased expression of genes associated with focal adhesion disassembly and extracellular matrix remodeling, suggests a global reinforcement of epithelial barrier structure. Taken together, our findings propose that LA promotes epithelial stability through both post-translational (small GTPase- or kinase-mediated) and transcriptional regulation of the cytoskeletal and junctional networks.
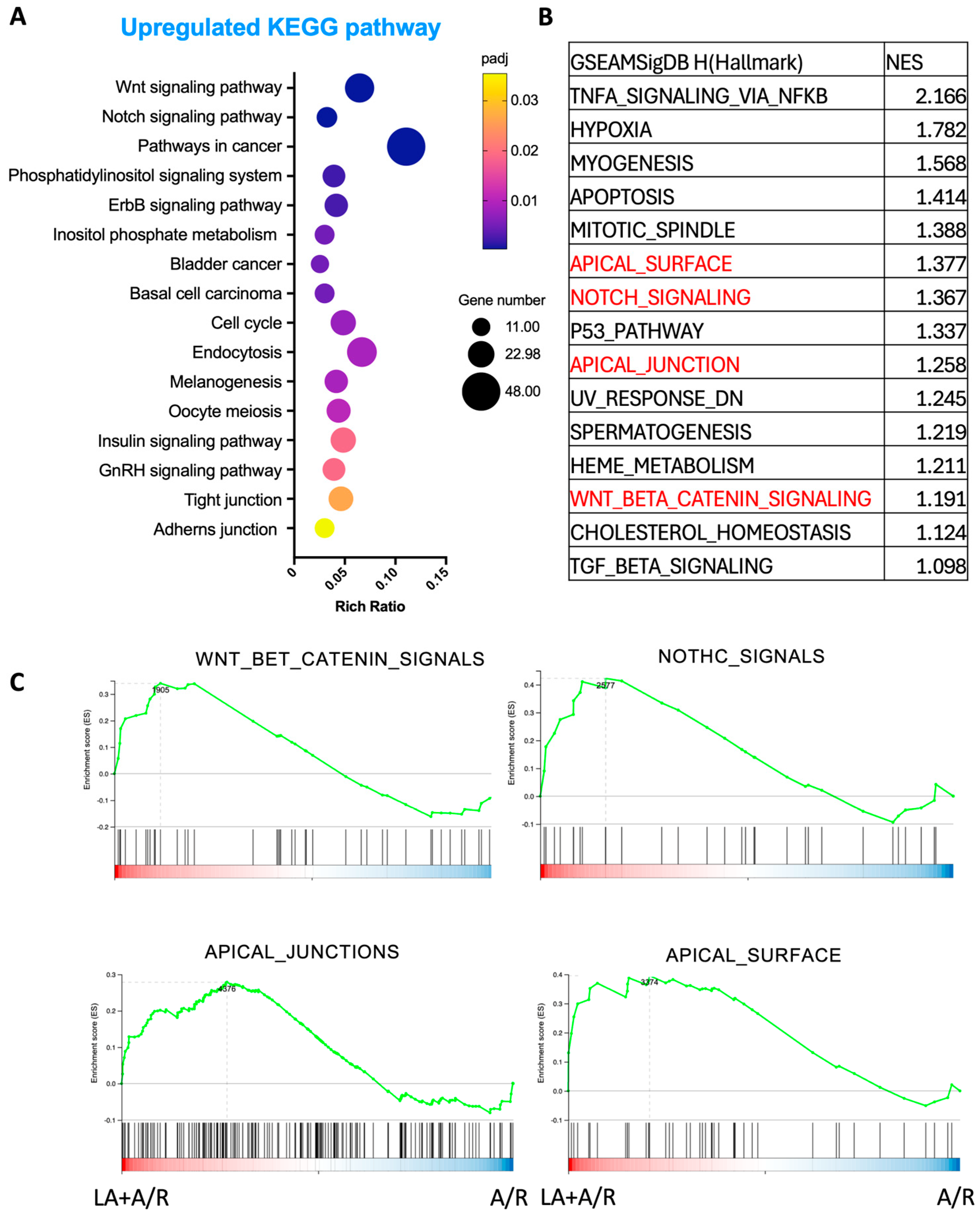
Importantly, transcriptomic data also pointed to the activation of epithelial proliferation pathways. KEGG and GSEA pathway analyses revealed significant upregulation of Wnt and Notch signaling pathways—both of which are central to epithelial renewal and stem cell maintenance. These findings were functionally validated by in vitro proliferation assays, which demonstrated a significant increase in epithelial cell growth following LA treatment (
Figure 7A). The upregulated expression of genes such as EGR1, DDIT4, and AMOTL2, known regulators of cell proliferation and differentiation, supports LA’s dual role in both maintaining junctional integrity and enhancing epithelial restitution. This proliferative effect may be particularly advantageous in pathological contexts where epithelial turnover is impaired, further underscoring LA’s therapeutic potential. This would make LA a promising therapeutic option for mucosal healing in mucosal inflammatory disorders, such as inflammatory bowel disease and necrotizing enterocolitis. However, it is important to consider the potential long-term implications of stimulating this pathway. Chronic intestinal inflammation or repeated injury cycles, which demand sustained proliferative repair, have been linked to an increased risk of dysplasia and tumorigenesis. Therefore, while LA’s pro-proliferative effects are advantageous for acute barrier repair, further investigation is warranted to understand its long-term safety profile in chronic conditions that involve persistent mucosal injury and repair.
Furthermore, the A/R model used in our study is highly relevant to a number of critical human gastrointestinal disorders characterized by ischemia–reperfusion (I/R) injury. Clinical conditions such as NEC in premature infants, acute mesenteric ischemia resulting from arterial or venous occlusion, and complications from major surgical procedures like intestinal transplantation and aortic aneurysm repair all share I/R injury as a core pathophysiological mechanism. In these settings, the temporary loss of blood flow (ischemia) followed by its restoration (reperfusion) triggers a potent inflammatory cascade and the production of reactive oxygen species, leading to severe damage to the intestinal mucosal barrier. This breach in the barrier allows for the translocation of luminal bacteria and toxins into the bloodstream, which can result in systemic inflammatory response syndrome, sepsis, and multi-organ failure [
22,
23]. Our findings suggest that LA could be a valuable therapeutic agent in these clinical scenarios by stabilizing tight junctions and preserving barrier integrity during the critical reperfusion phase, thereby mitigating the severe downstream consequences of I/R injury.
While our study provides valuable insights into the protective mechanisms of Larazotide Acetate on intestinal enterocytes, we must acknowledge the limitations of our in vitro Caco-2 and IPEC-J2 model. These monoculture systems, though robust, do not fully recapitulate the complex cellular and structural architecture of the native intestinal barrier, which includes a diverse array of cell types that contribute to the mucosal defense and repair processes. Therefore, future work is essential to validate these findings in more physiologically sophisticated systems. The logical progression would be to utilize 3D intestinal organoids to study these intercellular interactions, followed by in vivo studies in animal models of ischemia–reperfusion injury. Another limitation is our prophylactic design. We showed that pre-treatment with Larazotide Acetate protects the barrier from injury, but not whether it can therapeutically repair existing damage. Future studies should assess post-injury application to determine its full clinical utility. Such research will be critical for confirming the therapeutic efficacy of LA and advancing its potential translation into clinical applications for acute intestinal injuries.