Hydrogel-Based Formulations to Deliver Analgesic Drugs: A Scoping Review of Applications and Efficacy
Abstract
1. Introduction
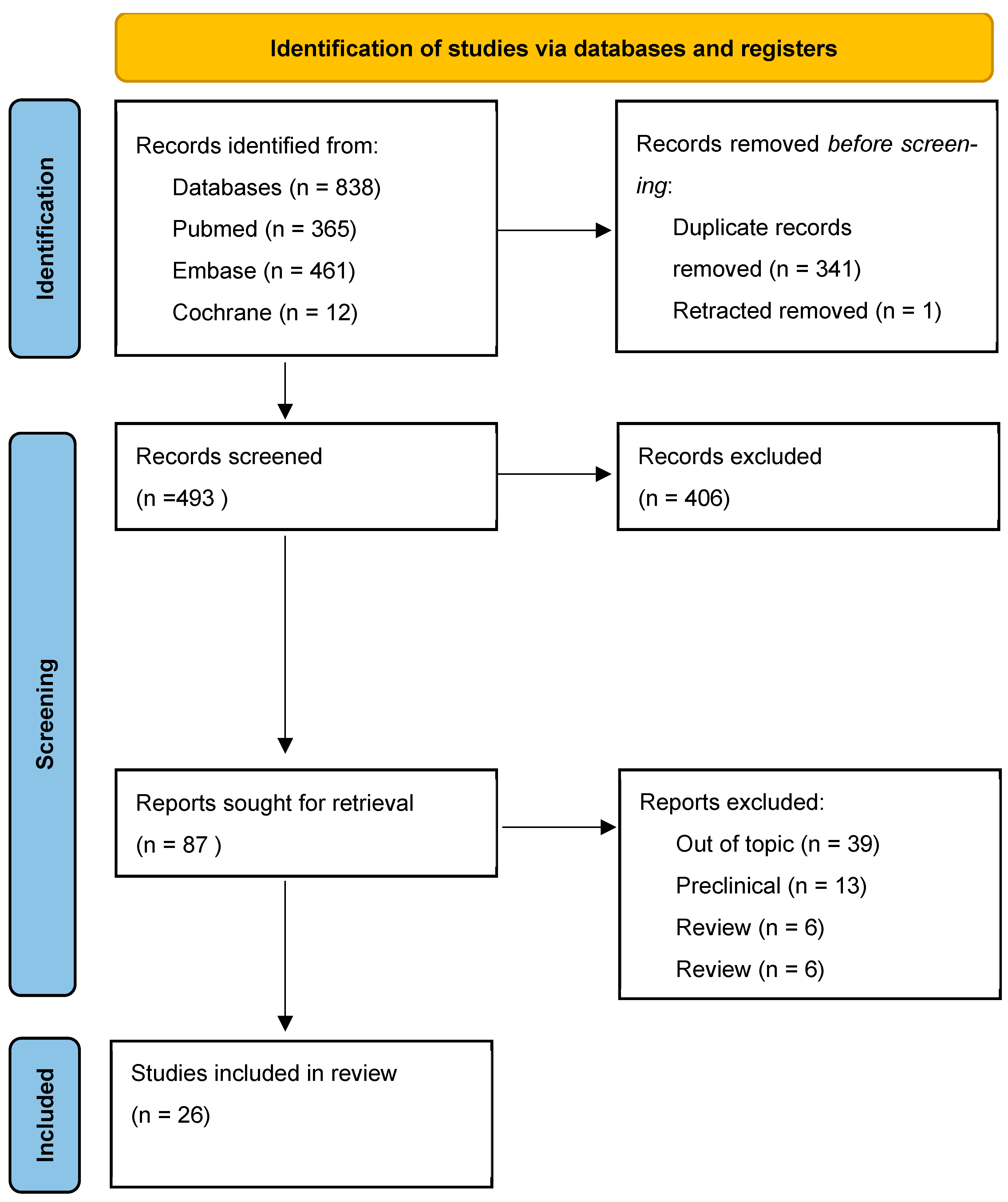
2. Materials and Methods
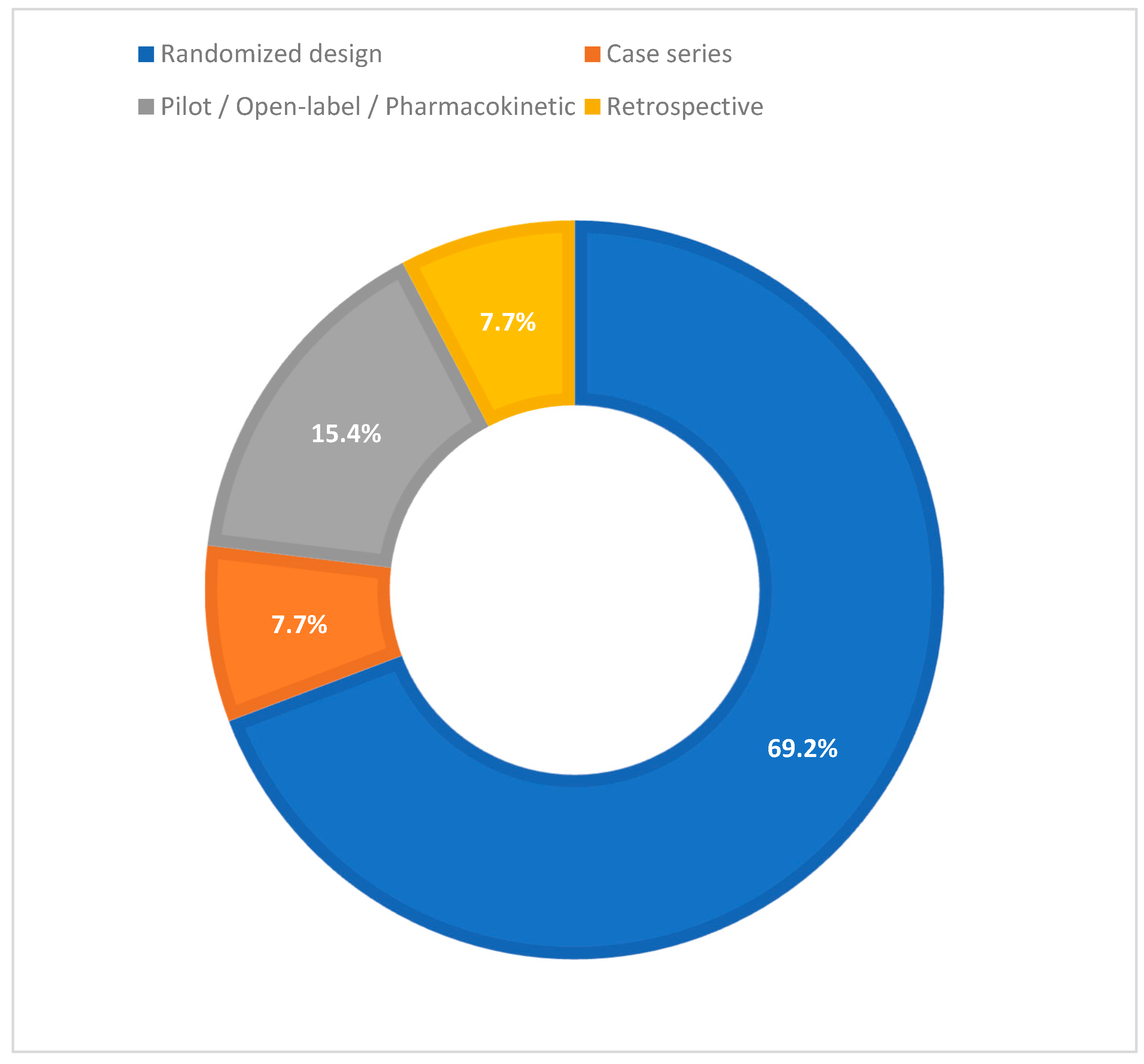
3. Results
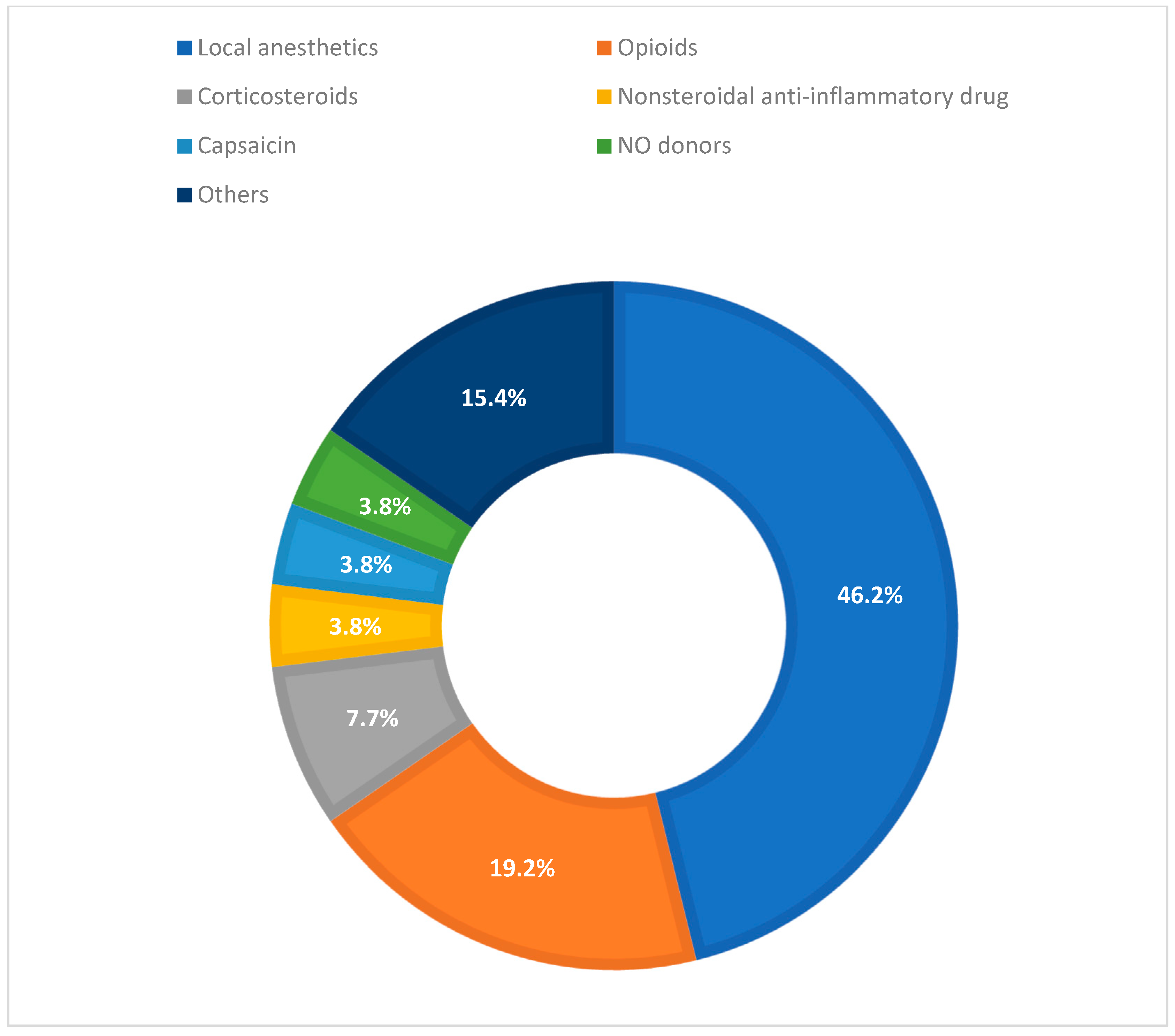
3.1. Drug Classes
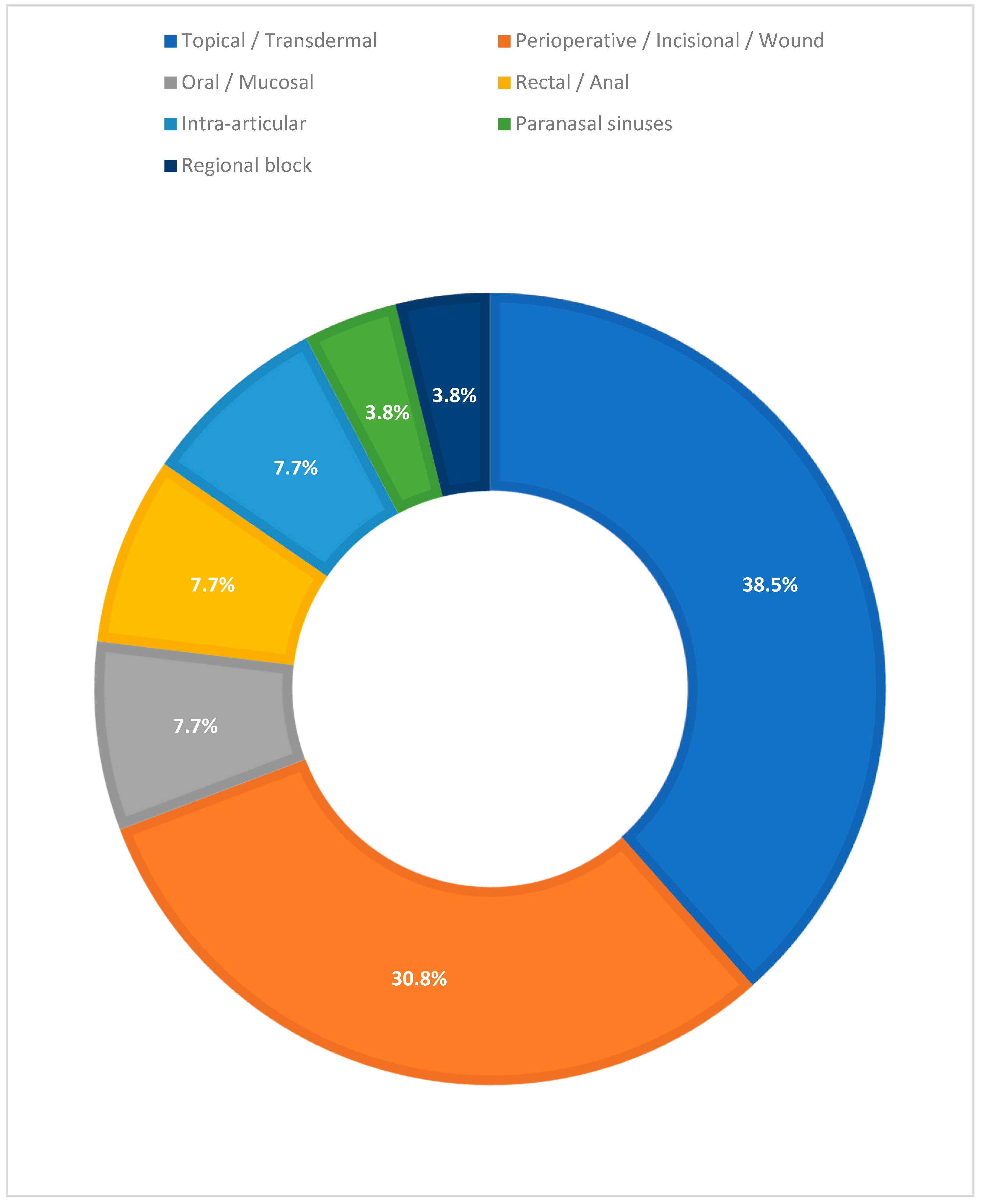
- Local Anesthetics (12/26; 46.2%). Designs were mostly randomized; predominant routes of administration were perioperative, incisional, or wound injection (eight studies), with contributions from topical/transdermal (two), paranasal sinuses (one), and regional block (one). Outcomes included reduction in VAS/NRS, decrease in rescue medication use, and prolonged duration of analgesic effect; 10 of these 12 studies that analyzed local anesthetics in pain management reported great pain-solving effect, while 1 study was neutral and 1 was not definable.
- Opioids and Peripheral Opioid Receptor Modulators (5/26; 19.2%). To administer these drugs, there was a prevalence of topical and transdermal application (three), with evidence also for rectal/anal (one) and oral/mucosal (one) routes. The main endpoints were pain release and need for rescue medication; four of five studies reported improvement in pain management, and one was not definable.
- Intra-articular Corticosteroids (2/26; 7.7%). Intra-articular (i.e., knee infiltration) in an osteoarthritis context; both studies described pain relief and better clinical outcomes.
- NSAIDs (1/26; 3.8%). Topical/transdermal application guaranteed good pain control and outcomes.
- Topical Neuromodulator (i.e., capsaicin) (1/26; 3.8%). Topical/transdermal application of capsaicin improved pain relief if the pain was related to local symptoms.
- Other/Not Classified (4/26; 15.4%). Heterogeneous contexts (topical/transdermal, oral/mucosal, and perioperative/incisional/wound); three studies reported good pain management, and one was not definable.
3.2. Route and Site of Administration
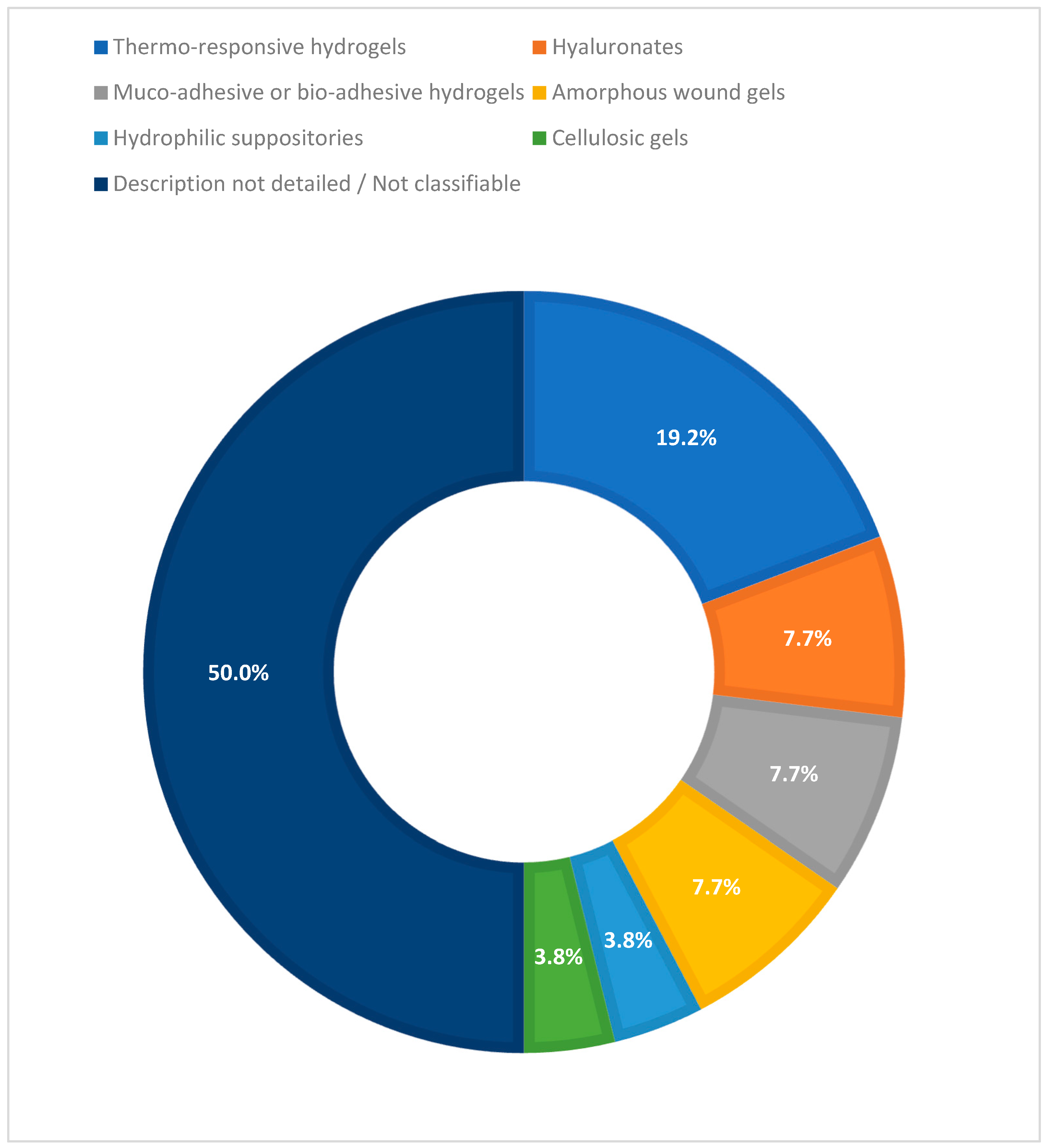
3.3. Hydrogel Technology
3.4. Non-DDS Clinical Studies (Pain Modulation Without Drug Release)
3.4.1. Ocular
3.4.2. Burns and Wounds
3.4.3. Musculoskeletal and Discal
3.4.4. Other Contexts (Lubrication/Barrier)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | Activities of Daily Living |
| AEs | Adverse Events |
| AMT | Thermoresponsive hydrogel formulation (AMT-143) |
| APIs | Active Pharmaceutical Ingredients |
| BCL | Bandage Contact Lens |
| DDS | Drug Delivery System(s) |
| EHO | Experimental Hydrogel with Olea europaea extract (EHO-85) |
| ENT | Ear, Nose, and Throat |
| HA | Hyaluronic Acid |
| LD | Lidocaine |
| NO | Nitric Oxide |
| NRS | Numerical Rating Scale |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| OA | Osteoarthritis |
| PF | Poloxamer Formulation (e.g., PF72 hydrogel) |
| PK | Pharmacokinetics |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses—Scoping Review extension |
| PRK | Photorefractive Keratectomy |
| RCT | Randomized Controlled Trial |
| RCTs | Randomized Controlled Trials |
| TA | Triamcinolone Acetonide |
| TLA | Topical Local Anesthetic |
| VAS | Visual Analog Scale |
Appendix A
| Database | Search String | Results |
|---|---|---|
| Pubmed | (“hydrogel s”[All Fields] OR “hydrogelating”[All Fields] OR “hydrogelation”[All Fields] OR “hydrogelations”[All Fields] OR “hydrogelator”[All Fields] OR “hydrogelators”[All Fields] OR “hydrogels”[MeSH Terms] OR “hydrogels”[All Fields] OR “hydrogel”[All Fields]) AND (“drug delivery systems”[MeSH Terms] OR (“drug”[All Fields] AND “delivery”[All Fields] AND “systems”[All Fields]) OR “drug delivery systems”[All Fields] OR (“drug”[All Fields] AND “delivery”[All Fields]) OR “drug delivery”[All Fields]) AND (“pain”[MeSH Terms] OR “pain”[All Fields]) | 365 |
| Cochrane | hydrogels and pain | 12 |
| Embase | (‘analgesia’/exp OR ‘analgaesia’ OR ‘analgesia’ OR ‘pain management’ OR ‘pain relief’ OR ‘sequential analgetic analgesia’ OR ‘surgical analgesia’) AND (‘hydrogel’/exp OR ‘gamma polymerized hydrogel’ OR ‘hydrogel’ OR ‘hydrogels’) | 461 |
Appendix B
| Year | Setting | Application Site | Drug | Hydrogel | Direction of Effect | Safety | Reference |
|---|---|---|---|---|---|---|---|
| 2015 | Knee osteoarthritis | Intra-articular (knee) | Triamcinolone acetonide | Hyaluronic acid hydrogels | Improved vs. control/baseline | Unclear/Not stated | [29] |
| 2012 | Knee osteoarthritis | Intra-articular (knee) | Triamcinolone acetonide | Hyaluronic acid + triamcinolone hydrogel | Improved vs. control/baseline | Unclear/Not stated | [30] |
| 2014 | Purulent frontal sinusitis | Sinus cavity | Dioxidine, epsilon-aminocaproic acid, lidocaine | Sodium alginate hydrogel | Improved vs. control/baseline | Unclear/Not stated | [13] |
| 2024 | Post-craniotomy pain | Scalp incision site | Lidocaine | Lidocaine 5% hydrogel plaster | No difference/Neutral | Unclear/Not stated | [14] |
| 2021 | Localized neuropathic pain | Transdermal | Lidocaine | Topical system 1.8% vs. 5% patch | Improved vs. control/baseline | Unclear/Not stated | [15] |
| 2009 | Postherpetic neuralgia | Topical | Lidocaine | Lidocaine medicated plaster (5%) | Improved vs. control/baseline | Favorable safety/tolerability | [16] |
| 2022 | Minimally invasive colorectal surgery | Surgical wound | Ropivacaine or Bupivacaine | Gel infusion kit | Improved vs. control/baseline | Unclear/Not stated | [17] |
| 2024 | Inguinal hernia repair | Surgical site infiltration | Ropivacaine | AMT-143 thermosensitive hydrogel | Unclear/Not stated | Unclear/Not stated | [18] |
| 2024 | Bimaxillary surgery | Surgical site (orthognathic surgery) | Ropivacaine | PF 72 thermoresponsive hydrogel | Improved vs. control/baseline | Unclear/Not stated | [19] |
| 2024 | Ear reconstruction surgery | Donor site (iliac crest) | Ropivacaine | Sodium carboxymethylcellulose hydrogel | Improved vs. control/baseline | Unclear/Not stated | [20] |
| 2024 | Postoperative pain relief | Surgical site (breast augmentation) | Ropivacaine | PF72 thermoresponsive hydrogel | Improved vs. control/baseline | Unclear/Not stated | [21] |
| 2022 | Postoperative pain management | Surgical incision site | Ropivacaine | PF 72 thermoresponsive hydrogel | Improved vs. control/baseline | Unclear/Not stated | [22] |
| 2022 | Thoracoscopic surgery | Thoracic port site | Ropivacaine | Poloxamer 407-based hydrogel | Improved vs. control/baseline | Unclear/Not stated | [23] |
| 2021 | Laparoscopic nephrectomy | Regional block | Ropivacaine or Bupivacaine | Unspecified | Improved vs. control/baseline | Unclear/Not stated | [24] |
| 2002 | Anal fissure | Anal | Glyceryl trinitrate | Topical nitrate ointment | Improved vs. control/baseline | Unclear/Not stated | [31] |
| 2024 | Chronic low back pain | Topical (lower back) | Loxoprofen | Loxoprofen sodium hydrogel patch | Improved vs. control/baseline | Favorable safety/tolerability | [32] |
| 1996 | Pediatric pain management | Rectal | Morphine | Hydrophilic hydrogel suppository | Improved vs. control/baseline | Unclear/Not stated | [25] |
| 2011 | Chronic leg ulcers | Topical (leg ulcer) | Morphine | Topical morphine gel | Improved vs. control/baseline | Unclear/Not stated | [26] |
| 2009 | Arterial leg ulcers | Topical | Morphine | Topical morphine hydrogel | Improved vs. control/baseline | Unclear/Not stated | [27] |
| 2020 | Oral mucositis pain | Oral mucosa | Morphine (topical) | Mucoadhesive oral hydrogel | Improved vs. control/baseline | Unclear/Not stated | [28] |
| 2021 | Painful dermal ulcers | Topical | Morphine and Loperamide | Intrasite gel | Unclear/Not stated | Unclear/Not stated | [51] |
| 2001 | Aphthous ulcers | Oral | 2-octyl cyanoacrylate | Bioadhesive hydrogel device | Unclear/Not stated | Unclear/Not stated | [33] |
| 2013 | Burn wounds | Topical | Aloe vera vs. silver sulphadiazine | Aloe vera gel | Improved vs. control/baseline | Unclear/Not stated | [34] |
| 2022 | Chronic ulcer treatment | Topical (chronic ulcers) | Olea europaea leaf extract | EHO-85 amorphous hydrogel | Improved vs. control/baseline | Unclear/Not stated | [35] |
| 2024 | Onychocryptosis | Topical (toenail) | Ozoile (ozonized oil) | Ozoile-based hydrogel | Improved vs. control/baseline | Unclear/Not stated | [36] |
| 2012 | Myofascial neck pain | Topical (neck) | Capsaicin 0.1% | Capsaicin hydrogel patch | Improved vs. control/baseline | Unclear/Not stated | [37] |
References
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Getachew, M.; Tesfaye, H.; Yihunie, W.; Ayenew, T.; Alemu, S.; Dagnew, E.M.; Biyazin, Y.; Abebe, D.; Degefu, N.; Abebaw, A. Sustained release local anesthetics for pain management: Relevance and formulation approaches. Front. Pain Res. 2024, 5, 1383461. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Li, P.; Shi, L.; Zhang, S.; Guo, Q.; Yang, Y. Advances in the use of local anesthetic extended-release systems in pain management. Drug Deliv. 2024, 31, 2296349. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Choi, W.; Kohane, D.S. Hybrid Nanoparticle-Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Kim, J. Wound Pain Management: The Present and the Future. J. Wound Manag. Res. 2024, 20, 199–211. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chow, D.W.Y.; Lau, C.M.L.; Zhou, G.; Back, W.; Xu, J.; Carim, S.; Chau, Y. A bioinspired synthetic soft hydrogel for the treatment of dry eye. Bioeng. Transl. Med. 2021, 6, e10227. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Abu-Ghazaleh, S.; Sloan, J.A.; vanHaelst-Pisani, C.; Hammer, A.M.; Rowland, K.M., Jr.; Law, M.; Windschitl, H.E.; Kaur, J.S.; Ellison, N. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J. Clin. Oncol. 1997, 15, 969–973. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.; Lee, M.; Hahn, S.; Jeon, M.J. Effect of a pH-Balanced Vaginal Gel on Dyspareunia and Sexual Function in Breast Cancer Survivors Who Were Premenopausal at Diagnosis: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 129, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Khar’Kova, N.A.; Gerasimenko, M.Y.; Egorova, E.A. The use of hydrogel materials coletex-adl and colegel in the treatment of purulent frontal sinusitis. Sovrem. Tehnol. Med. 2014, 6, 176–180. [Google Scholar]
- Han, X.; Yang, Y.; Ren, T.; Ji, N.; Luo, F. Efficacy of Preemptive Topical Lidocaine 5% Plaster in the Prevention of Post-Craniotomy Pain, a Randomized Clinical Trial. J. Pain Res. 2024, 17, 4251–4261. [Google Scholar] [CrossRef] [PubMed]
- Gudin, J.; Webster, L.R.; Greuber, E.; Vought, K.; Patel, K.; Kuritzky, L. Open-Label Adhesion Performance Studies of a New Lidocaine Topical System 1.8% versus Lidocaine Patches 5% and Lidocaine Medicated Plaster 5% in Healthy Subjects. J. Pain Res. 2021, 14, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Hans, G.; Sabatowski, R.; Binder, A.; Boesl, I.; Rogers, P.; Baron, R. Efficacy and tolerability of a 5% lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: Results of a long-term study. Curr. Med. Res. Opin. 2009, 25, 1295–1305. [Google Scholar] [CrossRef]
- Shin, J.K.; Jeong, H.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A.; Sim, W.S.; Kim, H.C. Efficacy of a local anesthetic gel infusion kit for pain relief after minimally invasive colorectal surgery: An open-label, randomized clinical trial. Sci. Rep. 2022, 12, 17429. [Google Scholar] [CrossRef]
- NCT. Postsurgical Analgesia After Hernia Repair. 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06709612 (accessed on 3 October 2025).
- Yun, C.W.; Kim, K.H.; Lee, W.; Kim, S.H. Comparative Analysis of Temperature-Responsive Hydrogel (PF 72) for Postoperative Pain After Bimaxillary Surgery: A Retro-spective Study. Aesthetic Plast. Surg. 2024, 48, 1271–1275. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, B.; Jiang, H.; Luan, W.; Pan, B.; Li, C. Sodium Carboxymethylcellulose Serves as Sustained-Release Agents for Providing Analgesia at the Donor Site After Costal Cartilage Harvesting in Ear Reconstruction. Aesthetic Plast. Surg. 2025, 49, 2350–2356. [Google Scholar] [CrossRef]
- Cho, J.; Kim, K.H.; Lee, W.; Go, J.Y.; Kim, S.H. Effectiveness of a Novel Temperature-Responsive Hydrogel (PF72) for Postoperative Pain Relief in Breast Augmentation. J. Clin. Med. 2023, 13, 110. [Google Scholar] [CrossRef]
- Choi, B.M.; Hwang, C.S.; Yoon, Y.S.; Park, I.J.; Yoo, M.W.; Kim, B.S. Novel temperature-responsive hydrogel injected to the incision site for postoperative pain relief in laparoscopic abdominal surgery: A single-blind, randomized, pivotal clinical trial. Surg. Endosc. 2022, 36, 5794–5802. [Google Scholar] [CrossRef]
- Jeon, J.H.; Seong, Y.W.; Han, J.E.; Cho, S.; Kim, J.H.; Jheon, S.; Kim, K. Randomized Trial of Poloxamer 407-Based Ropivacaine Hydrogel After Thoracoscopic Pulmonary Resection. Ann. Thorac. Surg. 2022, 114, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Dam, M.; Hansen, C.; Poulsen, T.D.; Azawi, N.H.; Laier, G.H.; Wolmarans, M.; Chan, V.; Bendtsen, T.F.; Borglum, J. Transmuscular quadratus lumborum block reduces opioid consumption and prolongs time to first opioid demand after laparoscopic nephrectomy. Reg. Anesth. Pain Med. 2021, 46, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lundeberg, S.; Beck, O.; Olsson, G.L.; Boreus, L.O. Rectal administration of morphine in children. Pharmacokinetic evaluation after a single-dose. Acta Anaesthesiol. Scand. 1996, 40, 445–451. [Google Scholar] [CrossRef]
- Huptas, L.; Rompoti, N.; Herbig, S.; Korber, A.; Klode, J.; Schadendorf, D.; Dissemond, J. A new topically applied morphine gel for the pain treatment in patients with chronic leg ulcers: First results of a clinical investigation. Hautarzt 2011, 62, 280–286. [Google Scholar] [CrossRef]
- Jansen, M.M.; van der Horst, J.C.; van der Valk, P.G.; Kuks, P.F.; Zylicz, Z.; van Sorge, A.A. Pain-relieving properties of topically applied morphine on arterial leg ulcers: A pilot study. J. Wound Care 2009, 18, 306–311. [Google Scholar] [CrossRef]
- Hood, A.; Scullion, B.; McGuire, H. QP2: Challenges Treating Severe Oral Mucositis Pain in Patients at High Risk for Aspiration. J. Pain Palliat. Care Pharmacother. 2021, 34, 173–174. [Google Scholar] [CrossRef]
- Petrella, R.J.; Emans, P.J.; Alleyne, J.; Dellaert, F.; Gill, D.P.; Maroney, M. Safety and performance of Hydros and Hydros-TA for knee osteoarthritis: A prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet. Disord. 2015, 16, 57. [Google Scholar] [CrossRef]
- Petrella, R.J.; Eamans, P.; Alleyne, J.; Maroney, M. A prospective, multi-center, randomized, double-blind feasibility study to evaluate the safety and performance of hydros joint therapy and Hydros-TA joint therapy for management of pain associated with osteoarthritis in the knee. Osteoarthr. Cartil. 2012, 20, S172–S173. [Google Scholar] [CrossRef]
- Tankova, L.; Yoncheva, K.; Muhtarov, M.; Kadyan, H.; Draganov, V. Topical mononitrate treatment in patients with anal fissure. Aliment. Pharmacol. Ther. 2002, 16, 101–103. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, X.; Wang, J.; Yan, F.; Gao, J.; Sun, T. Efficacy and Safety of Loxoprofen Sodium Hydrogel Patch in Patients with Chronic Inflammatory Pain: A 4-Week Real-World Study. J. Pain Res. 2024, 17, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kutcher, M.J.; Ludlow, J.B.; Samuelson, A.D.; Campbell, T.; Pusek, S.N. Evaluation of a bioadhesive device for the management of aphthous ulcers. J. Am. Dent. Assoc. 2001, 132, 368–376. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ahmed, N. Effectiveness of Aloe Vera gel compared with 1% silver sulphadiazine cream as burn wound dressing in second degree burns. J. Pak. Med. Assoc. 2013, 63, 225–230. [Google Scholar] [PubMed]
- Verdu-Soriano, J.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Caballero-Villarraso, J.; Moreno-Moreno, P.; Casado-Diaz, A.; Berenguer-Perez, M.; Guler-Caamano, I.; Laosa-Zafra, O.; et al. Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 1260. [Google Scholar] [CrossRef]
- Francavilla, V.; Secolo, G.; D’Armetta, M.; Toscano, R.; Campo, A.; Catanzaro, V.; Manno, M.; Secolo, I.; Messina, G. Onychocryptosis: A retrospective study of clinical aspects, inflammation treatment and pain management using Ozoile as a hydrogel and cream formulation. Eur. J. Transl. Myol. 2024, 34, 165–171. [Google Scholar] [CrossRef]
- Cho, J.H.; Brodsky, M.; Kim, E.J.; Cho, Y.J.; Kim, K.W.; Fang, J.Y.; Song, M.Y. Efficacy of a 0.1% capsaicin hydrogel patch for myofascial neck pain: A double-blinded randomized trial. Pain Med. 2012, 13, 965–970. [Google Scholar] [CrossRef]
- Taylor, K.R.; Molchan, R.P.; Townley, J.R.; Caldwell, M.C.; Panday, V.A. The effect of silicone hydrogel bandage soft contact lens base curvature on comfort and outcomes after photorefractive keratectomy. Eye Contact Lens 2015, 41, 77–83. [Google Scholar] [CrossRef]
- Taylor, K.R.; Caldwell, M.C.; Payne, A.M.; Apsey, D.A.; Townley, J.R.; Reilly, C.D.; Panday, V.A. Comparison of 3 silicone hydrogel bandage soft contact lenses for pain control after photorefractive keratectomy. J. Cataract Refract. Surg. 2014, 40, 1798–1804. [Google Scholar] [CrossRef]
- Holbert, M.D.; Griffin, B.R.; McPhail, S.M.; Ware, R.S.; Foster, K.; Bertoni, D.C.; Kimble, R.M. Effectiveness of a hydrogel dressing as an analgesic adjunct to first aid for the treatment of acute paediatric thermal burn injuries: Study protocol for a randomised controlled trial. Trials 2019, 20, 13. [Google Scholar] [CrossRef]
- Surowiecka, A.; Struzyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Far, R.; Veilleux, C.; Swamy, G.; Yang, M.M.H. Delayed percutaneous intradiscal hydrogel herniation causing neurological injury after minor trauma: Illustrative case. J. Neurosurg. Case Lessons 2024, 8, CASE24394. [Google Scholar] [CrossRef]
- Sacks, G.; DeStefano, V.; Parker, C.; Lebens, R.; Mushlin, H. The Artificial Disc Nucleus and Other Strategies for Replacement of the Nucleus Pulposus: Past, Present and Future Designs for an Emerging Surgical Solution. Eng. Regen. 2024, 5, 269–281. [Google Scholar] [CrossRef]
- Nasir, N.A.M.; Saad, A.Z.M.; Bachok, N.S.; Rashid, A.H.A.; Ujang, Z.; Noorsal, K.; Yusof, N.; Hashim, K.; Nor, F.M.; Imran, F.H.; et al. A Prospective Multicenter Randomized Controlled Trial to Evaluate the Efficacy of Chitosan Hydrogel Paste in Comparison to Commercial Hydroactive Gel as a Wound Bed Preparation. Indian J. Plast. Surg. 2023, 56, 44–52. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Bastami, S.; Frödin, T.; Ahlner, J.; Uppugunduri, S. Topical morphine gel in the treatment of painful leg ulcers, a double-blind, placebo-controlled clinical trial: A pilot study. Int. Wound J. 2012, 9, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Steigleman, W.A.; Rose-Nussbaumer, J.; Al-Mohtaseb, Z.; Santhiago, M.R.; Lin, C.C.; Pantanelli, S.M.; Kim, S.J.; Schallhorn, J.M. Management of Pain after Photorefractive Keratectomy: A Report by the American Academy of Ophthalmology. Ophthalmology 2023, 130, 87–98. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; Romano, S.; Margarucci, S.; Petillo, O.; Calarco, A. Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases. Gels 2024, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Chronopoulou, L.; Palocci, C. Liposome-Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10, 284. [Google Scholar] [CrossRef]
- Jyothi, B.; Mitragotri, M.V.; Kurugodiyavar, M.D.; Shaikh, S.I.; Korikanthimath, V.V. Morphine Versus Loperamide with Intrasite Gel in the Treatment of Painful Dermal Ulcers: A Randomized, Crossover Study. Pain Physician 2021, 24, E37–E44. [Google Scholar]
| Delivered Drugs | Number of Studies (RCTs) | Effect | Hydrogel Technology | References | ||
|---|---|---|---|---|---|---|
| Improved | Neutral | Unclear | ||||
| Local anesthetics | 12 (10) | 10 | 1 | 1 | Thermoresponsive, Other/Unspecified | [13,14,15,16,17,18,19,20,21,22,23,24] |
| Opioids | 5 (1) | 4 | 0 | 1 | Amorphous wound gel, Unspecifed | [25,26,27,28] |
| Corticosteroids | 2 (2) | 2 | 0 | 0 | Hyaluronic acid–based | [29,30] |
| Nitric Oxide | 1 (1) | 1 | 0 | 0 | Topical nitrate ointment | [31] |
| NSAIDs | 1 (1) | 1 | 0 | 0 | Unspecified | [32] |
| 2-octyl cyanoacrylate, Aloe vera, Olea europea, Ozonized oil, Capsaicin | 5 (1) | 4 | 0 | 1 | Other, Unspecified, Muco/Bioadhesive | [33,34,35,36,37] |
| Hydrogel Group | Number of Studies (RCTs) | Role in Pain Control | Common Indications | Representative Hydrogels | Representative Citations |
|---|---|---|---|---|---|
| Ocular silicone hydrogels | 12 (5) | Mechanical shield and lubrication of corneal epithelium after PRK; decreases nociceptive stimulation and blinking friction. | Acute ocular, Chronic ocular discomfort | Lotrafilcon A, Balafilcon A, Balafilcon A (silicone hydrogels) | [38,39] |
| Topical wound/burn hydrogels | 12 (7) | Cooling/heat-sink, moist occlusive environment, and atraumatic removal reduce procedural and dressing-change pain. | Acute burn, Chronic wound | Burnaid, Oxyzyme, SockIt!, (hydrogel dressings) | [40,41] |
| Intradiscal hydrogel implants | 6 (0) | Hydraulic cushioning and disk height support aim to reduce mechanical nociception; safety depends on implant stability. | Chronic disk pain, Chronic back | GelStix, Polyethylene glycol, Hyalodisc (hydrogel implants) | [42,43] |
| Other non-DDS hydrogels | 8 (3) | Physical/biophysical modulation (barrier, lubrication, moisture) rather than pharmacological delivery. | Chronic hallux rigidus, Chronic knee Osteoarthritis | Polyvinyl alcohol hydrogel implant, hyaluronic acid hydrogel, Cross-linked sodium hyaluronate | [44] |
| Aspect | Key Findings | Notes |
|---|---|---|
| Hydrogel classification | DDS hydrogels (contain and release analgesic APIs) vs. non-DDS hydrogels (pain modulation via physical/biophysical mechanisms) | Non-DDS cataloged separately for transparency |
| Drug classes delivered | Local anesthetics (46%), opioids (19%), corticosteroids, NSAIDs, neuromodulators, nitric oxide donors | Predominantly perioperative, topical/mucosal, intra-articular |
| Hydrogel technologies | Thermo-responsive (poloxamer), hyaluronate-based, muco/bio-adhesive, amorphous wound gels, hydrophilic suppositories, cellulosic gels | No hybrid nanotechnology included in clinical studies |
| Administration sites | Topical/transdermal, perioperative/incisional, oral/mucosal, rectal/anal, intra-articular, sinus cavity, regional block | Broad distribution across acute and chronic pain settings |
| Analgesic effects | 85% of DDS studies reported improved pain outcomes; reduced rescue medication; prolonged analgesic effect | More consistent benefits in acute/severe pain, variable in chronic/milder pain |
| Safety and tolerability | Generally favorable, but incomplete reporting of adverse events | Need for standardized safety registries |
| Non-DDS evidence | Physical pain modulation (cooling, barrier, lubrication) in burns, ocular, discogenic and mucosal conditions | Supports multimodal strategies combining matrix effects with pharmacological delivery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Franco, S.; Alfieri, A.; Sansone, P.; Pota, V.; Coppolino, F.; Frangiosa, A.; Maffei, V.; Pace, M.C.; Passavanti, M.B.; Fiore, M. Hydrogel-Based Formulations to Deliver Analgesic Drugs: A Scoping Review of Applications and Efficacy. Biomedicines 2025, 13, 2465. https://doi.org/10.3390/biomedicines13102465
Di Franco S, Alfieri A, Sansone P, Pota V, Coppolino F, Frangiosa A, Maffei V, Pace MC, Passavanti MB, Fiore M. Hydrogel-Based Formulations to Deliver Analgesic Drugs: A Scoping Review of Applications and Efficacy. Biomedicines. 2025; 13(10):2465. https://doi.org/10.3390/biomedicines13102465
Chicago/Turabian StyleDi Franco, Sveva, Aniello Alfieri, Pasquale Sansone, Vincenzo Pota, Francesco Coppolino, Andrea Frangiosa, Vincenzo Maffei, Maria Caterina Pace, Maria Beatrice Passavanti, and Marco Fiore. 2025. "Hydrogel-Based Formulations to Deliver Analgesic Drugs: A Scoping Review of Applications and Efficacy" Biomedicines 13, no. 10: 2465. https://doi.org/10.3390/biomedicines13102465
APA StyleDi Franco, S., Alfieri, A., Sansone, P., Pota, V., Coppolino, F., Frangiosa, A., Maffei, V., Pace, M. C., Passavanti, M. B., & Fiore, M. (2025). Hydrogel-Based Formulations to Deliver Analgesic Drugs: A Scoping Review of Applications and Efficacy. Biomedicines, 13(10), 2465. https://doi.org/10.3390/biomedicines13102465











