Efficacy and Safety of Topical 5% Cannabidiol Plus Myrcene for the Treatment of Vestibulodynia: A Multi-Centric Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Women at least 18 years of age and premenopausal (absence of menstruation for 12 months);
- Experience moderate to severe pain, a minimum of 4/10 on a numeric rating scale with respect to at least 1 of 3 parameters assessed (pain, dyspareunia, and swab test) for at least 90% of sexual intercourse attempts;
- Pain limited to the vestibule during vaginal intercourse and during activities exerting pressure on the vestibule (tampon insertion, tight jeans or pants, cycling, and horseback riding).
- Presence of VBD for at least 6 months and diagnosed according to the standardized gynecological examination protocol.
- Active vulvo-vaginal infections at the time of their gynecological examination;
- Genital bleeding of unknown origin;
- Patients concomitantly included in different interventional clinical trials;
- Unwillingness to provide informed consent to the trial;
- Women who used topical drugs in the past 30 days.
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VBD | Vestibulodynia; |
| THC | Tetrahydrocannabinol; |
| CBD | Cannabidiol; |
| VAS | Visual analogue scale; |
| MD | Mean difference; |
| CBs | Cannabinoid receptors; |
| TRPV1 | Transient vanilloid receptor potential channels subtype 1; |
| RCT | Randomized controlled trial; |
| LNP | Localized neuropathic pain. |
References
- Bornstein, J.; Goldstein, A.T.; Stockdale, C.K.; Bergeron, S.; Pukall, C.; Zolnoun, D.; Coady, D.; International Society for the Study of Vulvovaginal Disease (ISSVD); International Society for the Study of Women’s Sexual Health (ISSWSH); International Pelvic Pain Society (IPPS). 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistent Vulvar Pain and Vulvodynia. J. Sex. Med. 2016, 13, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Graziottin, A.; Murina, F.; Gambini, D.; Taraborrelli, S.; Gardella, B.; Campo, M.; VuNet Study Group. Vulvar pain: The revealing scenario of leading comorbidities in 1183 cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, S.; Reed, B.D.; Wesselmann, U.; Bohm-Starke, N. Vulvodynia. Nat. Rev. Dis. Primers. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Tonc, E.; Omwanda, G.K.; Tovar, K.A.; Golden, X.M.E.; Chatterjea, D. Immune mechanisms in vulvodynia: Key roles for mast cells and fibroblasts. Front. Cell. Infect. Microbiol. 2023, 13, 1215380. [Google Scholar] [CrossRef]
- McLean, L.; Antonio, F.I.; Rodrigues, M.P.; Pukall, C. Pelvic floor muscle activation amplitude at rest, during voluntary contraction, and during Valsalva maneuver-a comparison between those with and without provoked vestibulodynia. J. Sex. Med. 2025, 22, 570–578. [Google Scholar] [CrossRef]
- Santangelo, G.; Ruggiero, G.; Murina, F.; Di Donato, V.; Perniola, G.; Palaia, I.; Fischetti, M.; Casorelli, A.; Giannini, A.; Di Dio, C.; et al. Vulvodynia: A practical guide in treatment strategies. Int. J. Gynaecol. Obstet. 2023, 163, 510–520. [Google Scholar] [CrossRef]
- Rosen, N.O.; Dawson, S.J.; Brooks, M.; Kellogg-Spadt, S. Treatment of Vulvodynia: Pharmacological and Non-Pharmacological Approaches. Drugs 2019, 79, 483–493. [Google Scholar] [CrossRef]
- Lefebvre, È.; Tawil, N.; Yahia, L. Transdermal Delivery of Cannabidiol for the Management of Acute Inflammatory Pain: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2024, 25, 5858. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef]
- Alayoubi, M.; Rodrigues, A.; Wu, C.; Whitehouse, E.; Nguyen, J.; Cooper, Z.D.; O’Neill, P.R.; Cahill, C.M. Elucidating interplay between myrcene and cannabinoid receptor 1 receptors to produce antinociception in mouse models of neuropathic pain. Pain 2025, 166, 2140–2151. [Google Scholar] [CrossRef]
- Wu, C.; Goldstein, A.; Klebanoff, J.S.; Moawad, G.N. Surgical management of neuroproliferative-associated vestibulodynia: A tutorial on vestibulectomy with vaginal advancement flap. Am. J. Obstet. Gynecol. 2019, 221, 525.e1–525.e2. [Google Scholar] [CrossRef]
- Thapa, R.; Ang, D. Nociplastic pain: A practical guide to chronic pain management in the primary care setting. Clevel. Clin. J. Med. 2025, 92, 236–247. [Google Scholar] [CrossRef]
- Cortez-Resendiz, A.; Leiter, T.J.; Riela, S.M.; Graziane, N.M.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacology of Cannabinoids in Chronic Pain. Med. Cannabis Cannabinoids 2025, 8, 31–46. [Google Scholar] [CrossRef]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef] [PubMed]
- Bih, C.I.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.L.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Pang, Z. TRP Channels in Skin Biology and Pathophysiology. Pharmaceuticals 2016, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Bekauri, T.; Fischer, S.; Honn, K.V.; Maddipati, K.R.; Love, T.; Little, C.; Wood, R.W.; Bonham, A.D.; Linder, M.A.; Yule, D.I.; et al. Inflammation, lipid dysregulation, and transient receptor potential cation channel subfamily V member 4 signaling perpetuate chronic vulvar pain. Pain 2024, 165, 820–837. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- Sivesind, T.E.; Maghfour, J.; Rietcheck, H.; Kamel, K.; Malik, A.S.; Dellavalle, R.P. Cannabinoids for the Treatment of Dermatologic Conditions. JID Innov. 2022, 2, 100095. [Google Scholar] [CrossRef]
- Xu, D.H.; Cullen, B.D.; Tang, M.; Fang, Y. The Effectiveness of Topical Cannabidiol Oil in Symptomatic Relief of Peripheral Neuropathy of the Lower Extremities. Curr. Pharm. Biotechnol. 2020, 21, 390–402. [Google Scholar] [CrossRef]
- Mick, G.; Baron, R.; Finnerup, N.B.; Hans, G.; Kern, K.-U.; Brett, B.; Dworkin, R.H. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manag. 2012, 2, 71–77. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Active Treatment n = 20 | Placebo n = 20 | p-Value |
|---|---|---|---|
| Age (years) | 27.5 (20.0–49.0) | 29.5 (22.0–49.0) | 0.39 |
| Duration of the disease (months) | 22.0 (6.0–48.0) | 24.0 (6.0–120.0) | 0.61 |
| Current use of hormonal contraceptive | 4 (20.0) | 6 (30.0) | 0.72 |
| Burning/pain (VAS) | 6.1 ± 1.6 | 5.9 ± 1.9 | 0.65 |
| Dyspareunia (VAS) | 7.3 ± 1.4 | 5.9 ± 1.9 | 0.02 |
| Cotton swab test (VAS) | 6.9 ± 1.6 | 6.2 ± 1.7 | 0.22 |
| Parameter | Active Treatment n = 20 | Placebo n = 20 | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | p-value | Baseline | Follow-up | p-value | |
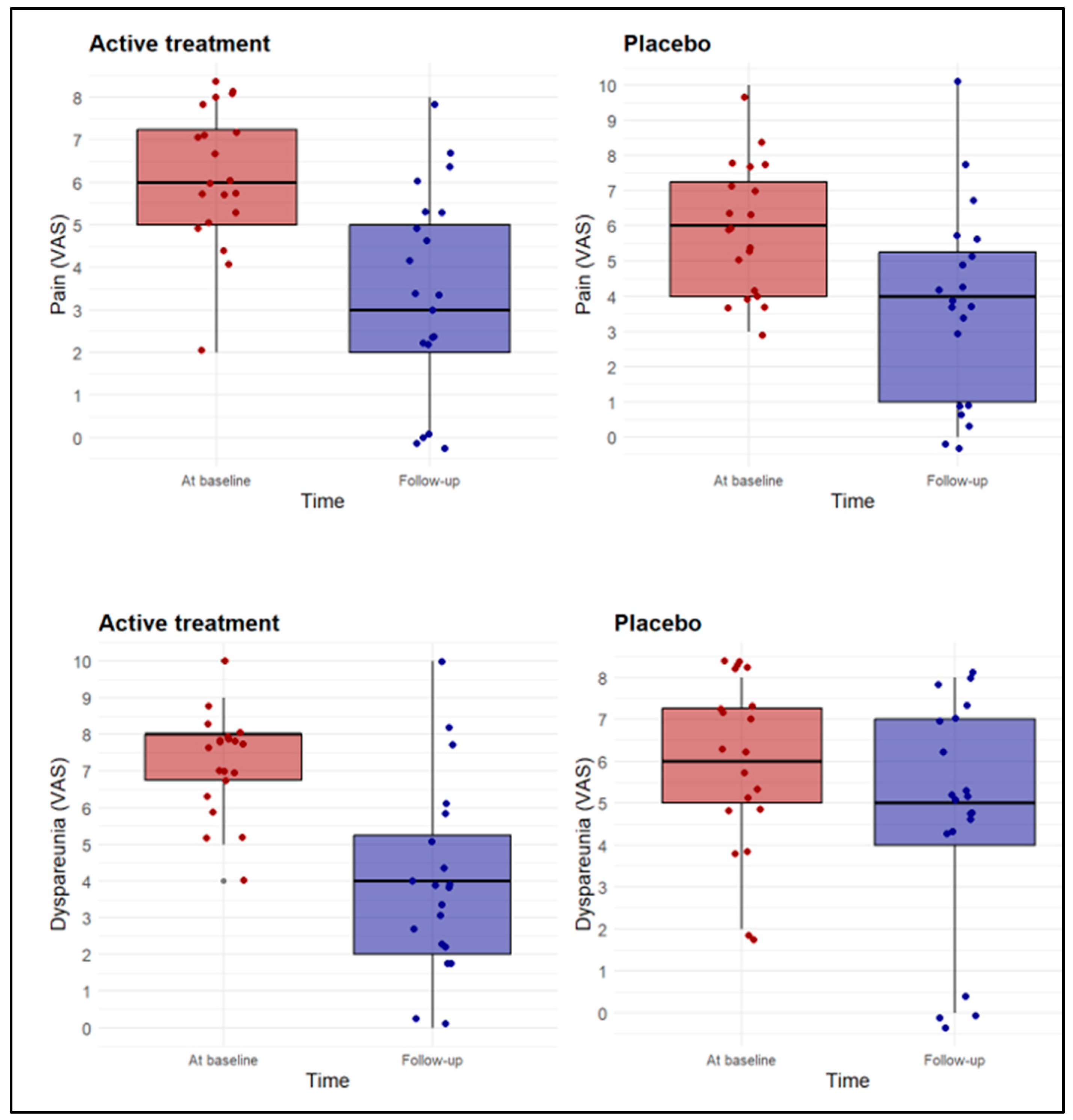
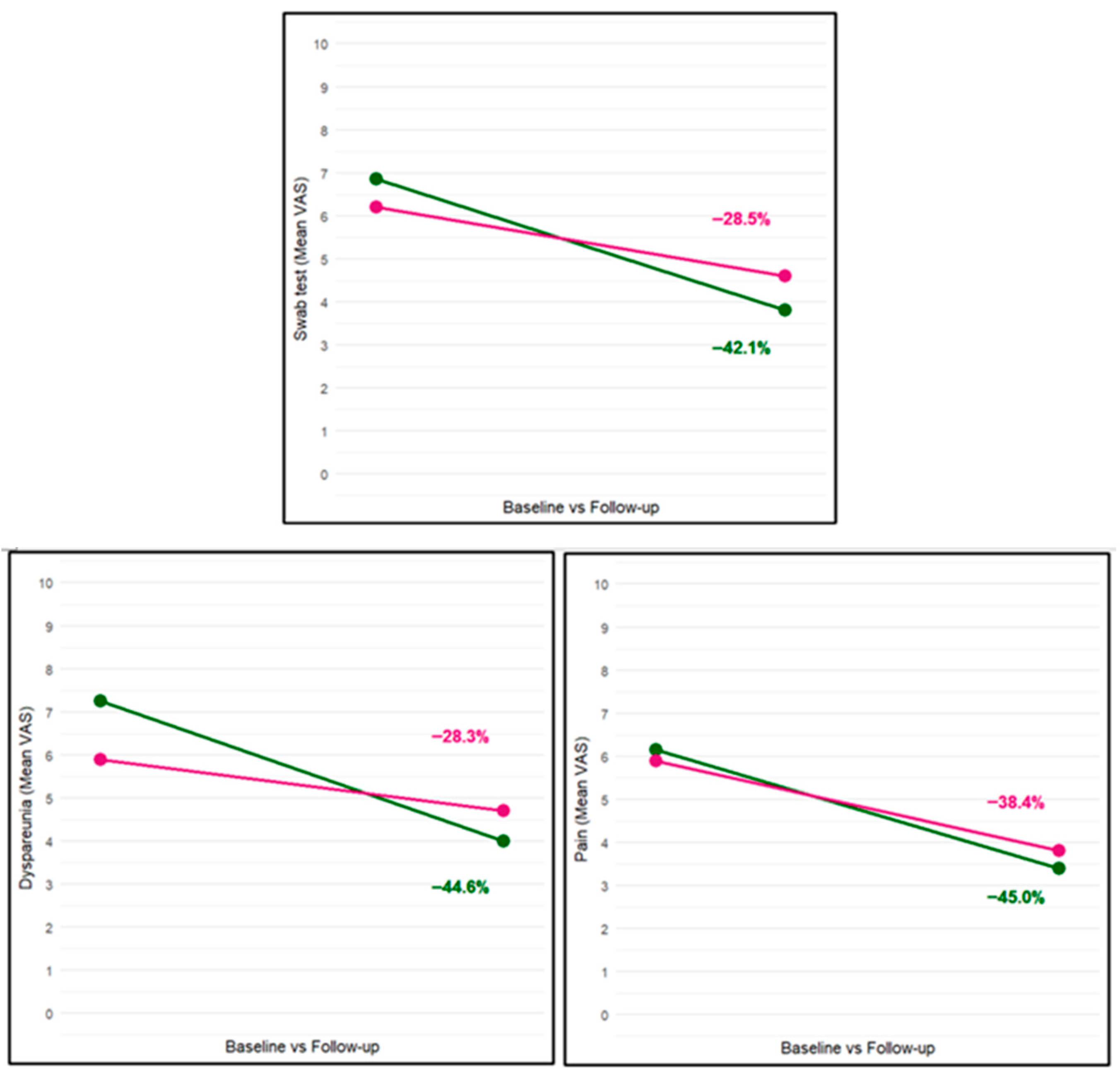
| Burning/pain (VAS) | 6.1 ± 1.6 | 3.4 ± 2.4 | <0.01 | 5.9 ± 1.9 | 3.8 ± 2.8 | <0.01 |
| Dyspareunia (VAS) | 7.3 ± 1.4 | 4.0 ± 2.6 | <0.01 | 5.9 ± 1.9 | 4.7 ± 2.7 | <0.01 |
| Cotton swab test (VAS) | 6.9 ± 1.6 | 3.8 ± 2.4 | <0.01 | 6.2 ± 1.7 | 4.6 ± 2.4 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murina, F.; Ettore, G.; Fochesato, C.; Castiglione, M.G.; Caruso, M.; Fonti, I.; Savasi, V. Efficacy and Safety of Topical 5% Cannabidiol Plus Myrcene for the Treatment of Vestibulodynia: A Multi-Centric Randomized Controlled Trial. Biomedicines 2025, 13, 2440. https://doi.org/10.3390/biomedicines13102440
Murina F, Ettore G, Fochesato C, Castiglione MG, Caruso M, Fonti I, Savasi V. Efficacy and Safety of Topical 5% Cannabidiol Plus Myrcene for the Treatment of Vestibulodynia: A Multi-Centric Randomized Controlled Trial. Biomedicines. 2025; 13(10):2440. https://doi.org/10.3390/biomedicines13102440
Chicago/Turabian StyleMurina, Filippo, Giuseppe Ettore, Cecilia Fochesato, Maria Grazia Castiglione, Melania Caruso, Ilenia Fonti, and Valeria Savasi. 2025. "Efficacy and Safety of Topical 5% Cannabidiol Plus Myrcene for the Treatment of Vestibulodynia: A Multi-Centric Randomized Controlled Trial" Biomedicines 13, no. 10: 2440. https://doi.org/10.3390/biomedicines13102440
APA StyleMurina, F., Ettore, G., Fochesato, C., Castiglione, M. G., Caruso, M., Fonti, I., & Savasi, V. (2025). Efficacy and Safety of Topical 5% Cannabidiol Plus Myrcene for the Treatment of Vestibulodynia: A Multi-Centric Randomized Controlled Trial. Biomedicines, 13(10), 2440. https://doi.org/10.3390/biomedicines13102440







