Successful Delivery of Small Non-Coding RNA Molecules into Human iPSC-Derived Lung Spheroids in 3D Culture Environment
Abstract
1. Introduction
2. Materials and Methods
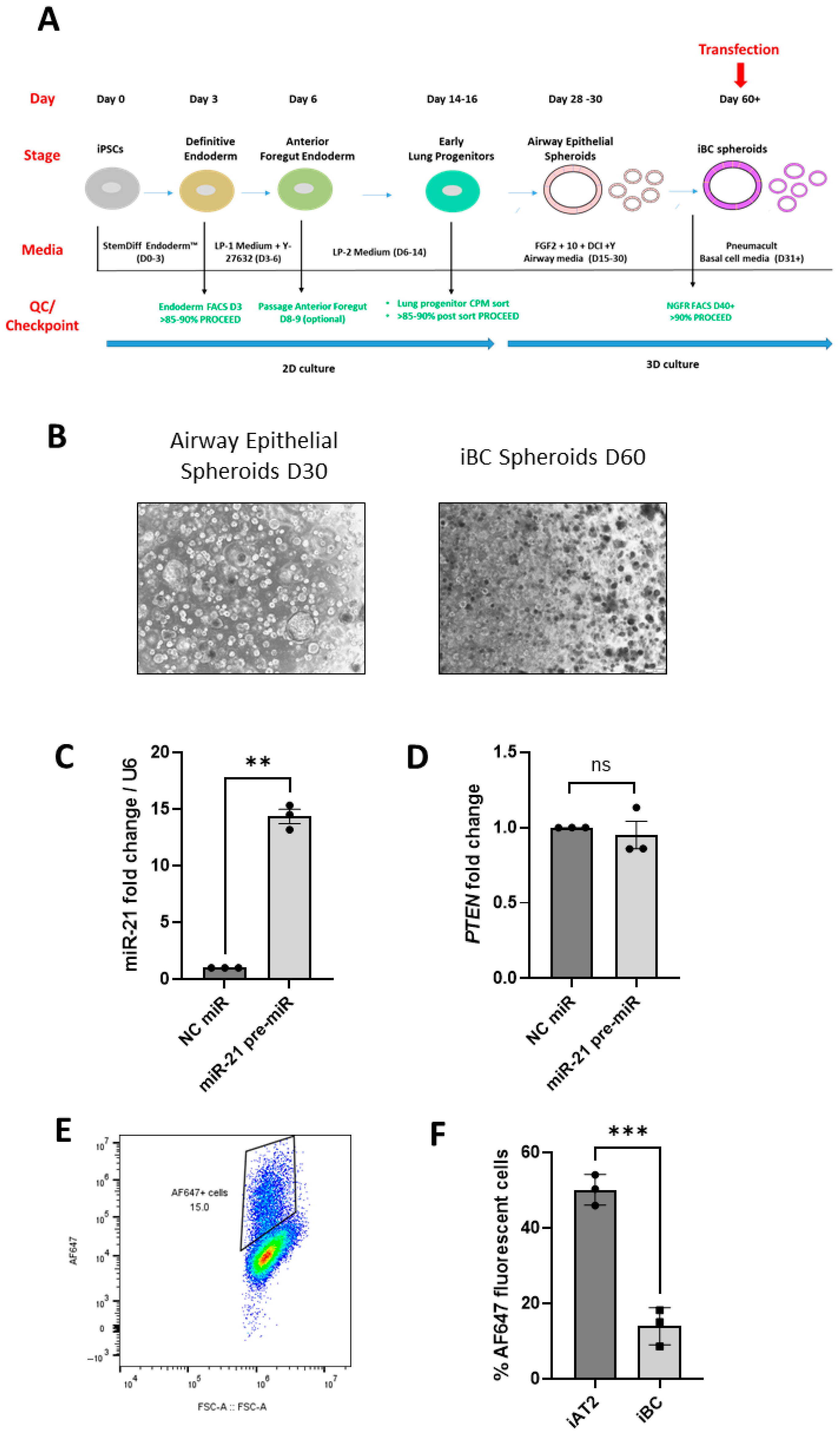
2.1. Spheroid Cell Culture
2.2. Distal Lung Spheroids (iAT2 Spheroids)
2.3. Proximal Lung Spheroids (iBC Spheroids)
2.4. Transfection of iPSC-Derived Lung Spheroids with Fluorescently Labelled siRNA
2.5. Flow Cytometry
2.6. Cytotoxicity/LDH Assay
2.7. Confocal Microscopy
2.8. siRNA and miRNA Studies
2.9. Gene Expression Analysis—Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Protein Analysis—Western Blotting
2.11. Statistical Analyses
3. Results
3.1. iPSC-Derived AT2 Spheroids Can Successfully Be Transfected as Whole Spheroids in 3D Matrigel Without Serum Supplementation
3.2. Successful ‘In Situ’ SF Transfection of iAT2 and iBC Spheroids with siRNAs and miRNA Mimics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef]
- McCauley, K.B.; Hawkins, F.; Serra, M.; Thomas, D.C.; Jacob, A.; Kotton, D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 2017, 20, 844–857.e6. [Google Scholar] [CrossRef]
- Hawkins, F.J.; Suzuki, S.; Beermann, M.L.; Barillà, C.; Wang, R.; Villacorta-Martin, C.; Berical, A.; Jean, J.C.; Le Suer, J.; Matte, T.; et al. Derivation of Airway Basal Stem Cells from Human Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 79–95.e8. [Google Scholar] [CrossRef] [PubMed]
- Hurley, K.; Ding, J.; Villacorta-Martin, C.; Herriges, M.J.; Jacob, A.; Vedaie, M.; Alysandratos, K.D.; Sun, Y.L.; Lin, C.; Werder, R.B.; et al. Reconstructed Single-Cell Fate Trajectories Define Lineage Plasticity Windows during Differentiation of Human PSC-Derived Distal Lung Progenitors. Cell Stem Cell 2020, 26, 593–608.e8. [Google Scholar] [CrossRef] [PubMed]
- Alysandratos, K.D.; Russo, S.J.; Petcherski, A.; Taddeo, E.P.; Acín-Pérez, R.; Villacorta-Martin, C.; Jean, J.C.; Mulugeta, S.; Rodriguez, L.R.; Blum, B.C.; et al. Patient-specific iPSCs carrying an SFTPC mutation reveal the intrinsic alveolar epithelial dysfunction at the inception of interstitial lung disease. Cell Rep. 2021, 36, 109636. [Google Scholar] [CrossRef] [PubMed]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef]
- Gaebler, A.M.; Hennig, A.; Buczolich, K.; Weitz, J.; Welsch, T.; Stange, D.E.; Pape, K. Universal and Efficient Electroporation Protocol for Genetic Engineering of Gastrointestinal Organoids. J. Vis. Exp. 2020, 156, 60704. [Google Scholar] [CrossRef]
- Pelofy, S.; Bousquet, H.; Gibot, L.; Rols, M.P.; Golzio, M. Transfer of small interfering RNA by electropermeabilization in tumor spheroids. Bioelectrochemistry 2021, 141, 107848. [Google Scholar] [CrossRef]
- Schwank, G.; Andersson-Rolf, A.; Koo, B.K.; Sasaki, N.; Clevers, H. Generation of BAC transgenic epithelial organoids. PLoS ONE 2013, 8, e76871. [Google Scholar] [CrossRef]
- Morgan, R.G.; Chambers, A.C.; Legge, D.N.; Coles, S.J.; Greenhough, A.; Williams, A.C. Optimized delivery of siRNA into 3D tumor spheroid cultures in situ. Sci. Rep. 2018, 8, 7952. [Google Scholar] [CrossRef]
- Huang, S.-T.; Chen, Y.-F.; Chen, Y.-C.; Lin, J.-T.; Yao, C.-L.; Jan, J.-S. Star-shaped copolypeptide-mediated transfection of 3D organoids. Eur. Polym. J. 2025, 232, 113932. [Google Scholar] [CrossRef]
- Riching, A.S.; Malloy, A.; Anderson, E.M.; Sheard, J.; Mikkonen, P.; van Brabant Smith, A.; Strezoska, Z.; Levenga, J. A Facile, Transfection-Free Approach to siRNA Delivery in In Vitro 3D Spheroid Models. Curr. Protoc. 2024, 4, e1121. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Morley, M.; Hawkins, F.; McCauley, K.B.; Jean, J.C.; Heins, H.; Na, C.L.; Weaver, T.E.; Vedaie, M.; Hurley, K.; et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 2017, 21, 472–488.e10. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Vedaie, M.; Roberts, D.A.; Thomas, D.C.; Villacorta-Martin, C.; Alysandratos, K.D.; Hawkins, F.; Kotton, D.N. Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3303–3332. [Google Scholar] [CrossRef]
- Suzuki, S.; Hawkins, F.J.; Barillà, C.; Beermann, M.L.; Kotton, D.N.; Davis, B.R. Differentiation of human pluripotent stem cells into functional airway basal stem cells. STAR Protoc. 2021, 2, 100683. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Xie, T.; Liang, J.; Geng, Y.; Liu, N.; Kurkciyan, A.; Kulur, V.; Leng, D.; Deng, N.; Liu, Z.; Song, J.; et al. MicroRNA-29c Prevents Pulmonary Fibrosis by Regulating Epithelial Cell Renewal and Apoptosis. Am. J. Respir. Cell Mol. Biol. 2017, 57, 721–732. [Google Scholar] [CrossRef]
- Oglesby, I.; Santi, C.D.; Schweikert, A.; Cryan, S.-A.; Hurley, K. Novel strategies for genetic manipulation of human iPSC-derived organoid platforms. In Proceedings of the European Respiratory Society Congress, Barcelona, Spain, 4–6 September 2022; p. 2368. [Google Scholar]
- Gotoh, S.; Ito, I.; Nagasaki, T.; Yamamoto, Y.; Konishi, S.; Korogi, Y.; Matsumoto, H.; Muro, S.; Hirai, T.; Funato, M.; et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 2014, 3, 394–403. [Google Scholar] [CrossRef]
- Sun, Y.L.; Hurley, K.; Villacorta-Martin, C.; Huang, J.; Hinds, A.; Gopalan, K.; Caballero, I.S.; Russo, S.J.; Kitzmiller, J.A.; Whitsett, J.A.; et al. Heterogeneity in Human Induced Pluripotent Stem Cell-derived Alveolar Epithelial Type II Cells Revealed with ABCA3/SFTPC Reporters. Am. J. Respir. Cell Mol. Biol. 2021, 65, 442–460. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sitaraman, S.; Alysandratos, K.D.; Wambach, J.A.; Limberis, M.P. Gene Therapeutics for Surfactant Dysfunction Disorders: Targeting the Alveolar Type 2 Epithelial Cell. Hum. Gene Ther. 2022, 33, 1011–1022. [Google Scholar] [CrossRef]
- Arlene Glasgow, S.V.; De Santi, C.; Raoof, R.; Henshall, D.; Linnane, B.; McNally, P.; Greene, C. An investigation of miR-21 and the TLR4/PDCD4 axis in CF bronchial epithelium. In Proceedings of the European Respiratory Society Congress, Online, 7–9 September 2020; p. 915. [Google Scholar]
- Oglesby, A.G.I.; Greene, C.; Hurley, K. Investigating miRNA regulation of PTEN in Cystic Fibrosis using airway epithelial cells derived from patient specific iPSC. In Proceedings of the European Respiratory Society Congress, Milan, Italy, 9–13 September 2023; p. PA1865. [Google Scholar]
- Meng, F.; Henson, R.; Wehbe-Janek, H.; Ghoshal, K.; Jacob, S.T.; Patel, T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007, 133, 647–658. [Google Scholar] [CrossRef]
- Hawkins, F.; Kramer, P.; Jacob, A.; Driver, I.; Thomas, D.C.; McCauley, K.B.; Skvir, N.; Crane, A.M.; Kurmann, A.A.; Hollenberg, A.N.; et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. J. Clin. Investig. 2017, 127, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Schwank, G.; Koo, B.K.; Sasselli, V.; Dekkers, J.F.; Heo, I.; Demircan, T.; Sasaki, N.; Boymans, S.; Cuppen, E.; van der Ent, C.K.; et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013, 13, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Birmingham, A.; Wiemann, S.; Beijersbergen, R.L.; Hornung, V.; Smith, A. Advances in CRISPR-Cas9 genome engineering: Lessons learned from RNA interference. Nucleic Acids Res. 2015, 43, 3407–3419. [Google Scholar] [CrossRef] [PubMed]
- Artegiani, B.; Hendriks, D.; Beumer, J.; Kok, R.; Zheng, X.; Joore, I.; Chuva de Sousa Lopes, S.; van Zon, J.; Tans, S.; Clevers, H. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat. Cell Biol. 2020, 22, 321–331. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Zhao, G.; Zeng, Y.; Cheng, W.; Karkampouna, S.; Papadopoulou, P.; Hu, B.; Zang, S.; Wezenberg, E.; Forn-Cuní, G.; Lopes-Bastos, B.; et al. Peptide-Modified Lipid Nanoparticles Boost the Antitumor Efficacy of RNA Therapeutics. ACS Nano 2025, 19, 13685–13704. [Google Scholar] [CrossRef]
- Sakib, S.; Zou, S. Attenuation of Chronic Inflammation in Intestinal Organoids with Graphene Oxide-Mediated Tumor Necrosis Factor-α_Small Interfering RNA Delivery. Langmuir 2024, 40, 3402–3413. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweikert, A.; De Santi, C.; Teoh, X.J.; Xin Yang, F.L.; O’Sullivan, E.; Greene, C.M.; Hurley, K.; Oglesby, I.K. Successful Delivery of Small Non-Coding RNA Molecules into Human iPSC-Derived Lung Spheroids in 3D Culture Environment. Biomedicines 2025, 13, 2419. https://doi.org/10.3390/biomedicines13102419
Schweikert A, De Santi C, Teoh XJ, Xin Yang FL, O’Sullivan E, Greene CM, Hurley K, Oglesby IK. Successful Delivery of Small Non-Coding RNA Molecules into Human iPSC-Derived Lung Spheroids in 3D Culture Environment. Biomedicines. 2025; 13(10):2419. https://doi.org/10.3390/biomedicines13102419
Chicago/Turabian StyleSchweikert, Anja, Chiara De Santi, Xi Jing Teoh, Frederick Lee Xin Yang, Enya O’Sullivan, Catherine M. Greene, Killian Hurley, and Irene K. Oglesby. 2025. "Successful Delivery of Small Non-Coding RNA Molecules into Human iPSC-Derived Lung Spheroids in 3D Culture Environment" Biomedicines 13, no. 10: 2419. https://doi.org/10.3390/biomedicines13102419
APA StyleSchweikert, A., De Santi, C., Teoh, X. J., Xin Yang, F. L., O’Sullivan, E., Greene, C. M., Hurley, K., & Oglesby, I. K. (2025). Successful Delivery of Small Non-Coding RNA Molecules into Human iPSC-Derived Lung Spheroids in 3D Culture Environment. Biomedicines, 13(10), 2419. https://doi.org/10.3390/biomedicines13102419






