Harnessing Plant Bioactive Compounds in Biomaterial Scaffolds for Advanced Wound Healing: A Comprehensive Review
Abstract
1. Introduction
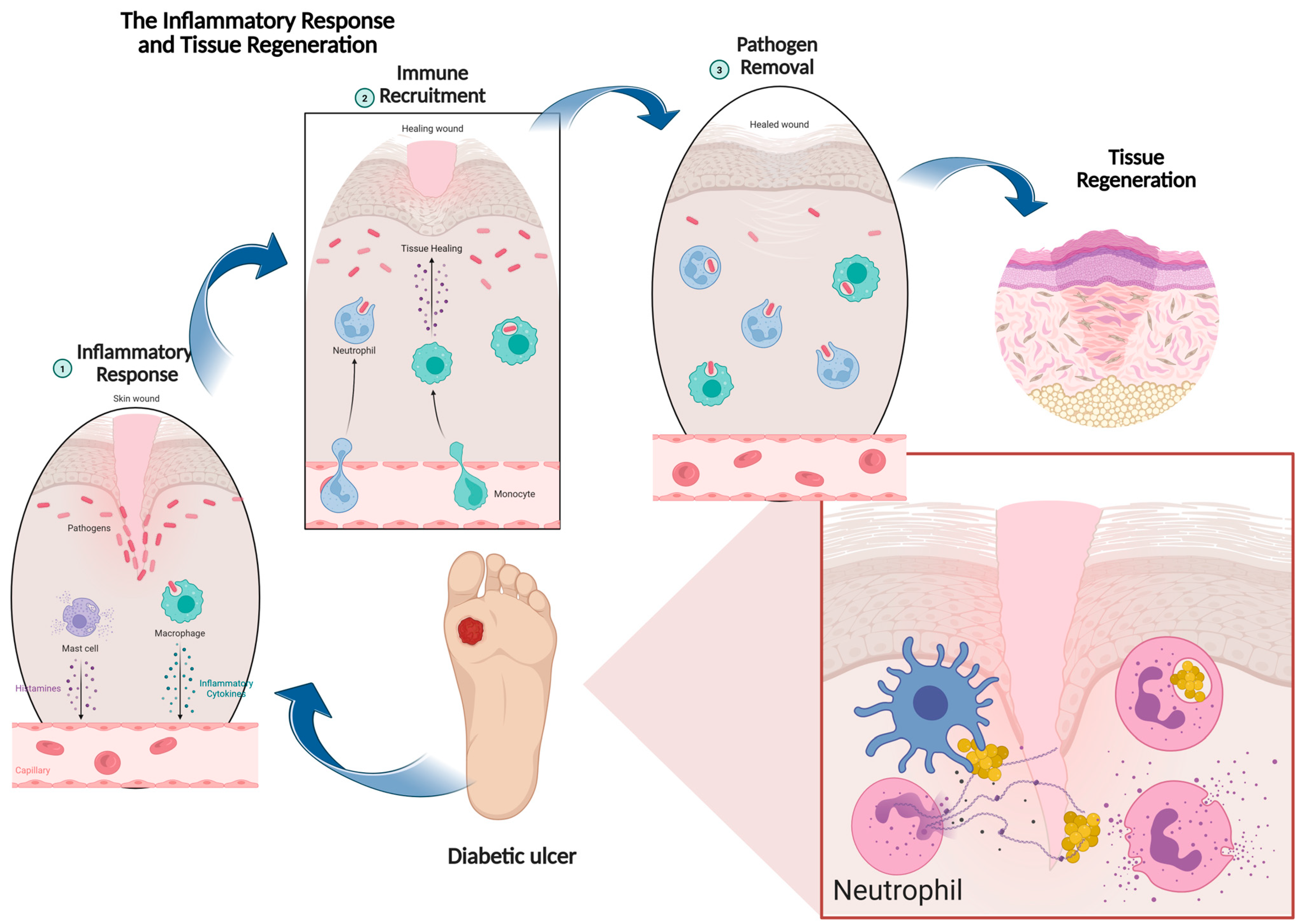
2. Physiology of Wound Healing
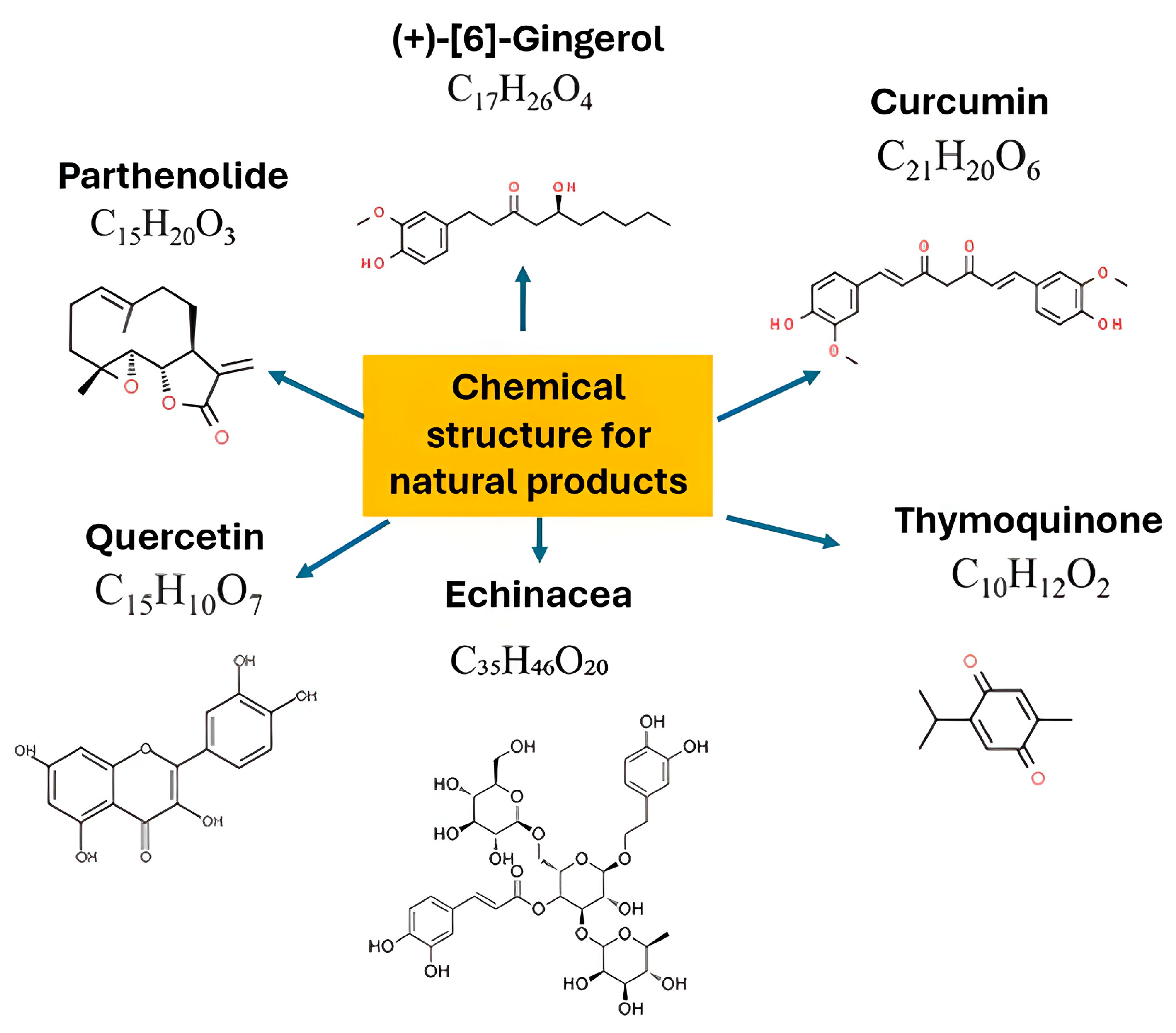
3. Pro-Healing Plant Bioactive Compounds
3.1. Polyphenol
3.2. Terpenoids
3.3. Alkaloid
4. Properties of Plant Bioactive Compounds for Wound Healing and Their Mechanism
4.1. Antimicrobial Properties
4.2. Antioxidant Activity
4.3. Anti-Inflammatory Effects
4.4. Pro-Angiogenesis
4.5. Collagen Synthesis
5. Role of Biomaterials as Delivery Systems in Wound Healing
6. Strategies for Incorporating Plant Bioactive Compounds into Various Scaffolds
6.1. Electrospinning
| Fabrication Method | Description | Incorporated Plant Bioactives | Healing Benefits | References |
|---|---|---|---|---|
| Electrospinning | Uses electrostatic forces to produce nanofibrous scaffolds with ECM-like pores. | Grape seed extract, P. oleracea, A. vera, flavonoids, tannins, terpenoids. | High surface area for cell attachment and proliferation; sustained release of bioactives; antioxidant, antimicrobial, anti-inflammatory activity; tunable mechanical properties; portable in situ electrospinning enables direct wound dressing. | [91,92,93,94,95,96,97] |
| 3D Bioprinting | Precise fabrication of customized scaffolds mimicking tissue architecture. | Flavonoids, polyphenols, essential oils (in alginate/gelatin bioinks). | Allows spatial control of cells and bioactives; customizable scaffold shape; enhanced antimicrobial, anti-inflammatory, and angiogenic activity; gradient delivery of compounds improves diabetic wound healing. | [98,99,100] |
| Hydrogel Formulation | Hydrophilic polymer networks (90% water) with high elasticity and moisture retention. | Cocoa extract, plantain peel, A. vera, Calendula officinalis, curcumin, Kunzea ericoides. | Maintains moist environment; absorbs exudates; supports keratinocyte migration and fibroblast proliferation; controlled degradation; sustained release of bioactives; enhanced antibacterial, antioxidant, immunoregulatory effects. | [101,102,103,104,105,106,107,108,109] |
| Lyophilization | Freeze-drying at low temperature to preserve phytochemicals and create porous scaffolds. | Croton oblongifolius, Spinacia oleracea, Cissus quadrangularis | Produces highly porous scaffolds (>90% porosity); excellent fluid absorption; stable phytochemicals; sustained release; improved biocompatibility, antioxidant, antibacterial, and bone tissue repair properties. | [110,111,112,113] |
6.2. 3D-Bioprinting
6.3. Hydrogel Formulation
6.4. Lyophilization
7. Conclusion, Challenges, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecinska-Pirog, J.; Walecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-based strategies to advance therapies for chronic skin wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. A brief history of wound care. Plast. Reconstr. Surg. 2006, 117 (Suppl. 7), 6S–11S. [Google Scholar] [CrossRef] [PubMed]
- Gonfa, Y.H.; Beshah, F.; Tadesse, M.G.; Bachheti, A.; Bachheti, R.K. Phytochemical investigation and potential pharmacologically active compounds of Rumex nepalensis: An appraisal. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 18. [Google Scholar] [CrossRef]
- Agarwal, T.; Tan, S.-A.; Onesto, V.; Law, J.X.; Agrawal, G.; Pal, S.; Lim, W.L.; Sharifi, E.; Moghaddam, F.D.; Maiti, T.K. Engineered herbal scaffolds for tissue repair and regeneration: Recent trends and technologies. Biomed. Eng. Adv. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 680354. [Google Scholar] [CrossRef]
- Hashemi, S.-S.; Rezaeian, R.; Rafati, A.; Sanati, P.; Mehrabani, D.; Eshaghi Malekshah, R.; Moghaddam, A.; Khonakdar, H.A. A review on application of herbals and their polymer composites in wound healing. Arab. J. Chem. 2024, 17, 105820. [Google Scholar] [CrossRef]
- Tsourdi, E.; Barthel, A.; Rietzsch, H.; Reichel, A.; Bornstein, S.R. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed. Res. Int. 2013, 2013, 385641. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis-from Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair. Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998, 30, 1019–1030. [Google Scholar] [CrossRef]
- Profyris, C.; Tziotzios, C.; Do Vale, I. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics: Part I. The molecular basis of scar formation. J. Am. Acad. Dermatol. 2012, 66, 1–10. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2016, 20, 1689–1699. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 3–44. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.; Forster-Carneiro, T.; Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Carvalho, M.T.B.; Araujo-Filho, H.G.; Barreto, A.S.; Quintans-Junior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar]
- Marinelli, L.; Cacciatore, I.; Eusepi, P.; Dimmito, M.P.; Di Rienzo, A.; Reale, M.; Costantini, E.; Borrego-Sanchez, A.; Garcia-Villen, F.; Viseras, C.; et al. In Vitro Wound-Healing Properties of Water-Soluble Terpenoids Loaded on Halloysite Clay. Pharmaceutics 2021, 13, 1117. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef]
- Geng, X.; Wang, Y.; Li, H.; Song, L.; Luo, C.; Gu, X.; Zhong, H.; Chen, H.; Chen, X.; Wang, J.; et al. Total iridoid glycoside extract of Lamiophlomis rotata (Benth) Kudo accelerates diabetic wound healing by the NRF2/COX2 axis. Chin. Med. 2024, 19, 53. [Google Scholar] [CrossRef]
- Roy, A. A review on the alkaloids an important therapeutic compound from plants. Int. J. Plant Biotechnol. 2017, 3, 1–9. [Google Scholar]
- Kamarul Zaman, M.A.; Mohamad Azzeme, A. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2018, 6, 021–029. [Google Scholar] [CrossRef]
- Azzazy, H.M.E.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Sandar, W.-P.; Saw, S.; Kumar, A.M.; Camara, B.S.; Sein, M.-M. Wounds, antimicrobial resistance and challenges of implementing a surveillance system in Myanmar: A mixed-methods study. Trop. Med. Infect. Dis. 2021, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Geoff, S. Antimicrobial resistance relating to wound management and infection. Wound Pract. Res. 2016, 24, 224–227. [Google Scholar]
- Piskláková, L.; Skuhrovcová, K.; Bártová, T.; Seidelmannová, J.; Vondrovic, Š.; Velebný, V. Trends in the Incorporation of Antiseptics into Natural Polymer-Based Nanofibrous Mats. Polymers 2024, 16, 664. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun fiber pads of cellulose acetate and essential oils with antimicrobial activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef]
- Rashid, N.; Khalid, S.H.; Ullah Khan, I.; Chauhdary, Z.; Mahmood, H.; Saleem, A.; Umair, M.; Asghar, S. Curcumin-loaded bioactive polymer composite film of pva/gelatin/tannic acid downregulates the pro-inflammatory cytokines to expedite healing of full-thickness wounds. ACS Omega 2023, 8, 7575–7586. [Google Scholar] [CrossRef]
- Yaseen, H.S.; Asif, M.; Saadullah, M.; Mahrukh; Asghar, S.; Shams, M.U.; Bazmi, R.R.; Saleem, M.; Yousaf, H.M.; Yaseen, M. Methanolic extract of Ephedra ciliata promotes wound healing and arrests inflammatory cascade in vivo through downregulation of TNF-α. Inflammopharmacology 2020, 28, 1691–1704. [Google Scholar] [CrossRef]
- Kováč, J.; Slobodníková, L.; Trajčíková, E.; Rendeková, K.; Mučaji, P.; Sychrová, A.; Bittner Fialová, S. Therapeutic potential of flavonoids and tannins in management of oral infectious diseases—A review. Molecules 2022, 28, 158. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Sumayya, S.S.; Lubaina, A.S.; Murugan, K. Bactericidal Potentiality of Purified Terpenoid Extracts from the Selected Sea Weeds and its Mode of Action. J. Trop. Life Sci. 2020, 10, 197–205. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Vadhana, P.; Singh, B.R.; Bharadwaj, M.; Singh, S.V. Emergence of herbal antimicrobial drug resistance in clinical bacterial isolates. Pharm. Anal. Acta 2015, 6, 434. [Google Scholar] [CrossRef]
- Atta, S.; Waseem, D.; Fatima, H.; Naz, I.; Rasheed, F.; Kanwal, N. Antibacterial potential and synergistic interaction between natural polyphenolic extracts and synthetic antibiotic on clinical isolates. Saudi J. Biol. Sci. 2023, 30, 103576. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Ambika; Singh, P.P.; Chauhan, S. Activity-guided isolation of antioxidants from the leaves of Terminalia arjuna. Nat. Prod. Res. 2014, 28, 760–763. [Google Scholar] [CrossRef]
- Fernandez, O.; Capdevila, J.Z.; Dalla, G.; Melchor, G. Efficacy of Rhizophora mangle aqueous bark extract in the healing of open surgical wounds. Fitoterapia 2000, 73, 564–568. [Google Scholar] [CrossRef]
- Tie, L.; An, Y.; Han, J.; Xiao, Y.; Xiaokaiti, Y.; Fan, S.; Liu, S.; Chen, A.F.; Li, X. Genistein accelerates refractory wound healing by suppressing superoxide and FoxO1/iNOS pathway in type 1 diabetes. J. Nutr. Biochem. 2013, 24, 88–96. [Google Scholar] [CrossRef]
- Savoia, P.; Raina, G.; Camillo, L.; Farruggio, S.; Mary, D.; Veronese, F.; Graziola, F.; Zavattaro, E.; Tiberio, R.; Grossini, E. Anti-oxidative effects of 17 β-estradiol and genistein in human skin fibroblasts and keratinocytes. J. Dermatol. Sci. 2018, 92, 62–77. [Google Scholar] [CrossRef]
- Duchnik, E.; Kruk, J.; Baranowska-Bosiacka, I.; Pilutin, A.; Maleszka, R.; Marchlewicz, M. Effects of the soy isoflavones, genistein and daidzein, on male rats’ skin. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2019, 36, 760–766. [Google Scholar] [CrossRef]
- Maier, C.M.; Chan, P.H. Book review: Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, R.; Karam, C.; Fostok, S.; El-Jouni, W.; Barbour, E.K. Anti-Inflammatory Bioactivities in Plant Extracts. J. Med. Food 2007, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Scalise, A.; Bianchi, A.; Tartaglione, C.; Bolletta, E.; Pierangeli, M.; Torresetti, M.; Marazzi, M.; Di Benedetto, G. Microenvironment and Microbiology of Skin Wounds: The Role of Bacterial Biofilms and Related Factors; Seminars in Vascular Surgery; Elsevier: Amsterdam, The Netherlands, 2015; pp. 151–159. [Google Scholar]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Complement. Altern. Med. 2012, 12, 226. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Monteforte, A.J.; Lam, B.; Das, S.; Mukhopadhyay, S.; Wright, C.S.; Martin, P.E.; Dunn, A.K.; Baker, A.B. Glypican-1 nanoliposomes for potentiating growth factor activity in therapeutic angiogenesis. Biomaterials 2016, 94, 45–56. [Google Scholar] [CrossRef]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential Proteomic Analysis Distinguishes Tissue Repair Biomarker Signatures in Wound Exudates Obtained from Normal Healing and Chronic Wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Samukawa, K.; Satake, N.; Sakanaka, M. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur. J. Pharmacol. 2008, 584, 415–423. [Google Scholar] [CrossRef]
- Phaechamud, T.; Yodkhum, K.; Charoenteeraboon, J.; Tabata, Y. Chitosan-aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 50, 210–225. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Hu, W. Flavonoids accelerate wound healing of pressure sore by promoting angiogenesis: Potential mechanism. Pak. J. Pharm. Sci. 2022, 35, 1647–1654. [Google Scholar] [PubMed]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 2018, 17, 399. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Chiu, J.-H.; Wu, I.H.; Wang, B.-W.; Pan, C.-M.; Chen, Y.-H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1α. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef]
- Cai, H.-A.; Huang, L.; Zheng, L.-J.; Fu, K.; Wang, J.; Hu, F.-D.; Liao, R.-Y. Ginsenoside (Rg-1) promoted the wound closure of diabetic foot ulcer through iNOS elevation via miR-23a/IRF-1 axis. Life Sci. 2019, 233, 116525. [Google Scholar] [CrossRef]
- Chithra, P.; Sajithlal, G.; Chandrakasan, G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol. Cell. Biochem. 1998, 181, 71–76. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Lin, D.-W.; Jiang, Y.-W.; Wu, C.; Zhang, H.; Li, Y.-Z.; Wang, Y.-S. Quercetin Alleviates Cardiac Fibrosis via Regulating the SIRT3 Signaling Pathway. Cardiovasc. Drugs Ther. 2025, 39, 737–748. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The role of biomaterials-based scaffolds in advancing skin tissue construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Cole, A.; Strachan, F. Biocomposite Scaffolds for Tissue Engineering: Materials, Fabrication Techniques and Future Directions. Materials 2024, 17, 5577. [Google Scholar] [CrossRef] [PubMed]
- Monia, T. Sustainable natural biopolymers for biomedical applications. J. Thermoplast. Compos. Mater. 2024, 37, 2505–2524. [Google Scholar] [CrossRef]
- Jabbari, E. Challenges for Natural Hydrogels in Tissue Engineering. Gels 2019, 5, 30. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Biazar, E. Decellularized Scaffolds for Tissue Regeneration: Techniques and Applications. In Advances in Regenerative Medicine and Tissue Engineering; IntechOpen: London, UK, 2024. [Google Scholar]
- Brouki Milan, P.; Masoumi, F.; Biazar, E.; Zare Jalise, S.; Mehrabi, A. Exploiting the Potential of Decellularized Extracellular Matrix (ECM) in Tissue Engineering: A Review Study. Macromol. Biosci. 2025, 25, e2400322. [Google Scholar] [CrossRef]
- Rheima, A.M.; Abdul-Rasool, A.A.; Al-Sharify, Z.T.; Zaidan, H.K.; Athair, D.M.; Mohammed, S.H.; Kianfar, E. Nano bioceramics: Properties, applications, hydroxyapatite, nanohydroxyapatite and drug delivery. Case Stud. Chem. Environ. Eng. 2024, 10, 100869. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-Based Scaffolds for Soft-Tissue Engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Kumar, A.; Jacob, A. Techniques in scaffold fabrication process for tissue engineering applications: A review. J. Appl. Biol. Biotechnol. 2022, 10, 163–176. [Google Scholar] [CrossRef]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D bioprinting and its innovative approach for biomedical applications. MedComm (2020) 2023, 4, e194. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Health Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B: Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef]
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Taneva, S.; Momchilova, S.; Georgieva, A.I. Physico-Chemical, Mechanical, and Biological Properties of Polylactide/Portulaca Oleracea Extract Electrospun Fibers. Membranes 2023, 13, 298. [Google Scholar] [CrossRef]
- Zahedi, E.; Esmaeili, A.; Eslahi, N.; Shokrgozar, M.A.; Simchi, A. Fabrication and Characterization of Core-Shell Electrospun Fibrous Mats Containing Medicinal Herbs for Wound Healing and Skin Tissue Engineering. Mar. Drugs 2019, 17, 27. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wright, M.E.E.; Cheng, N.; Oh, H.H.; Wang, Y.; Datu, A.; Santerre, J.P.; Amini-Nik, S.; Jeschke, M.G. Electrospun Polyurethane–Gelatin Composite: A New Tissue-Engineered Scaffold for Application in Skin Regeneration and Repair of Complex Wounds. Acs Biomater. Sci. Eng. 2019, 6, 505–516. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun Nanofibers as Dressings for Chronic Wound Care: Advances, Challenges, and Future Prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Yue, Y.; Gong, X.; Jiao, W.; Li, Y.; Yin, X.; Si, Y.; Yu, J.; Ding, B. In-situ electrospinning of thymol-loaded polyurethane fibrous membranes for waterproof, breathable, and antibacterial wound dressing application. J. Colloid. Interface Sci. 2021, 592, 310–318. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Maduna, L.; Patnaik, A. Challenges Associated with the Production of Nanofibers. Processes 2024, 12, 2100. [Google Scholar] [CrossRef]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.Y.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Teng, Y.; Shi, J.; Yang, X.; Ding, Z.; Guo, X.; Hou, S.; Lv, Q. 3D Bioprinting: Opportunities for Wound Dressing Development. Biomed. Mater. 2023, 18, 052001. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tang, L.; Hu, Y.; Jiang, S.; Luo, Y.; Liu, C.; Guo, W.; Zhang, J.; Xu, T.; Zhu, L. 3D Bioprinting of Integral ADSCs-NO Hydrogel Scaffolds to Promote Severe Burn Wound Healing. Regen. Biomater. 2021, 8, rbab014. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Chekir, L.; Ghoul, M. Effect of Heat Treatment and Light Exposure on the Antioxidant Activity of Flavonoids. Processes 2020, 8, 1078. [Google Scholar] [CrossRef]
- Nasser, S. Phytochemicals properties of herbal extracts for ultraviolet protection and skin health: A narrative review. J. Radiat. Res. Appl. Sci. 2023, 16, 100729. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Liu, J.-C.; Zhang, Z.-Y.; Xu, C.-X. A review on cell damage, viability, and functionality during 3D bioprinting. Mil. Med. Res. 2022, 9, 70. [Google Scholar] [CrossRef]
- Mathur, V.; Agarwal, P.; Kasturi, M.; Srinivasan, V.; Seetharam, R.N.; Vasanthan, K.S. Innovative bioinks for 3D bioprinting: Exploring technological potential and regulatory challenges. J. Tissue Eng. 2025, 16, 20417314241308022. [Google Scholar] [CrossRef]
- Bei, Z.; Zheng, J. Recent advances in the application of functional hydrogels in skin wound healing. MedComm—Biomater. Appl. 2024, 3, e101. [Google Scholar] [CrossRef]
- Bullock, A.; Pickavance, P.; Haddow, D.; Rimmer, S.; MacNeil, S. Development of a calcium-chelating hydrogel for treatment of superficial burns and scalds. Regen. Med. 2010, 5, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Jin, S.; Newton, M.A.A.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The Formation Mechanism of Hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, V.; Agarwal, M.; Pant, A.; Kaur, H.; Singh, M. Controllable transdermal drug delivery of Theobroma cacao extract based polymeric hydrogel against dermal microbial and oxidative damage. Food Nutr. Sci. 2019, 10, 1212–1235. [Google Scholar] [CrossRef]
- Jessy Mercy, D.; Thirumalai, A.; Udayakumar, S.; Deepika, B.; Janani, G.; Girigoswami, A.; Girigoswami, K. Enhancing Wound Healing with Nanohydrogel-Entrapped Plant Extracts and Nanosilver: An In Vitro Investigation. Molecules 2024, 29, 5004. [Google Scholar] [CrossRef]
- He, X.; Gao, S.; Li, H.; Liu, H.; Zhao, S.; Wang, H.; Qin, S.; Li, J.; Zhou, F.; Xie, J. Hybrid hydrogel loaded with natural plant extract accelerates diabetic wound healing via ROS scavenging and immunoregulation. Chem. Eng. J. 2024, 499, 156388. [Google Scholar] [CrossRef]
- Kawasaki, H.; Shimanouchi, T.; Kimura, Y. Recent development of optimization of lyophilization process. J. Chem. 2019, 2019, 9502856. [Google Scholar] [CrossRef]
- Sutar, T.; Bangde, P.; Dandekar, P.; Adivarekar, R. Herbal hemostatic biopolymeric dressings of alginate/pectin coated with Croton oblongifolius extract. Carbohydr. Polym. Technol. Appl. 2021, 2, 100025. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Kirthika, S.; Keerthana, S.; Narasimhan, M.K.; Jeyanthi, J. Fabrication and Characterization of Spinacia Oleracea Extract Incorporated Alginate/Carboxymethyl Cellulose Microporous Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 156, 430–437. [Google Scholar] [CrossRef]
- Thongtham, N.; Chai-in, P.; Unger, O.; Boonrungsiman, S.; Suwantong, O. Fabrication of chitosan/collagen/hydroxyapatite scaffolds with encapsulated Cissus quadrangularis extract. Polym. Adv. Technol. 2020, 31, 1496–1507. [Google Scholar] [CrossRef]
- Tchessalov, S.; Shalaev, E.; Bhatnagar, B.; Nail, S.; Alexeenko, A.; Jameel, F.; Srinivasan, J.; Dekner, M.; Sahni, E.; Schneid, S.; et al. Best Practices and Guidelines (2022) for Scale-Up and Tech Transfer in Freeze-Drying Based on Case Studies. Part 1: Challenges during Scale Up and Transfer. AAPS PharmSciTech 2022, 24, 11. [Google Scholar] [CrossRef]



| Type of Scaffold | Example | Application for Wound Healing | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Synthetic Polymer | Polylactic acid (PLA), Polycaprolactone (PCL) | Wound dressings, controlled phytochemical release | Customizability in mechanical strength, degradation rate and surface characteristics | Potential toxicity, non-degradable byproduct | [77] |
| Natural Polymer | Chitosan, silk, alginates | Wound healing, antimicrobial and antioxidant bioactive delivery | Biocompatible, biodegradable, sustainable and versatile | Lower mechanical strength, limited scalability, variable degradation | [78,79] |
| Hydrogel | Agarose, alginate, collagen | Moist wound healing, controlled release of plant bioactives | Biocompatible, mimic the ECM, supports vascularization | Poor mechanical properties, difficult to sterilize | [80] |
| Decellularized matrices | Tissue extracellular matrix | Advanced wound dressing | Biocompatible, biomimetic, versatile, supports vascularization | Risk of immune rejection, complex production process, limited scalability | [81,82] |
| Ceramic | Hydroxyapatite, tricalcium phosphate, nanobioceramics | Chronic wound composite, for bone-related defects | Compressive strength, osteoconductivity, biocompatibility, enhanced tissue interaction | Brittle nature, low degradation rate, not suitable for soft tissue application | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabarudin, N.S.; Ab Ghani, N.; Ahmat, N.; Harlin, E.W.; Hao, L.Q.; Handajani, J.; Nor, F.M.; Md Fadilah, N.I.; Maarof, M.; Fauzi, M.B. Harnessing Plant Bioactive Compounds in Biomaterial Scaffolds for Advanced Wound Healing: A Comprehensive Review. Biomedicines 2025, 13, 2414. https://doi.org/10.3390/biomedicines13102414
Sabarudin NS, Ab Ghani N, Ahmat N, Harlin EW, Hao LQ, Handajani J, Nor FM, Md Fadilah NI, Maarof M, Fauzi MB. Harnessing Plant Bioactive Compounds in Biomaterial Scaffolds for Advanced Wound Healing: A Comprehensive Review. Biomedicines. 2025; 13(10):2414. https://doi.org/10.3390/biomedicines13102414
Chicago/Turabian StyleSabarudin, Nur Syazana, Norshazliza Ab Ghani, Nazeha Ahmat, Eka Wahyuni Harlin, Looi Qi Hao, Juni Handajani, Fatimah Mohd Nor, Nur Izzah Md Fadilah, Manira Maarof, and Mh Busra Fauzi. 2025. "Harnessing Plant Bioactive Compounds in Biomaterial Scaffolds for Advanced Wound Healing: A Comprehensive Review" Biomedicines 13, no. 10: 2414. https://doi.org/10.3390/biomedicines13102414
APA StyleSabarudin, N. S., Ab Ghani, N., Ahmat, N., Harlin, E. W., Hao, L. Q., Handajani, J., Nor, F. M., Md Fadilah, N. I., Maarof, M., & Fauzi, M. B. (2025). Harnessing Plant Bioactive Compounds in Biomaterial Scaffolds for Advanced Wound Healing: A Comprehensive Review. Biomedicines, 13(10), 2414. https://doi.org/10.3390/biomedicines13102414








