FAD012, a Ferulic Acid Derivative, Preserves Cerebral Blood Flow and Blood–Brain Barrier Integrity in the Rat Photothrombotic Stroke Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
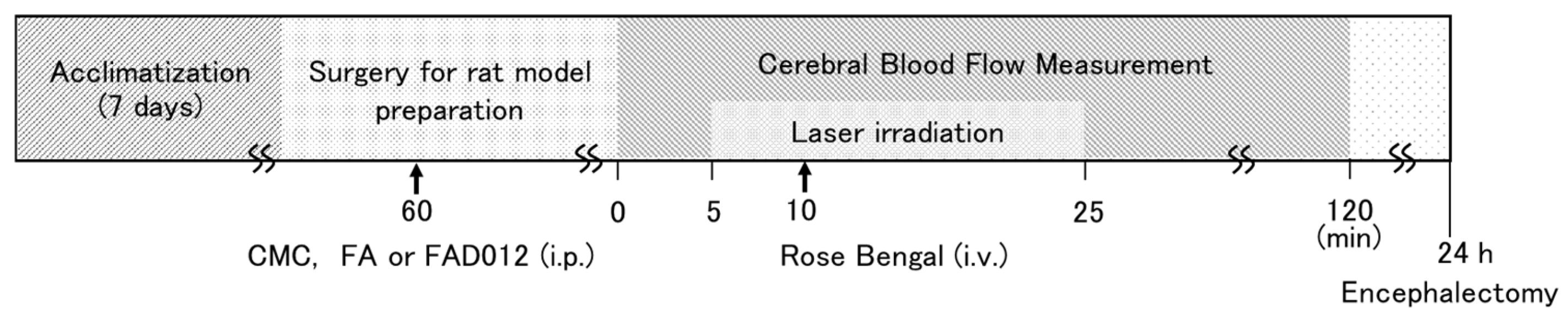
2.2. Treatment Schedule
2.3. Photothrombotic Stroke Model
2.4. 2,3,5-Triphenyl Tetrazolium Chloride Staining
2.5. Evans Blue Staining
2.6. Immunohistochemistry
2.7. In Silico Absorption, Distribution, Metabolism, Excretion, and Toxicity Prediction
2.8. Statistical Analysis
3. Results
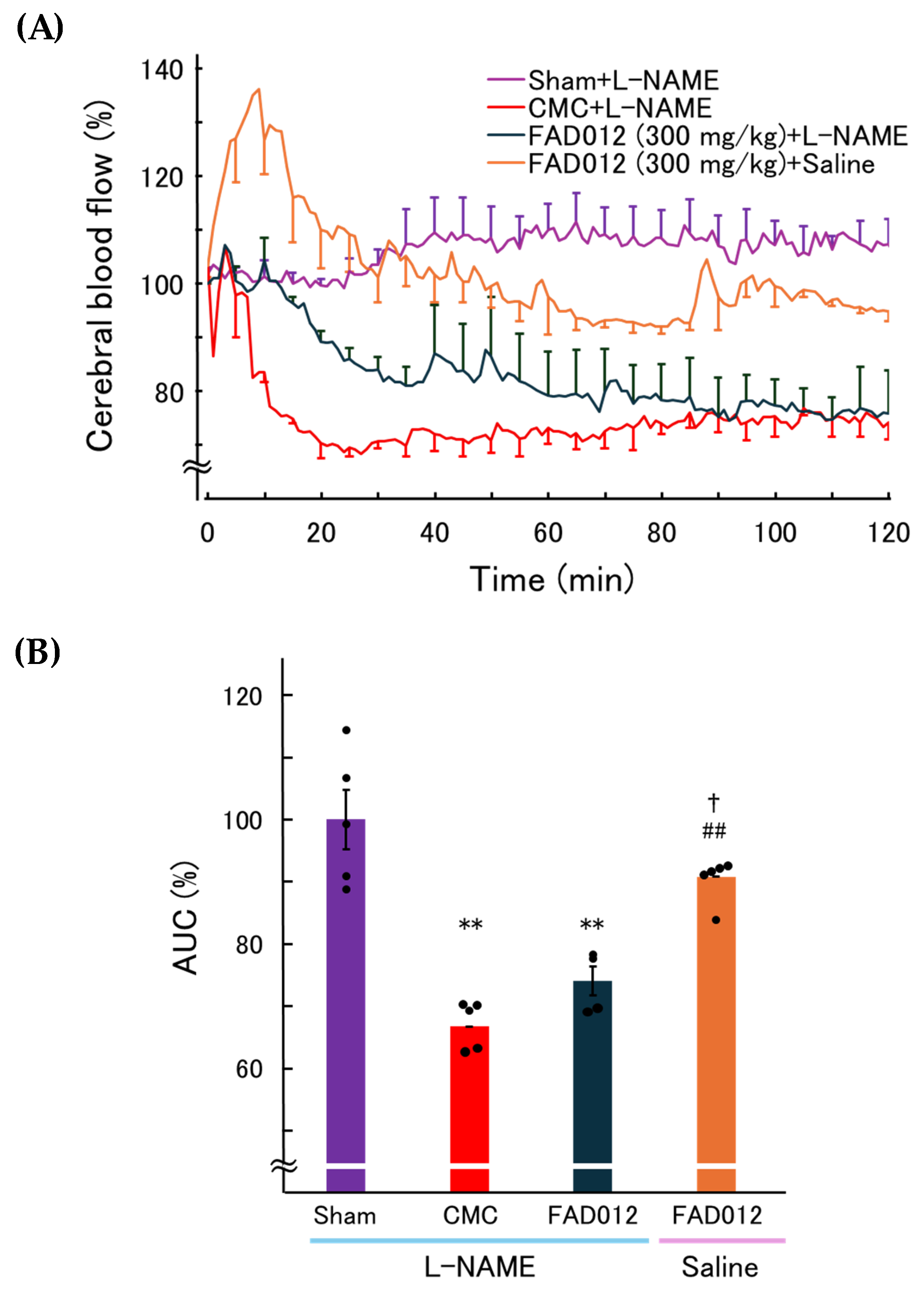
3.1. FAD012 Attenuates Photothrombosis-Induced Reduction in CBF
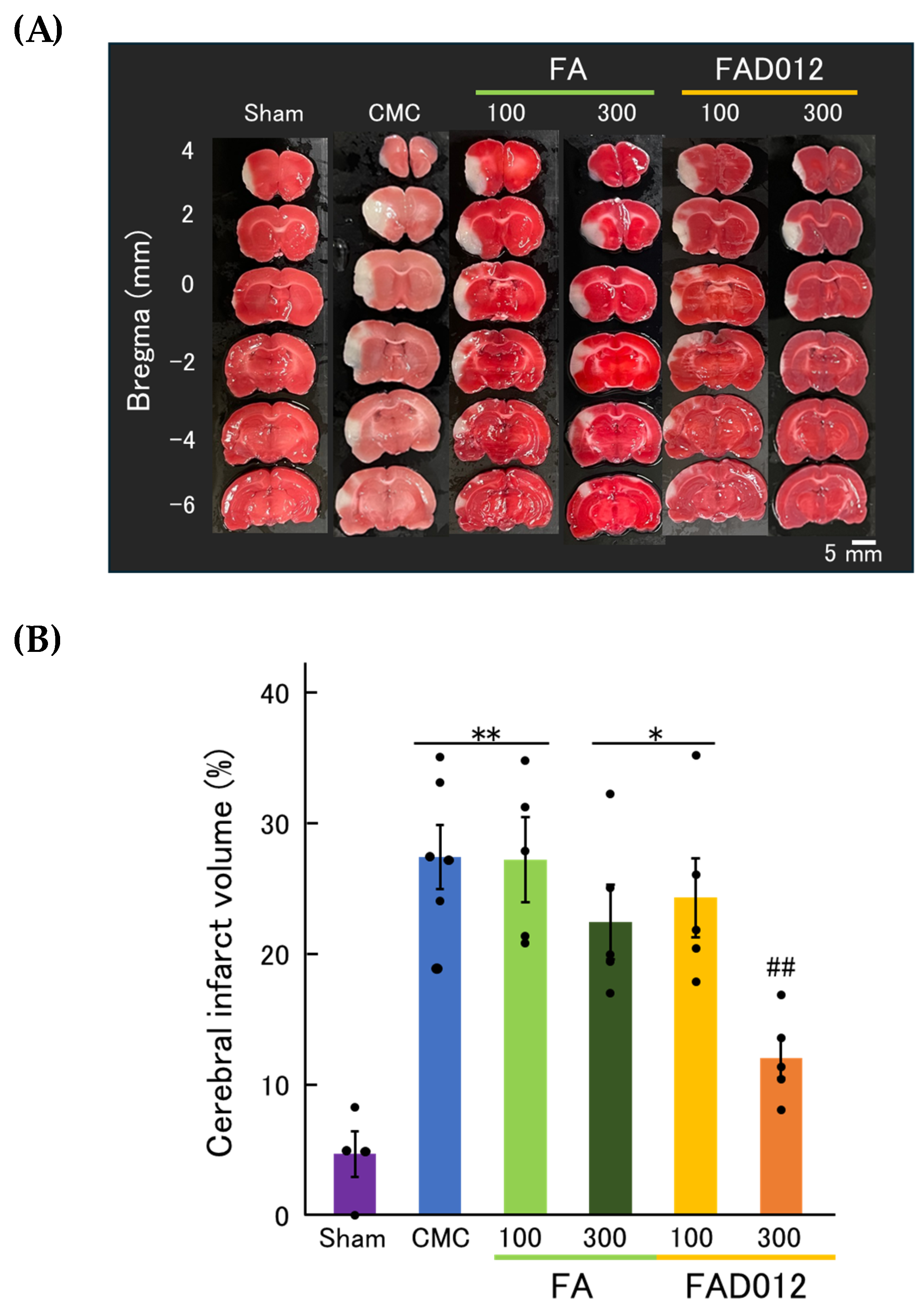
3.2. Neuroprotective Effects of FAD012 Assessed by TTC Staining
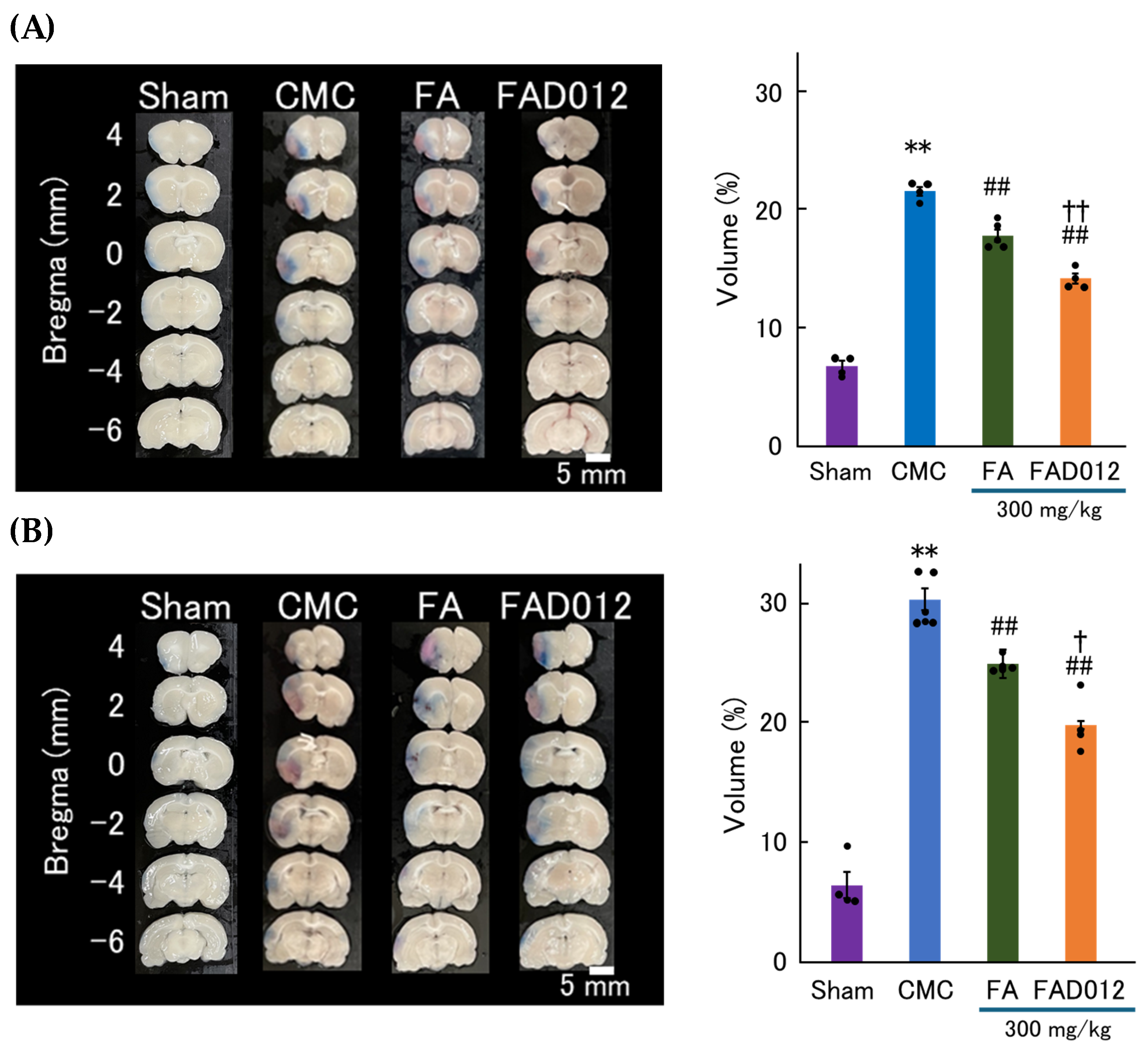
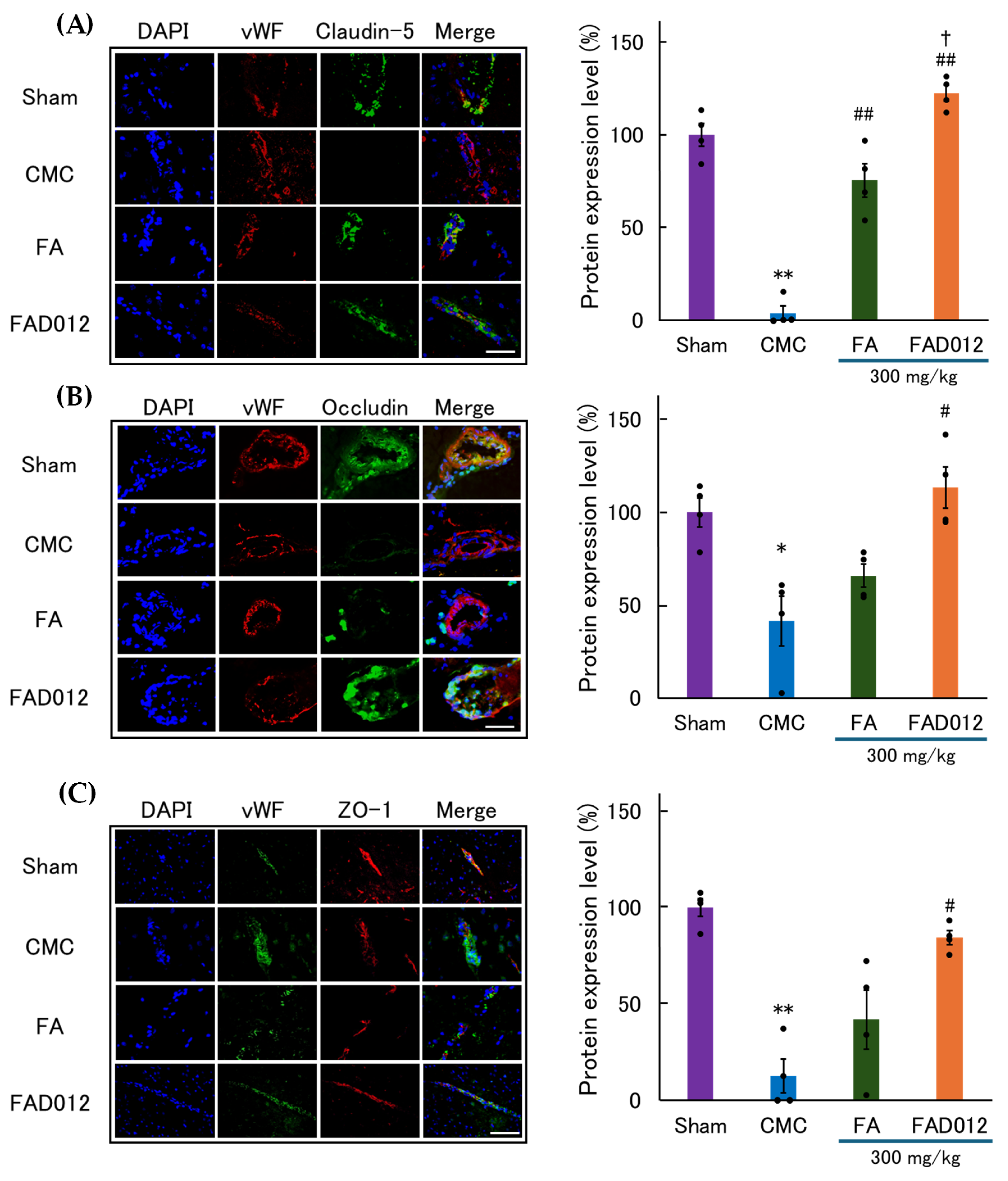
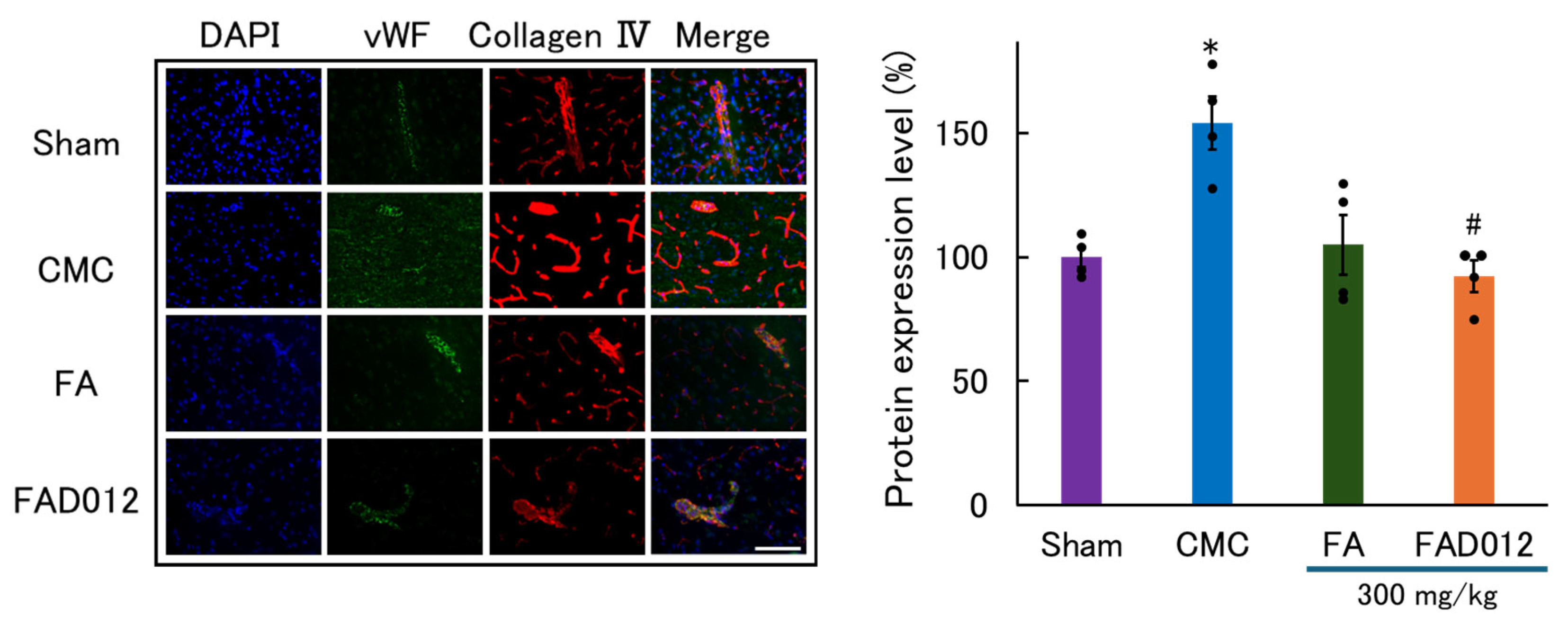
3.3. Preservation of BBB Integrity by FAD012 in Ischemic Stroke
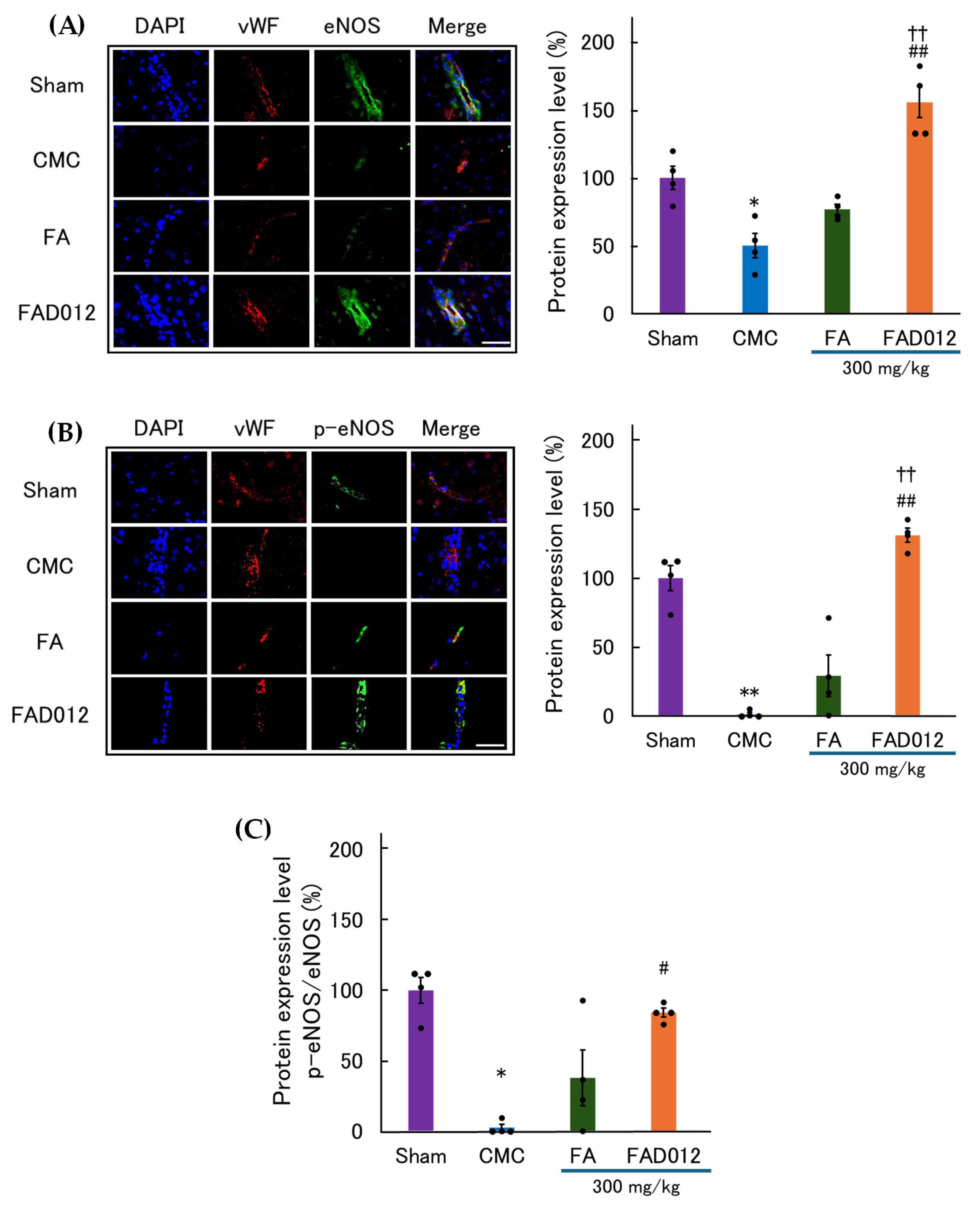
3.4. Involvement of NO in the CBF-Preserving Effect of FAD012
3.5. Absorption, Distribution, Metabolism, Excretion, and Toxicity Properties of FAD012
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| AMPK | AMP-activated protein kinase |
| ANOVA | Analysis of variance |
| AUC | Area under the curve |
| BBB | Blood–brain barrier |
| CBF | Cerebral blood flow |
| CMC | Carboxymethyl cellulose |
| CNS | Central nervous system |
| CYP | Cytochrome pigment |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| EB | Evans blue |
| eNOS | endothelial NOS |
| FA | Ferulic acid; 4-hydroxy-3-methoxycinnamic acid |
| FAD012 | 3,5-Dimethyl-4-hydroxy cinnamic acid |
| HO-1 | Heme oxygenase-1 |
| i.p. | Intraperitoneal |
| i.v. | Intravenous |
| LD50 | Lethal dose 50 |
| LOAEL | Lowest observed adverse effect level |
| L-NAME | NG-nitro-L-arginine methyl ester |
| MCAO/Re | Middle cerebral artery occlusion following reperfusion |
| MMPs | Matrix metalloproteinases |
| NO | Nitric oxide |
| NOS | NO synthase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PI3K | phosphoinositide 3-kinase |
| t-PA | Tissue plasminogen activator |
| TTC | 2,3,5-Triphenyl tetrazolium chloride |
| PBS | Phosphate-buffered saline |
| p-eNOS | phosphorylated eNOS |
| RBMVECs | Rat brain microvascular endothelial cells |
| ROS | Reactive oxygen species |
| TJ | Tight junction |
| vWF | von Willebrand factor |
References
- Lawes, C.M.; Bennett, D.A.; Feigin, V.L.; Rodgers, A. Blood pressure and stroke: An overview of published reviews. Stroke 2004, 35, 776–785, Erratum in Stroke 2004, 35, 1024. [Google Scholar] [CrossRef]
- Lawes, C.M.; Parag, V.; Bennett, D.A.; Suh, I.; Lam, T.H.; Whitlock, G.; Barzi, F.; Woodward, M. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care 2004, 27, 2836–2842. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, X.; Liu, Y.; Liu, S.Y. Mendelian randomization studies of lifestyle-related risk factors for stroke: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1379516. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Mika, Y.; Shunya, I. Cost of illness after stroke. J. Int. Univ. Health Welf. 2016, 21, 82–92. [Google Scholar]
- Ganti, L. Management of acute ischemic stroke in the emergency department: Optimizing the brain. Int. J. Emerg. Med. 2025, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ogasawara, K.; Kuroda, S.; Itabashi, R.; Toyoda, K.; Itoh, Y.; Iguchi, Y.; Shiokawa, Y.; Takagi, Y.; Ohtsuki, T.; et al. Japan Stroke Society Guideline 2021 for the Treatment of Stroke. Int. J. Stroke 2022, 17, 1039–1049. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H.; Travis, Z.D.; Zhang, J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current Progress of Inspection Technique, Mechanism, and Therapeutic Target. Curr. Neuropharmacol. 2020, 18, 1187–1212. [Google Scholar] [CrossRef]
- Wang, X.; Tsuji, K.; Lee, S.-R.; Ning, M.; Furie, K.L.; Buchan, A.M.; Lo, E.H. Mechanisms of Hemorrhagic Transformation After Tissue Plasminogen Activator Reperfusion Therapy for Ischemic Stroke. Stroke 2004, 35, 2726–2730. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Quan, X.; Han, Y.; Chen, J.; Yuan, M.; Chen, Y.; Zhou, M.; Yu, E.; Zhou, J.; et al. Shengui Sansheng San alleviates the worsening of blood-brain barrier integrity resulted from delayed tPA administration through VIP/VIPR1 pathway. Chin. Med. 2025, 20, 38. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Xuan, M.; Iwata, N.; Takayama, J.; Hayashi, K.; Kato, Y.; Aoyama, T.; Sugo, H.; Matsuzaki, H.; Yuan, B.; et al. Involvement of the Restoration of Cerebral Blood Flow and Maintenance of eNOS Expression in the Prophylactic Protective Effect of the Novel Ferulic Acid Derivative FAD012 against Ischemia/Reperfusion Injuries in Rats. Int. J. Mol. Sci. 2023, 24, 9663. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Matsuzaki, H.; Xuan, M.; Yuan, B.; Takayama, J.; Sakamoto, T.; Okazaki, M. Chronic Administration with FAD012 (3,5-Dimethyl-4-hydroxycinnamic Acid) Maintains Cerebral Blood Flow and Ameliorates Swallowing Dysfunction After Chronic Cerebral Hypoperfusion in Rats. Int. J. Mol. Sci. 2025, 26, 3277. [Google Scholar] [CrossRef] [PubMed]
- Mathias, K.; Machado, R.S.; Stork, S.; Dos Santos, D.; Joaquim, L.; Generoso, J.; Danielski, L.G.; Barichello, T.; Prophiro, J.S.; Petronilho, F. Blood-brain barrier permeability in the ischemic stroke: An update. Microvasc. Res. 2024, 151, 104621. [Google Scholar] [CrossRef]
- Wevers, N.R.; De Vries, H.E. Microfluidic models of the neurovascular unit: A translational view. Fluids Barriers CNS 2023, 20, 86. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Labat-gest, V.; Tomasi, S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. 2013, 76, e50370. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Minnerup, J.; Kleinschnitz, C.; Meuth, S.G.; Wiendl, H.; Bendszus, M.; Stoll, G. Photochemically induced ischemic stroke in rats. Exp. Transl. Stroke Med. 2012, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Wang, T.; Jiang, N.; Yu, P.; Chong, Y.; Fu, F. Ferulic acid ameliorates nerve injury induced by cerebral ischemia in rats. Exp. Ther. Med. 2015, 9, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Su, S.-Y.; Tang, N.-Y.; Ho, T.-Y.; Lo, W.-Y.; Hsieh, C.-L. Ferulic acid inhibits nitric oxide-induced apoptosis by enhancing GABAB1 receptor expression in transient focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2010, 31, 889–899. [Google Scholar] [CrossRef]
- Ding-Zhou, L.; Marchand-Verrecchia, C.; Croci, N.; Plotkine, M.; Margaill, I. L-NAME reduces infarction, neurological deficit and blood-brain barrier disruption following cerebral ischemia in mice. Eur. J. Pharmacol. 2002, 457, 137–146. [Google Scholar] [CrossRef]
- Cotrina, M.L.; Lou, N.; Tome-Garcia, J.; Goldman, J.; Nedergaard, M. Direct comparison of microglial dynamics and inflammatory profile in photothrombotic and arterial occlusion evoked stroke. Neuroscience 2017, 343, 483–494. [Google Scholar] [CrossRef]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar] [CrossRef]
- Uzdensky, A.B. Photothrombotic Stroke as a Model of Ischemic Stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, C.; Xu, W.; Li, W.; Feng, Z.; Zhang, X.; Zhao, K. Edaravone Dexborneol Downregulates Neutrophil Extracellular Trap Expression and Ameliorates Blood-Brain Barrier Permeability in Acute Ischemic Stroke. Mediat. Inflamm. 2022, 2022, 3855698. [Google Scholar] [CrossRef]
- Leten, C.; Struys, T.; Dresselaers, T.; Himmelreich, U. In vivo and ex vivo assessment of the blood brain barrier integrity in different glioblastoma animal models. J. Neuro-Oncol. 2014, 119, 297–306. [Google Scholar] [CrossRef]
- Nagaraja, T.N.; Keenan, K.A.; Fenstermacher, J.D.; Knight, R.A. Acute Leakage Patterns of Fluorescent Plasma Flow Markers after Transient Focal Cerebral Ischemia Suggest Large Openings in Blood-Brain Barrier. Microcirculation 2010, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-Brain Barrier Disruption Induced Cognitive Impairment Is Associated With Increase of Inflammatory Cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Mvondo, J.G.M.; Matondo, A.; Mawete, D.T.; Bambi, S.-M.N.; Mbala, B.M.; Lohohola, P.O. In Silico ADME/T Properties of Quinine Derivatives using SwissADME and pkCSM Webservers. Int. J. Trop. Dis. Health 2021, 42, 1–12. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Banks, W.A.; Rhea, E.M. The Blood-Brain Barrier, Oxidative Stress, and Insulin Resistance. Antioxidants 2021, 10, 1695. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative Stress-Mediated Blood-Brain Barrier (BBB) Disruption in Neurological Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Gu, Y.; Zheng, G.; Xu, M.; Li, Y.; Chen, X.; Zhu, W.; Tong, Y.; Chung, S.K.; Liu, K.J.; Shen, J. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J. Neurochem. 2012, 120, 147–156. [Google Scholar] [CrossRef]
- Candelario-Jalil, E. Injury and repair mechanisms in ischemic stroke: Considerations for the development of novel neurotherapeutics. Curr. Opin. Investig. Drugs 2009, 10, 644–654. [Google Scholar]
- Michalski, D.; Spielvogel, E.; Puchta, J.; Reimann, W.; Barthel, H.; Nitzsche, B.; Mages, B.; Jäger, C.; Martens, H.; Horn, A.K.E.; et al. Increased Immunosignals of Collagen IV and Fibronectin Indicate Ischemic Consequences for the Neurovascular Matrix Adhesion Zone in Various Animal Models and Human Stroke Tissue. Front. Physiol. 2020, 11, 575598. [Google Scholar] [CrossRef]
- Yamashita, T.; Kamiya, T.; Deguchi, K.; Inaba, T.; Zhang, H.; Shang, J.; Miyazaki, K.; Ohtsuka, A.; Katayama, Y.; Abe, K. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J. Cereb. Blood Flow Metab. 2009, 29, 715–725. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Vargas, M.R.; Johnson, D.A.; Sirkis, D.W.; Messing, A.; Johnson, J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008, 28, 13574–13581. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.; Gratton, J.P.; Sessa, W.C. Post-translational control of endothelial nitric oxide synthase: Why isn’t calcium/calmodulin enough? J. Pharmacol. Exp. Ther. 2001, 299, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Xue, Y.Q.; Ma, T.; Wang, X.; Li, J.J.; Lan, L.; Malik, K.U.; McDonald, M.P.; Dopico, A.M.; Liao, F.F. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol. Neurodegener. 2015, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Fabian, R.H.; Perez-Polo, J.R.; Kent, T.A. Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1809–H1814. [Google Scholar] [CrossRef]
- Khan, M.; Dhammu, T.S.; Qiao, F.; Kumar, P.; Singh, A.K.; Singh, I. S-Nitrosoglutathione Mimics the Beneficial Activity of Endothelial Nitric Oxide Synthase-Derived Nitric Oxide in a Mouse Model of Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 104470. [Google Scholar] [CrossRef]
- Godínez-Rubí, M.; Rojas-Mayorquín, A.E.; Ortuño-Sahagún, D. Nitric oxide donors as neuroprotective agents after an ischemic stroke-related inflammatory reaction. Oxidative Med. Cell. Longev. 2013, 2013, 297357. [Google Scholar] [CrossRef]
- Tsai, P.-Y.; Ka, S.-H.; Chang, C.-Y.; Lee, I.C.; Chen, C.-C.; Hung, L.-M.; Chiang, A.-S.; Chen, C.-H. Activation of AMPK/eNOS pathway by polyphenol-rich extract improves endothelial function and reduces atherosclerosis in ApoE-deficient mice. Sci. Rep. 2015, 5, 10290. [Google Scholar] [CrossRef]
- Nor, A.M.; Ahmad, F.; Ismail, N. Polyphenols and Endothelial Function: AMPK–PI3K–Akt–eNOS Pathway as a Central Mechanism. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Modulation of endothelial nitric oxide synthase by polyphenols: Mechanisms and implications. Int. J. Mol. Sci. 2023, 24, 985. [Google Scholar] [CrossRef]
- Koh, P.O. Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia. Lab. Anim. Res. 2012, 28, 273–278. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Z.-Z.; Liu, D.; Qi, X.-R. Pharmacokinetics, brain distribution, release and blood–brain barrier transport of Shunaoxin pills. J. Ethnopharmacol. 2014, 151, 1133–1140. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Huttunen, J.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur. J. Pharm. Sci. 2019, 129, 99–109. [Google Scholar] [CrossRef]


| Groups | Elapsed Times to CBF Nadir (Min) |
|---|---|
| CMC | 30.6 ± 3.8 |
| FA (100 mg/kg) | 42.8 ± 7.5 |
| FA (300 mg/kg) | 36.6 ± 2.8 |
| FAD012 (100 mg/kg) | 50.2 ± 7.1 |
| FAD012 (300 mg/kg) | 70.6 ± 1.6 **,† |
| FAD012 | FA | |
|---|---|---|
| Physicochemical Properties | ||
| Formula | C10H10O4 | C11H12O3 |
| Molecular weight | 192.214 | 194.186 |
| Topological Polar Surface Area (TPSA) (Å2) | 57.53 | 66.76 |
| Consensus LogP | 2.03 | 1.36 |
| Water solubility (log mol/L) | −2.57 | −2.81 |
| Number of H-bond acceptors | 3 | 4 |
| Number of H-bond donors | 2 | 2 |
| Lipinski’s rule | Yes; 0 violation | Yes; 0 violation |
| Ghose’s rule | Yes | Yes |
| Absorption | ||
| Caco-2 permeability (log Papp in 10−6 cm/s) | 1.20 | 0.19 |
| Intestinal absorption (human)(% Absorbed) | 97.2 | 95.7 |
| Skin permeation (log Kp; cm/s) | −5.83 | −6.41 |
| P-glycoprotein substrate | Yes | Yes |
| P-glycoprotein I inhibitor | No | No |
| P-glycoprotein II inhibitor | No | No |
| Distribution | ||
| BBB permeability (log BB) | −0.17 | −0.30 |
| CNS permeability (log PS) | −2.83 | −2.55 |
| Volume of distribution (log L/kg) | −0.79 | −0.90 |
| Metabolism | ||
| CYP2D6 substrate | No | No |
| CYP3A4 substrate | No | No |
| CYP1A2 inhibitor | No | No |
| CYP2C9 inhibitor | No | No |
| CYP2C9 inhibitor | No | No |
| CYP2D6 inhibitor | No | No |
| CYP3A4 inhibitor | No | No |
| Excretion | ||
| Total Clearance (log ml/min/kg) | 0.72 | 0.64 |
| Renal OCT2 substrate | No | No |
| Toxicity | ||
| AMES toxicity | No | No |
| Max. tolerated dose (human)(log mg/kg/day) (mg/kg/day, calculated from log mol/kg) | 1.70 (50.1) | 1.49 (30.9) |
| Oral Rat Acute Toxicity (LD50)(mol/kg) (mg/kg, calculated from log mol/kg) | 2.39 (4.7 × 107) | 2.52 (6.4 × 107) |
| Oral Rat Chronic Toxicity (LOAEL)(log mg/kg_bw/day) (mg/kg_bw/day, calculated from log mol/kg_bw/day) | 2.30 (200) | 1.94 (87.1) |
| Hepatotoxicity | No | No |
| Skin Sensitization | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugoh, H.; Matsuzaki, H.; Takayama, J.; Iwata, N.; Xuan, M.; Yuan, B.; Sakamoto, T.; Okazaki, M. FAD012, a Ferulic Acid Derivative, Preserves Cerebral Blood Flow and Blood–Brain Barrier Integrity in the Rat Photothrombotic Stroke Model. Biomedicines 2025, 13, 2403. https://doi.org/10.3390/biomedicines13102403
Sugoh H, Matsuzaki H, Takayama J, Iwata N, Xuan M, Yuan B, Sakamoto T, Okazaki M. FAD012, a Ferulic Acid Derivative, Preserves Cerebral Blood Flow and Blood–Brain Barrier Integrity in the Rat Photothrombotic Stroke Model. Biomedicines. 2025; 13(10):2403. https://doi.org/10.3390/biomedicines13102403
Chicago/Turabian StyleSugoh, Hiroshi, Hirokazu Matsuzaki, Jun Takayama, Naohiro Iwata, Meiyan Xuan, Bo Yuan, Takeshi Sakamoto, and Mari Okazaki. 2025. "FAD012, a Ferulic Acid Derivative, Preserves Cerebral Blood Flow and Blood–Brain Barrier Integrity in the Rat Photothrombotic Stroke Model" Biomedicines 13, no. 10: 2403. https://doi.org/10.3390/biomedicines13102403
APA StyleSugoh, H., Matsuzaki, H., Takayama, J., Iwata, N., Xuan, M., Yuan, B., Sakamoto, T., & Okazaki, M. (2025). FAD012, a Ferulic Acid Derivative, Preserves Cerebral Blood Flow and Blood–Brain Barrier Integrity in the Rat Photothrombotic Stroke Model. Biomedicines, 13(10), 2403. https://doi.org/10.3390/biomedicines13102403







