Experimental Airway Allogenic Transplantation Model with Decellularized Cryopreserved Tracheas
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Controls
2.3. Tracheal Decellularization
2.4. Calculation of Sample Size (n)
2.5. Implantation Technique
2.6. Structural Study
2.7. Biomechanical Study
2.7.1. Tensile Tests
2.7.2. Radial Compression Test
2.8. Statistical Analysis
3. Results
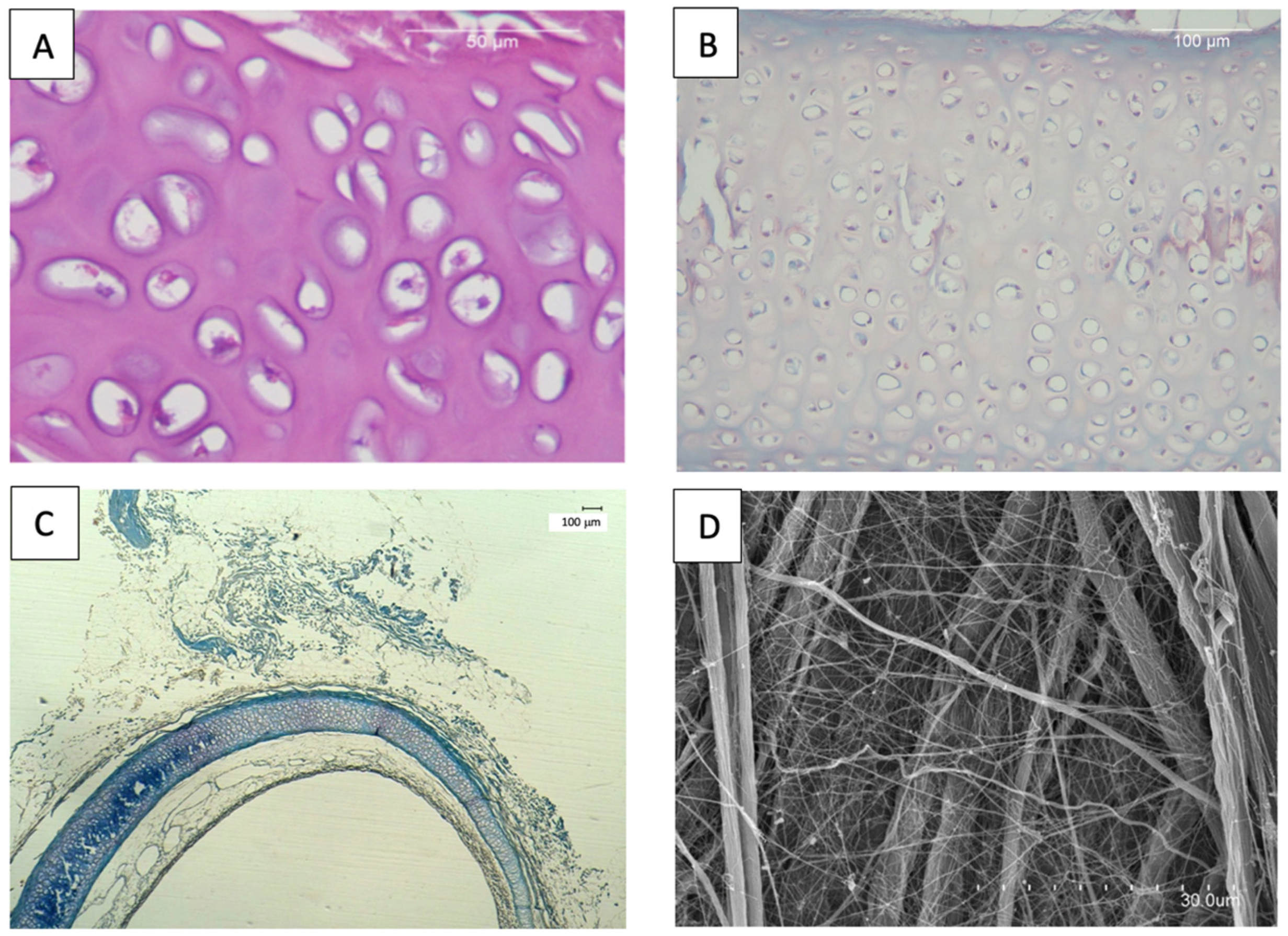
3.1. In Vitro Evaluation of Tracheal Decellularization
3.1.1. Histology
3.1.2. Biomechanics
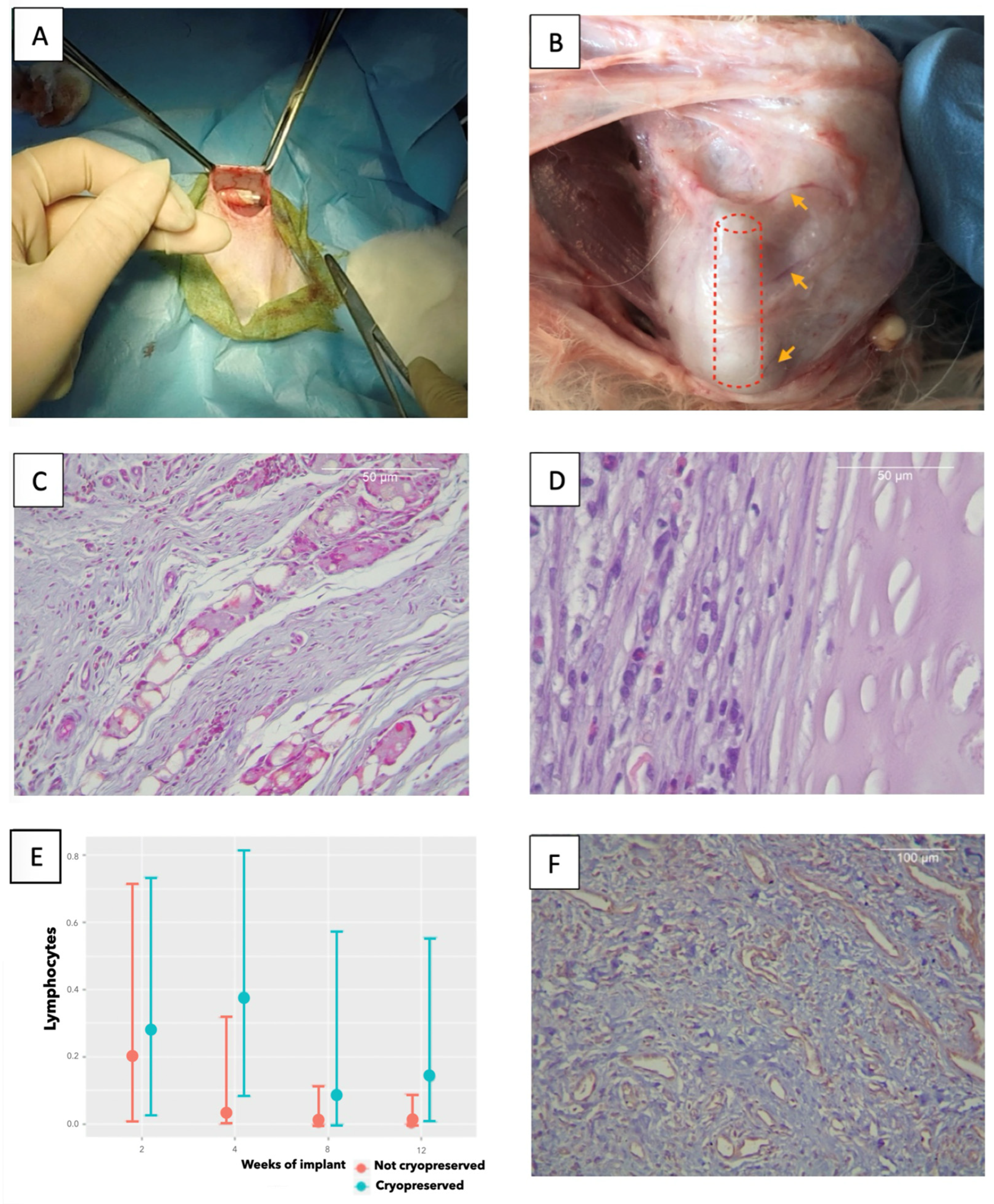
3.2. In Vivo Implantation
3.2.1. Histology
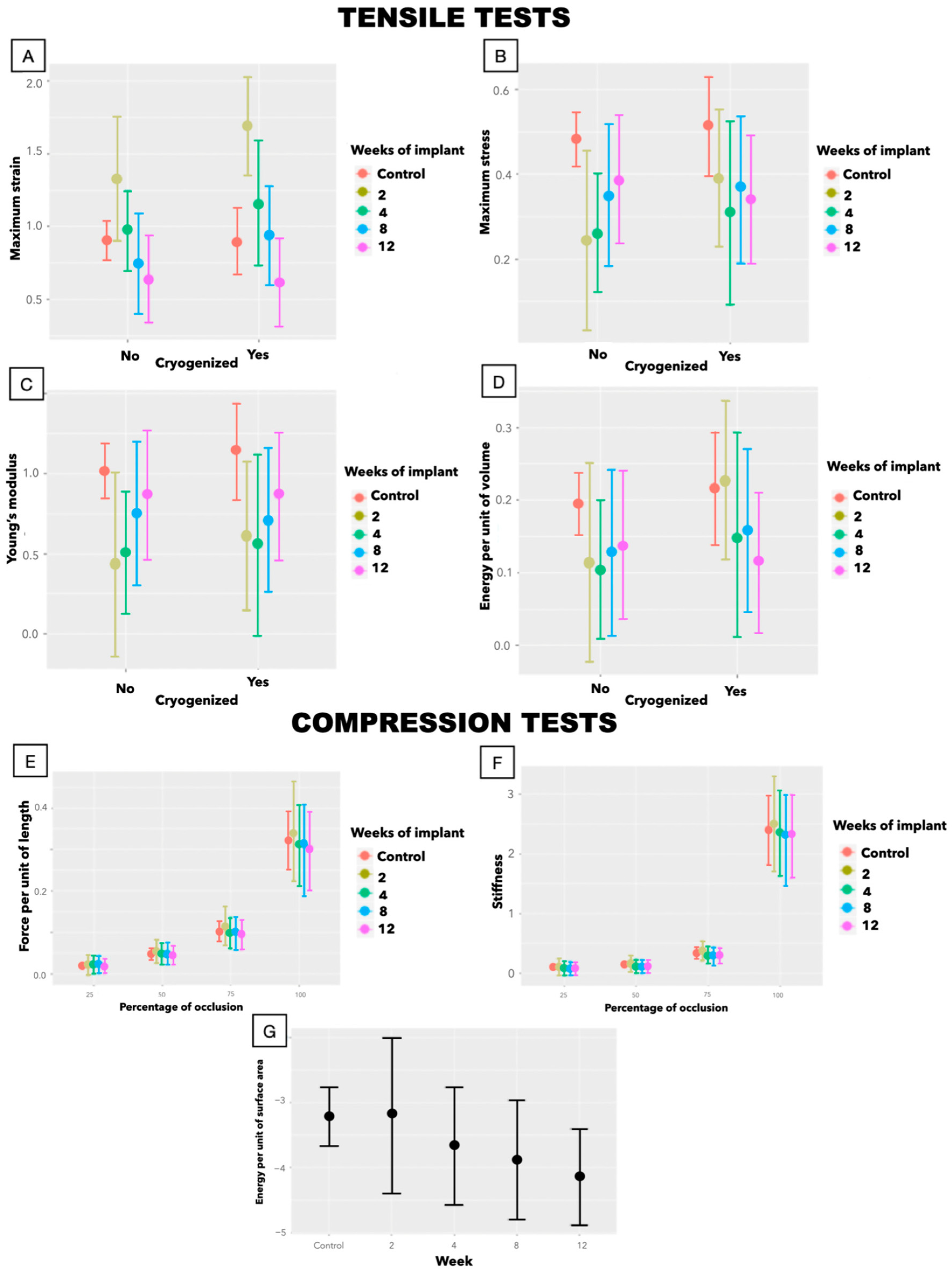
3.2.2. Biomechanics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Credible interval |
| DMSO | Dimethyl sulfoxide |
| E | Young’s modulus |
| ε | Strain |
| ƒ | Force tolerated per unit of length |
| FBS | Fetal bovine serum |
| H-E | Hematoxylin–eosin |
| MT | Masson’s trichrome |
| O | Orcein |
| OR | Odds ratio |
| PBS | Phosphate-buffered saline |
| Stiffness | |
| σ | Stress |
| SEM | Scanning electron microscope |
| UTM | Universal testing machine |
| W/S | Energy per unit of surface area |
| W/Vol | Energy stored per unit of volume |
References
- Grillo, H.C. Development of tracheal surgery: A historical review. Part 2: Treatment of tracheal diseases. Ann. Thorac. Surg. 2003, 75, 1039–1047. [Google Scholar] [CrossRef]
- Damiano, G.; Palumbo, V.D.; Fazzotta, S.; Curione, F.; Monte, G.L.; Brucato, V.M.B.; Monte, A.I.L. Current Strategies for Tracheal Replacement: A Review. Life 2021, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Belsey, R. Resection and reconstruction of the intrathoracic trachea. Br. J. Surg. 1950, 38, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ott, L.M.; Weatherly, R.A.; Detamore, M.S. Overview of tracheal tissue engineering: Clinical need drives the laboratory approach. Ann. Biomed. Eng. 2011, 39, 2091–2113. [Google Scholar] [CrossRef] [PubMed]
- Hysi, I.; Kipnis, E.; Fayoux, P.; Copin, M.C.; Zawadzki, C.; Jashari, R.; Hubert, T.; Ung, A.; Ramon, P.; Jude, B.; et al. Successful orthotopic transplantation of short tracheal segments without immunosuppressive therapy. Eur. J. Cardiothorac. Surg. 2014, 47, e54–e61. [Google Scholar] [CrossRef][Green Version]
- Delaere, P.; Lerut, T.; Van Raemdonck, D. Tracheal transplantation: State of the art and key role of blood supply in its success. Thorac. Surg. Clin. 2018, 28, 337–345. [Google Scholar] [CrossRef]
- Martinod, E.; Radu, D.M.; Onorati, I.; Portela, A.M.S.; Peretti, M.; Guiraudet, P.; Destable, M.-D.; Uzunhan, Y.; Freynet, O.; Chouahnia, K.; et al. Airway replacement using stented aortic matrices: Long-term follow-up and results of the TRITON-01 study in 35 adult patients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022, 22, 2961–2970. [Google Scholar] [CrossRef]
- Pribaz, J.J.; Fine, N.A. Prelamination: Defining the prefabricated flap--a case report and review. Microsurgery 1994, 15, 618–623. [Google Scholar] [CrossRef]
- Martínez-Hernández, N.J.; Díaz-Cuevas, A.; Milián-Medina, L.; Sancho-Tello, M.; Roselló-Ferrando, J.; Morcillo-Aixelá, A.; Campo-Cañaveral, J.L.; Roig-Bataller, A.; Mata-Roig, M. Decellularized tracheal prelamination implant: A proposed bilateral double organ technique. Artif. Organs 2021, 45, 1491–1500. [Google Scholar] [CrossRef]
- ISO 10993-6:2017; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects After Implantation. International Organization for Standardization: Geneva, Switzerland, 2017.
- Sancho-Tello, M.; Martorell, S.; Roig, M.M.; Milián, L.; Gámiz-González, M.A.; Ribelles, J.L.G.; Carda, C. Human platelet-rich plasma improves the nesting and differentiation of human chondrocytes cultured in stabilized porous chitosan scaffolds. J. Tissue Eng. 2017, 8, 2041731417697545. [Google Scholar] [CrossRef]
- Martínez-Hernández, N.J.; Mas-Estellés, J.; Milián-Medina, L.; Martínez-Ramos, C.; Cerón-Navarro, J.; Galbis-Caravajal, J.; Roig-Bataller, A.; Mata-Roig, M. A Standardised Approach to the Biomechanical Evaluation of Tracheal Grafts. Biomolecules 2021, 11, 1461. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Maughan, E.F.; Hynds, R.E.; Proctor, T.J.; Janes, S.M.; Elliott, M.; Birchall, M.A.; Lowdell, M.W.; De Coppi, P. Autologous Cell Seeding in Tracheal Tissue Engineering. Curr. Stem Cell Rep. 2017, 3, 279–289. [Google Scholar] [CrossRef]
- Wood, K.J.; Zaitsu, M.; Goto, R. Cell mediated rejection. Methods Mol. Biol. 2013, 1034, 71–83. [Google Scholar] [PubMed]
- Zang, M.; Zhang, Q.; Chang, E.I.; Mathur, A.B.; Yu, P. Decellularized tracheal matrix scaffold for tracheal tissue engineering: In vivo host response. Plast. Reconstr. Surg. 2013, 132, 549e–559e. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Yang, J.-R.; Chiang, N.-J.; Ma, H.; Tsay, R.-Y. Evaluation of decellularized extracellular matrix of skeletal muscle for tissue engineering. Int. J. Artif. Organs 2014, 37, 546–555. [Google Scholar] [CrossRef] [PubMed]
- De Wolf, J.; Brieu, M.; Zawadzki, C.; Ung, A.; Kipnis, E.; Jashari, R.; Hubert, T.; Fayoux, P.; Mariette, C.; Copin, M.C.; et al. Successful immunosuppressant-free heterotopic transplantation of tracheal allografts in the pig. Eur. J. Cardiothorac. Surg. 2017, 52, 248–255. [Google Scholar] [CrossRef]
- Reeves, A.R.D.; Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, G.; Kaplan, D.L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 2015, 73, 272–283. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol. 2011, 713, 97–111. [Google Scholar]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef]
- Johnson, C.; Sheshadri, P.; Ketchum, J.M.; Narayanan, L.K.; Weinberger, P.M.; Shirwaiker, R.A. In vitro characterization of design and compressive properties of 3D-biofabricated/decellularized hybrid grafts for tracheal tissue engineering. J. Mech. Behav. Biomed. Mater. 2016, 59, 572–585. [Google Scholar] [CrossRef]
| exp.Estimate | 95% CI | ||
|---|---|---|---|
| EOSINOPHILS | Cryopreservation | 1.818 | 0.089–31.706 |
| IT | 0.51 | 0.201–1.25 | |
| IT + Cryopreservation | 0.772 | 0.22–2.973 | |
| NEUTROPHILS | Cryopreservation | 1.593 | 0.049–37.667 |
| IT | 0.333 | 0.069–1.242 | |
| IT + Cryopreservation | 1.314 | 0.18–9.402 | |
| LYMPHOCYTES | Cryopreservation | 1.641 | 0.052–73.541 |
| IT | 0.049 | 0–0.995 | |
| IT + Cryopreservation | 14.638 | 0.569–2517.091 | |
| PLASMOCYTE | Cryopreservation | 1.34 | 0.053–25.632 |
| IT | 0.358 | 0.068–1.354 | |
| IT + Cryopreservation | 1.437 | 0.229–9.54 | |
| MACROPHAGES | Cryopreservation | 0.018 | 0–1.174 |
| IT | 0.29 | 0.079–0.891 | |
| IT + Cryopreservation | 10.487 | 1.603–97.327 | |
| GIANT CELLS | Cryopreservation | 0.261 | 0.018–3.957 |
| IT | 1.152 | 0.432–2.906 | |
| IT + Cryopreservation | 2.09 | 0.528–8.518 | |
| CONNECTIVE TISSUE | Cryopreservation | 3.833 | 0.322–52.99 |
| IT | 5.635 | 1.941–20.296 | |
| IT + Cryopreservation | 1.175 | 0.325–4.834 | |
| FATTY TISSUE | Cryopreservation | 0.003 | 0–2.747 |
| IT | 0.214 | 0.001–2.392 | |
| IT + Cryopreservation | 17.633 | 0.606–144618.768 | |
| NECROSIS | Cryopreservation | 2.079 | 0.154–27.353 |
| IT | 0.97 | 0.369–2.7 | |
| IT + Cryopreservation | 1.105 | 0.299–3.943 | |
| VASCULARIZATION | Cryopreservation | 1.146 | 0.696–1.841 |
| IT | 1.14 | 0.913–1.356 | |
| IT + Cryopreservation | 0.919 | 0.741–1.172 | |
| Estimate | 95% CI | ||
|---|---|---|---|
| Intercept | 0.902 | 0.773–1.032 | |
| 2 weeks | 0.426 | 0.012–0.847 | |
| 4 weeks | 0.074 | −0.17–0.323 | |
| 8 weeks | −0.156 | −0.479–0.16 | |
| 12 weeks | −0.268 | −0.541–0.004 | |
| 2 w + cryopreservation | 0.371 | −0.133–0.868 | |
| 4 w + cryopreservation | 0.186 | −0.293–0.675 | |
| 8 w + cryopreservation | 0.202 | −0.245–0.64 | |
| 12 w + cryopreservation | −0.012 | −0.393–0.347 | |
| σmax | Intercept | 0.481 | 0.418–0.544 |
| 2 weeks | −0.236 | −0.438–−0.037 | |
| 4 weeks | −0.221 | −0.349–−0.096 | |
| 8 weeks | −0.135 | −0.287–0.02 | |
| 12 weeks | −0.096 | −0.233–0.042 | |
| 2 w + cryopreservation | 0.112 | −0.141–0.359 | |
| 4 w + cryopreservation | 0.016 | −0.222–0.252 | |
| 8 w + cryopreservation | −0.01 | −0.214–0.209 | |
| 12 w + cryopreservation | −0.076 | −0.256–0.105 | |
| Intercept | 1.017 | 0.845–1.187 | |
| 2 weeks | −0.587 | −1.144–−0.035 | |
| 4 weeks | −0.512 | −0.855–−0.184 | |
| 8 weeks | −0.267 | −0.676–0.148 | |
| 12 weeks | −0.145 | −0.516–0.215 | |
| 2 w + cryopreservation | 0.057 | −0.602–0.735 | |
| 4 w + cryopreservation | −0.076 | −0.699–0.523 | |
| 8 w + cryopreservation | −0.17 | −0.727–0.388 | |
| 12 w + cryopreservation | −0.134 | −0.614–0.358 | |
| Intercept | 0.195 | 0.152–0.238 | |
| 2 weeks | −0.081 | −0.212–0.054 | |
| 4 weeks | −0.09 | −0.173–−0.006 | |
| 8 weeks | −0.067 | −0.173–0.038 | |
| 12 weeks | −0.059 | −0.147–0.034 | |
| 2 w + cryopreservation | 0.092 | −0.075–0.256 | |
| 4 w + cryopreservation | 0.023 | −0.13–0.177 | |
| 8 w + cryopreservation | 0.009 | −0.127–0.15 | |
| 12 w + cryopreservation | −0.042 | −0.165–0.077 |
| Variables | Estimate | Lower.95. | |
|---|---|---|---|
| Intercept | 0.019 | 0.01–0.029 | |
| 2 weeks | 0.006 | −0.026–0.04 | |
| 4 weeks | −0.004 | −0.03–0.02 | |
| 8 weeks | −0.005 | −0.039–0.016 | |
| 12 weeks | −0.006 | −0.033–0.011 | |
| Cryopreservation | −0.005 | −0.016–0.006 | |
| Intercept | 0.101 | 0.053–0.149 | |
| 2 weeks | 0.027 | −0.17–0.228 | |
| 4 weeks | −0.009 | −0.207–0.141 | |
| 8 weeks | −0.029 | −0.257–0.101 | |
| 12 weeks | −0.023 | −0.187–0.078 | |
| Cryopreservation | 0.027 | −0.17–0.228 | |
| Intercept | −2.683 | −3.14–−2.23 | |
| 2 weeks | 0.052 | −1.201–1.343 | |
| 4 weeks | −0.436 | −1.503–0.665 | |
| 8 weeks | −0.661 | −1.718–0.425 | |
| 12 weeks | −0.902 | −1.822–0.051 | |
| Cryopreservation | −0.28 | −0.85–0.287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, N.J.; Milián-Medina, L.; Mas-Estellés, J.; Roig-Bataller, A.; Hervás-Marín, D.; Mata-Roig, M. Experimental Airway Allogenic Transplantation Model with Decellularized Cryopreserved Tracheas. Biomedicines 2025, 13, 2401. https://doi.org/10.3390/biomedicines13102401
Martínez-Hernández NJ, Milián-Medina L, Mas-Estellés J, Roig-Bataller A, Hervás-Marín D, Mata-Roig M. Experimental Airway Allogenic Transplantation Model with Decellularized Cryopreserved Tracheas. Biomedicines. 2025; 13(10):2401. https://doi.org/10.3390/biomedicines13102401
Chicago/Turabian StyleMartínez-Hernández, Néstor J., Lara Milián-Medina, Jorge Mas-Estellés, Amparo Roig-Bataller, David Hervás-Marín, and Manuel Mata-Roig. 2025. "Experimental Airway Allogenic Transplantation Model with Decellularized Cryopreserved Tracheas" Biomedicines 13, no. 10: 2401. https://doi.org/10.3390/biomedicines13102401
APA StyleMartínez-Hernández, N. J., Milián-Medina, L., Mas-Estellés, J., Roig-Bataller, A., Hervás-Marín, D., & Mata-Roig, M. (2025). Experimental Airway Allogenic Transplantation Model with Decellularized Cryopreserved Tracheas. Biomedicines, 13(10), 2401. https://doi.org/10.3390/biomedicines13102401









