Intranasal 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Administration Hampered Contractile Response of Dopamine in Isolated Rat Ileum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and MPTP Administration Protocol
2.2. Tissue Collection and Processing
2.3. Functional Studies
2.4. HPLC Analysis of DA Content
2.5. Western Blot Analysis
2.6. Immunohistochemistry Analysis
2.7. Statistical Analysis
3. Results
3.1. Tonic Contractile Response to DA in Ilea Isolated from Rats Treated with Intranasal MPTP
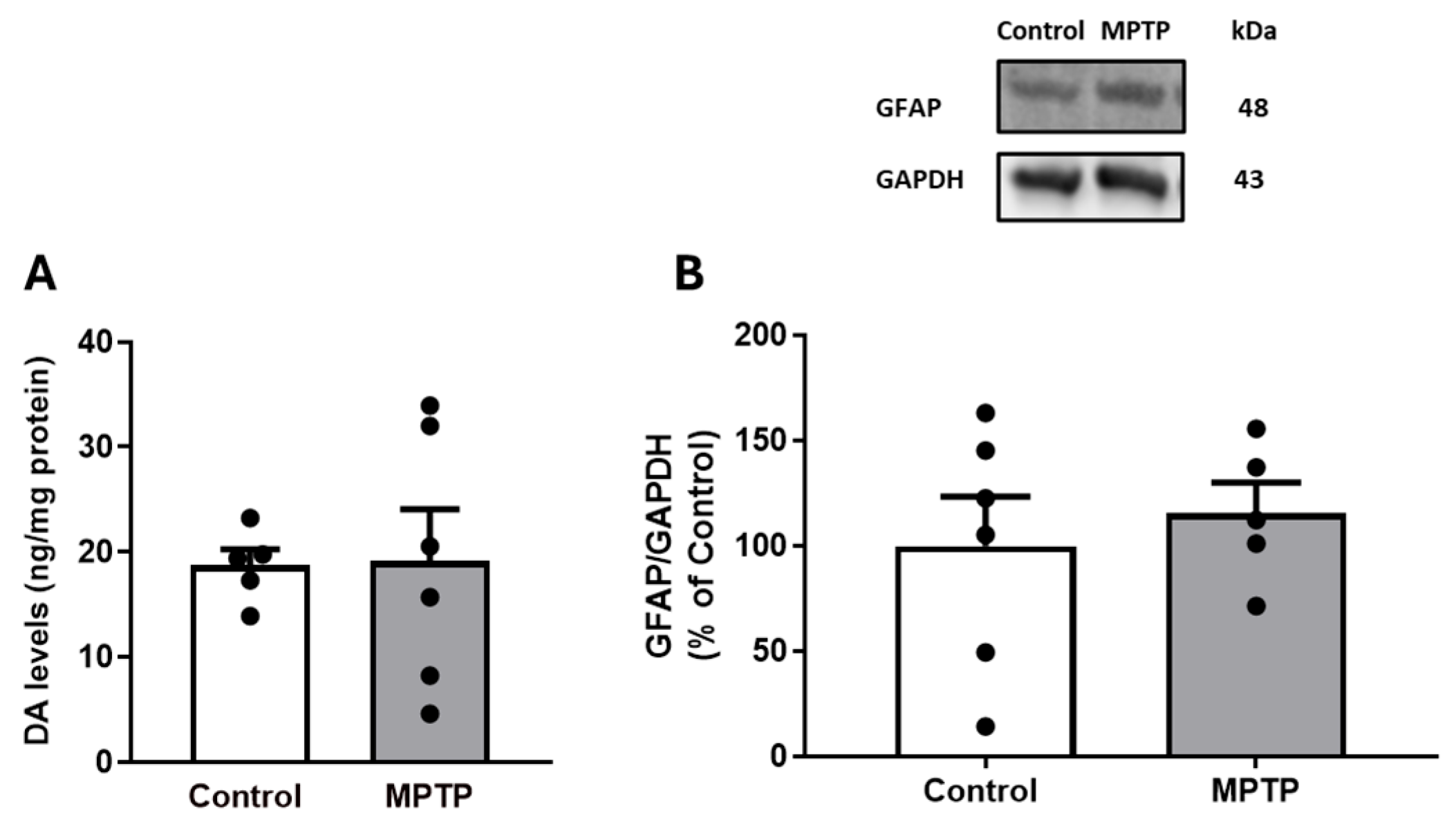
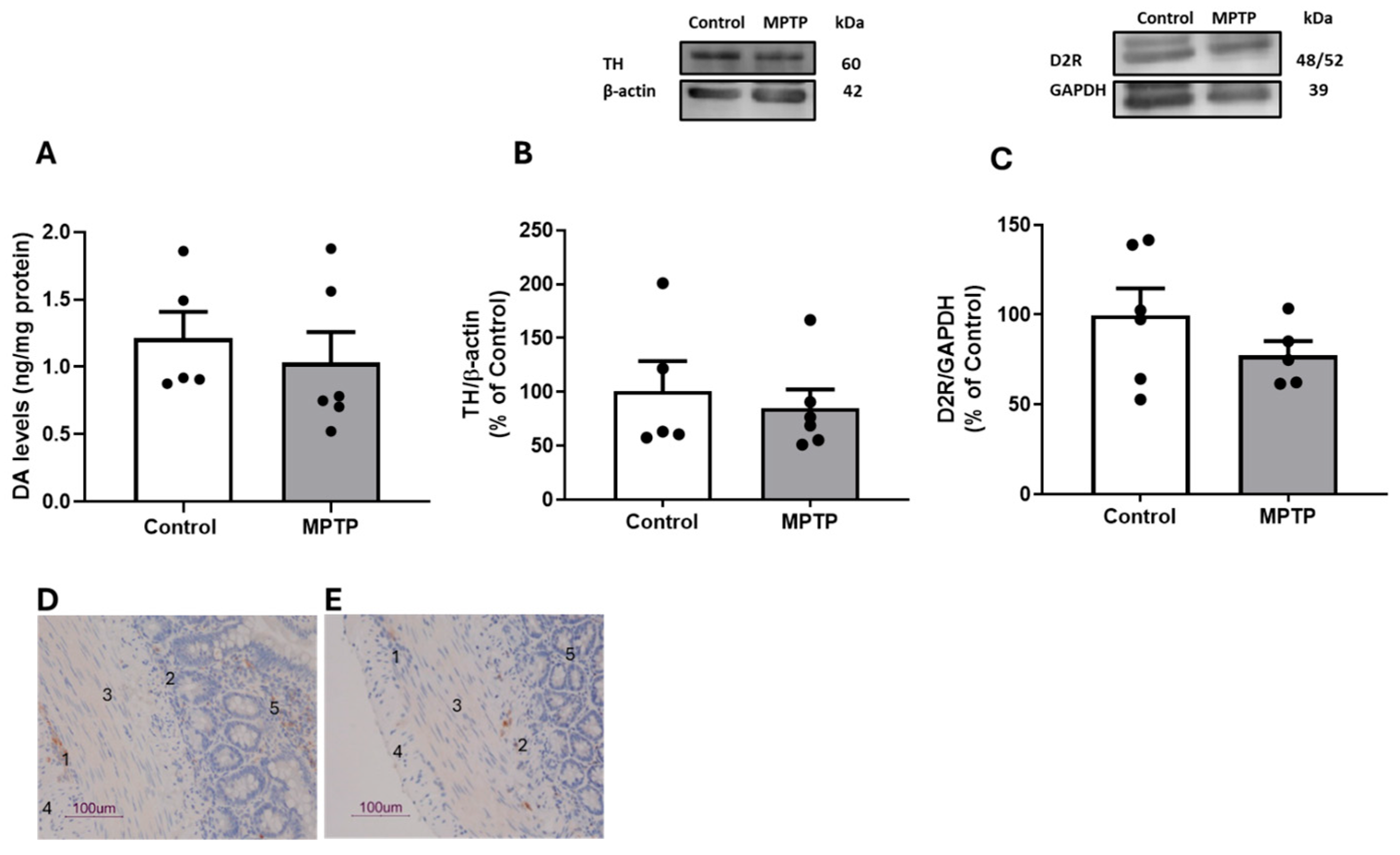
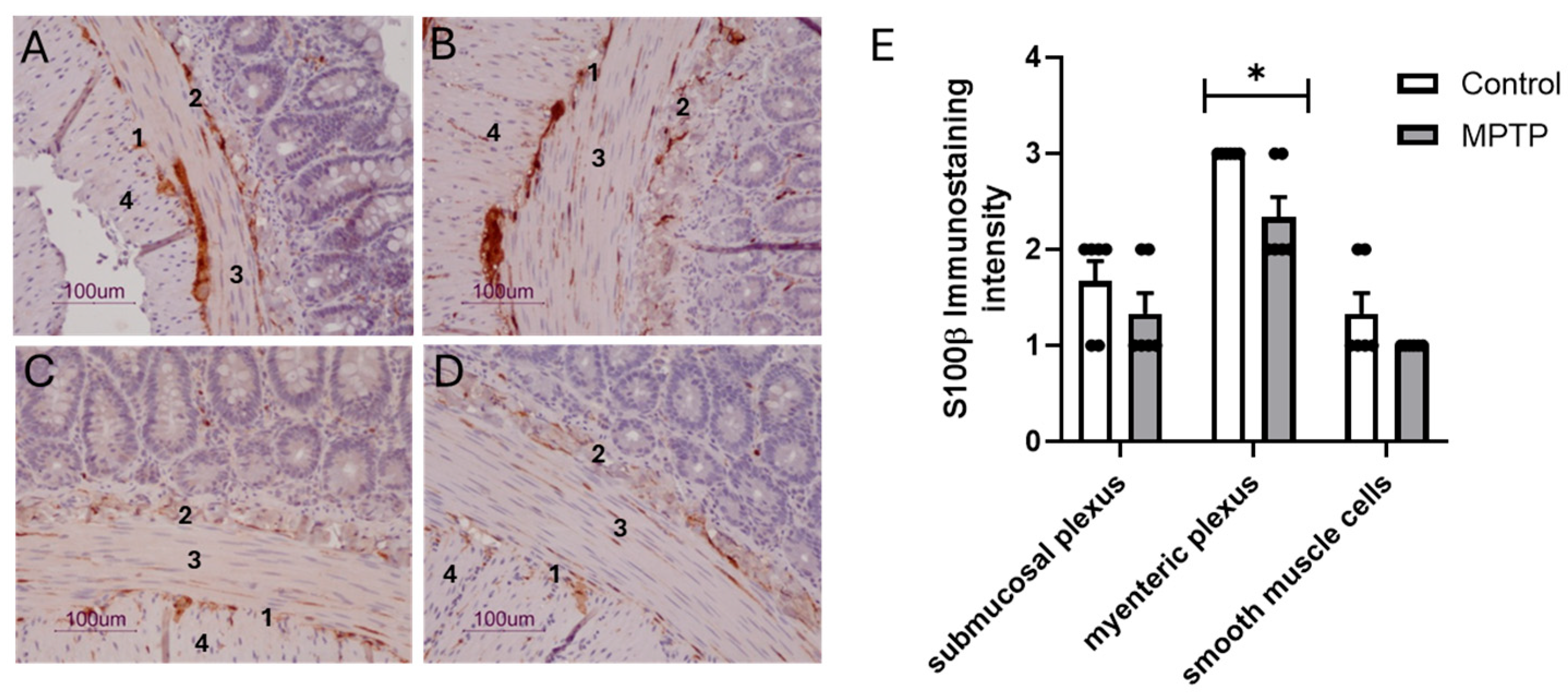
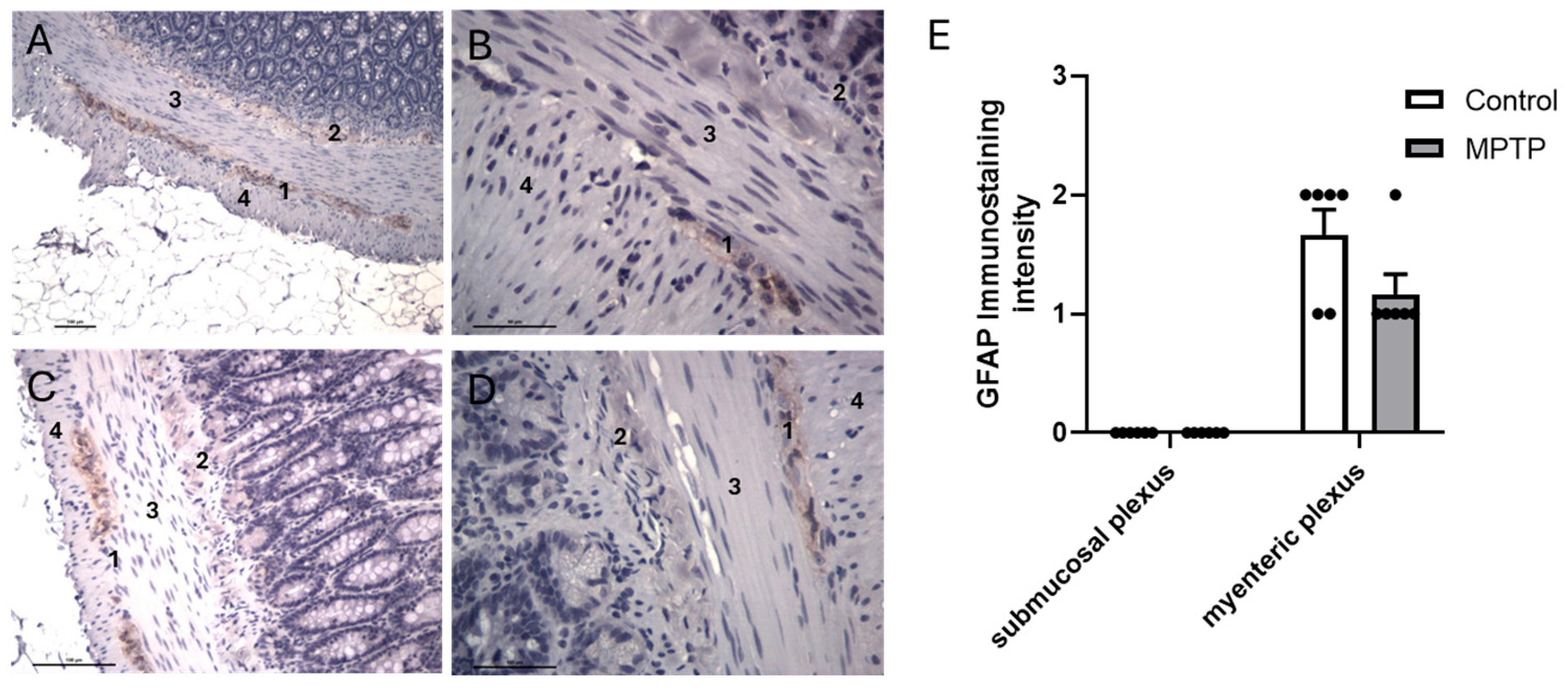
3.2. Dopaminergic and Gliosis Markers in Ilea of Rats Treated with Intranasal MPTP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forno, L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 259–272. [Google Scholar] [PubMed]
- Tanner, C.M.; Ostrem, J.L. Parkinson’s Disease. N. Engl. J. Med. 2024, 391, 442–452. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P. Quantitation of non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. S2), 2–7. [Google Scholar]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar]
- Hawkes, C.H. The prodromal phase of sporadic Parkinson’s disease: Does it exist and if so how long is it? Mov. Disord. 2008, 23, 1799–1807. [Google Scholar]
- Mizuno, Y.; Hattori, N.; Kubo, S.; Sato, S.; Nishioka, K.; Hatano, T.; Tomiyama, H.; Funayama, M.; Machida, Y.; Mochizuki, H. Progress in the pathogenesis and genetics of Parkinson’s disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 2215–2227. [Google Scholar]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Goetz, C.G.; Marin, C.; Kordower, J.H.; Rodriguez, M.; Hirsch, E.C.; Farrer, M.; Schapira, A.H.V.; Halliday, G. Missing pieces in the Parkinson’s disease puzzle. Nat. Med. 2010, 16, 653–661. [Google Scholar] [PubMed]
- Jost, W.H. Gastrointestinal motility problems in patients with Parkinson’s disease. Effects of antiparkinsonian treatment and guidelines for management. Drugs Aging. 1997, 10, 249–258. [Google Scholar]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Clin. Neurosci. 1998, 5, 136–146. [Google Scholar] [PubMed]
- Pasricha, T.S.; Guerrero-Lopez, I.L.; Kuo, B. Management of Gastrointestinal Symptoms in Parkinson’s Disease: A Comprehensive Review of Clinical Presentation, Workup, and Treatment. J. Clin. Gastroenterol. 2024, 58, 211–220. [Google Scholar] [PubMed]
- Warnecke, T.; Schafer, K.H.; Claus, I.; Del Tredici, K.; Jost, W.H. Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. npj Park. Dis. 2022, 8, 31. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003, 2, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Park. Relat. Disord. 2011, 17, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H. Gastrointestinal dysfunction in Parkinson’s Disease. J. Neurol. Sci. 2010, 289, 69–73. [Google Scholar] [CrossRef]
- Safarpour, D.; Stover, N.; Shprecher, D.R.; Hamedani, A.G.; Pfeiffer, R.F.; Parkman, H.P.; Quigley, E.M.; Cloud, L.J.; Other Non-motor Features Working Group; Parkinson Study Group. Consensus practice recommendations for management of gastrointestinal dysfunction in Parkinson disease. Park. Relat. Disord. 2024, 124, 106982. [Google Scholar] [CrossRef]
- Chalazonitis, A.; Rao, M.; Sulzer, D. Similarities and differences between nigral and enteric dopaminergic neurons unravel distinctive involvement in Parkinson’s disease. npj Park. Dis. 2022, 8, 50. [Google Scholar] [CrossRef]
- Li, Z.S.; Schmauss, C.; Cuenca, A.; Ratcliffe, E.; Gershon, M.D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: Analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 2006, 26, 2798–2807. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Bellanca, A.; Amato, A.; Serio, R. Opposite effects of dopamine on the mechanical activity of circular and longitudinal muscle of human colon. Neurogastroenterol. Motil. 2020, 32, e13811. [Google Scholar] [CrossRef] [PubMed]
- Singaram, C.; Ashraf, W.; Gaumnitz, E.A.; Torbey, C.; Sengupta, A.; Pfeiffer, R.; Quigley, E.M. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995, 346, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Annerino, D.M.; Arshad, S.; Taylor, G.M.; Adler, C.H.; Beach, T.G.; Greene, J.G. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012, 124, 665–680. [Google Scholar] [CrossRef]
- Lebouvier, T.; Chaumette, T.; Damier, P.; Coron, E.; Touchefeu, Y.; Vrignaud, S.; Naveilhan, P.; Galmiche, J.-P.; Varannes, S.B.D.; Derkinderen, P.; et al. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 2008, 57, 1741–1743. [Google Scholar] [CrossRef]
- Lebouvier, T.; Coron, E.; Chaumette, T.; Paillusson, S.; Bruley des Varannes, S.; Neunlist, M.; Derkinderen, P. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol. Motil. 2010, 22, e11–e14. [Google Scholar] [PubMed]
- Corbille, A.G.; Coron, E.; Neunlist, M.; Derkinderen, P.; Lebouvier, T. Appraisal of the dopaminergic and noradrenergic innervation of the submucosal plexus in PD. J. Park. Dis. 2014, 4, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Hindeya Gebreyesus, H.; Gebrehiwot Gebremichael, T. The Potential Role of Astrocytes in Parkinson’s Disease (PD). Med. Sci. 2020, 8, 7. [Google Scholar] [CrossRef]
- Sathe, K.; Maetzler, W.; Lang, J.D.; Mounsey, R.B.; Fleckenstein, C.; Martin, H.L.; Schulte, C.; Mustafa, S.; Synofzik, M.; Vukovic, Z.; et al. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain 2012, 135 Pt 11, 3336–3347. [Google Scholar] [CrossRef]
- Gschmack, E.; Monoranu, C.M.; Marouf, H.; Meyer, S.; Lessel, L.; Idris, R.; Berg, D.; Maetzler, W.; Steigerwald, F.; Volkmann, J.; et al. Plasma autoantibodies to glial fibrillary acidic protein (GFAP) react with brain areas according to Braak staging of Parkinson’s disease. J. Neural Transm. 2022, 129, 545–555. [Google Scholar] [CrossRef]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef]
- Lin, J.; Ou, R.; Li, C.; Hou, Y.; Zhang, L.; Wei, Q.; Pang, D.; Liu, K.; Jiang, Q.; Yang, T.; et al. Plasma glial fibrillary acidic protein as a biomarker of disease progression in Parkinson’s disease: A prospective cohort study. BMC Med. 2023, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Des Varannes, S.B.; Naveilhan, P.; Nguyen, J.M. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar]
- Hajjeh, O.; Rajab, I.; Bdair, M.; Saife, S.; Zahran, A.; Nazzal, I.; AbuZahra, M.I.; Jallad, H.; Abukhalil, M.M.; Hallak, M.; et al. Enteric nervous system dysfunction as a driver of central nervous system disorders: The Forgotten brain in neurological disease. Neuroscience 2025, 572, 232–247. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, T.; Xu, C.; Liang, X.; Li, S.; Sun, Y.; Liu, F.; Wang, J.; Tang, Y. Disease progression in proposed brain-first and body-first Parkinson’s disease subtypes. npj Park. Dis. 2024, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Bloem, B.R. Parkinson’s Disease Is Predominantly an Environmental Disease. J. Park. Dis. 2024, 14, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef]
- Prediger, R.D.; Aguiar, A.S., Jr.; Moreira, E.L.; Matheus, F.C.; Castro, A.A.; Walz, R.; De Bem, A.F.; Latini, A.; Tasca, C.I.; Farina, M.; et al. The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): A new rodent model to test palliative and neuroprotective agents for Parkinson’s disease. Curr. Pharm. Des. 2011, 17, 489–507. [Google Scholar] [CrossRef]
- Prediger, R.D.; Aguiar, A.S., Jr.; Matheus, F.C.; Walz, R.; Antoury, L.; Raisman-Vozari, R.; Doty, R.L. Intranasal administration of neurotoxicants in animals: Support for the olfactory vector hypothesis of Parkinson’s disease. Neurotox. Res. 2012, 21, 90–116. [Google Scholar] [CrossRef]
- Fattinger, K.; Benowitz, N.L.; Jones, R.T.; Verotta, D. Nasal mucosal versus gastrointestinal absorption of nasally administered cocaine. Eur. J. Clin. Pharmacol. 2000, 56, 305–310. [Google Scholar] [CrossRef]
- Prediger, R.D.; Batista, L.C.; Medeiros, R.; Pandolfo, P.; Florio, J.C.; Takahashi, R.N. The risk is in the air: Intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Exp. Neurol. 2006, 202, 391–403. [Google Scholar] [PubMed]
- Spiller, R.C. Intestinal absorptive function. Gut 1994, 35 (Suppl. S1), S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Coupar, I.M. Evidence for functional alpha 2D-adrenoceptors in the rat intestine. Br. J. Pharmacol. 1996, 117, 787–792. [Google Scholar]
- Silva, S.A.; Ribeiro, C.A. Tachyphylaxis to the sumatriptan-induced contractile effect in the human uterine artery but not in human cerebral blood vessels: Pharmacological demonstration of the 5-HT(1B) receptor functionality loss. Pharmacology 2012, 89, 29–36. [Google Scholar]
- Caley, C.F.; Weber, S.S. Sulpiride: An antipsychotic with selective dopaminergic antagonist properties. Ann. Pharmacother. 1995, 29, 152–160. [Google Scholar] [CrossRef]
- Pereira, F.C.; Gough, B.; Macedo, T.R.; Ribeiro, C.F.; Ali, S.F.; Binienda, Z.K. Buprenorphine modulates methamphetamine-induced dopamine dynamics in the rat caudate nucleus. Neurotox. Res. 2011, 19, 94–101. [Google Scholar] [PubMed]
- Anderson, G.; Noorian, A.R.; Taylor, G.; Anitha, M.; Bernhard, D.; Srinivasan, S.; Greene, J.G. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2007, 207, 4–12. [Google Scholar] [CrossRef]
- Cote, M.; Poirier, A.A.; Aube, B.; Jobin, C.; Lacroix, S.; Soulet, D. Partial depletion of the proinflammatory monocyte population is neuroprotective in the myenteric plexus but not in the basal ganglia in a MPTP mouse model of Parkinson’s disease. Brain Behav. Immun. 2015, 46, 154–167. [Google Scholar]
- Choi, J.G.; Huh, E.; Ju, I.G.; Kim, N.; Yun, J.; Oh, M.S. 1-Methyl-4-phenyl-1,2,3,6 tetrahydropyridine/probenecid impairs intestinal motility and olfaction in the early stages of Parkinson’s disease in mice. J. Neurol. Sci. 2018, 392, 77–82. [Google Scholar]
- Castro, A.A.; Wiemes, B.P.; Matheus, F.C.; Lapa, F.R.; Viola, G.G.; Santos, A.R.; Tasca, C.I.; Prediger, R.D. Atorvastatin improves cognitive, emotional and motor impairments induced by intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration in rats, an experimental model of Parkinson’s disease. Brain Res. 2013, 1513, 103–116. [Google Scholar]
- Savall, A.S.P.; De Mello, J.D.; Fidelis, E.M.; Bortolotto, V.C.; Dahleh, M.M.M.; Guerra, G.P.; Prigol, M.; Puntel, R.; Boldori, J.R.; Denardin, C.C.; et al. Eugenia uniflora Effects on the Depressive-like Behavior of MPTP-Exposed Female Rats: Apoptosis and alpha-Synuclein Modulation. Brain Sci. 2025, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.S.; Queiroz, L.Y.; Canever, J.B.; Griebner, G.; Stahler, C.U.; Mansur, D.S.; Prediger, R.D.S.; Cimarosti, H.I. SENP3 knockdown improves motor and cognitive impairments in the intranasal MPTP rodent model of Parkinson’s disease. Physiol. Behav. 2025, 288, 114725. [Google Scholar] [CrossRef] [PubMed]
- Viana, S.D.; Pita, I.R.; Lemos, C.; Rial, D.; Couceiro, P.; Rodrigues-Santos, P.; Caramelo, F.; Carvalho, F.; Ali, S.F.; Prediger, R.D.; et al. The effects of physical exercise on nonmotor symptoms and on neuroimmune RAGE network in experimental parkinsonism. J. Appl. Physiol. (1985) 2017, 123, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Mannino, D.; Cucinotta, L.; Calapai, F.; Crupi, L.; Paterniti, I.; Esposito, E. Rebalance of mitophagy by inhibiting LRRK2 improves colon alterations in an MPTP in vivo model. iScience 2024, 27, 110980. [Google Scholar] [CrossRef]
- Travagli, R.A.; Browning, K.N.; Camilleri, M. Parkinson disease and the gut: New insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 673–685. [Google Scholar] [CrossRef]
- Kirschstein, T.; Dammann, F.; Klostermann, J.; Rehberg, M.; Tokay, T.; Schubert, R.; Kohling, R. Dopamine induces contraction in the proximal, but relaxation in the distal rat isolated small intestine. Neurosci. Lett. 2009, 465, 21–26. [Google Scholar] [CrossRef]
- Nezami, B.G.; Srinivasan, S. Enteric nervous system in the small intestine: Pathophysiology and clinical implications. Curr. Gastroenterol. Rep. 2010, 12, 358–365. [Google Scholar] [CrossRef]
- Johnson, L.R. Gastrointestinal Physiology, 9th ed.; Mosby Physiology Series; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Donowitz, M.; Cusolito, S.; Battisti, L.; Fogel, R.; Sharp, G.W. Dopamine stimulation of active Na and Cl absorption in rabbit ileum: Interaction with alpha 2-adrenergic and specific dopamine receptors. J. Clin. Investig. 1982, 69, 1008–1016. [Google Scholar] [CrossRef]
- Fleming, M.A., II; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef]
- Costa, M.; Spencer, N.J.; Brookes, S.J.H. The role of enteric inhibitory neurons in intestinal motility. Auton. Neurosci. 2021, 235, 102854. [Google Scholar] [CrossRef]
- Colucci, M.; Cervio, M.; Faniglione, M.; De Angelis, S.; Pajoro, M.; Levandis, G.; Tassorelli, C.; Blandini, F.; Feletti, F.; De Giorgio, R.; et al. Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton. Neurosci. 2012, 169, 77–86. [Google Scholar]
- Natale, G.; Kastsiushenka, O.; Fulceri, F.; Ruggieri, S.; Paparelli, A.; Fornai, F. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res. 2010, 1355, 195–206. [Google Scholar]
- Toy, W.A.; Petzinger, G.M.; Leyshon, B.J.; Akopian, G.K.; Walsh, J.P.; Hoffman, M.V.; Vučković, M.G.; Jakowec, M.W. Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol. Dis. 2014, 63, 201–209. [Google Scholar] [PubMed]
- Li, Z.S.; Pham, T.D.; Tamir, H.; Chen, J.J.; Gershon, M.D. Enteric dopaminergic neurons: Definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 2004, 24, 1330–1339. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, C.; Weng, M.; Zhang, Z.; Zhang, L.; Jiang, X.; Yue, F. Intestinal alterations of mucosal barrier integrity, motility and enteric nerve in cynomolgus monkey model of Parkinson’s disease. Exp. Neurol. 2025, 389, 115256. [Google Scholar] [PubMed]
- Xie, W.; Gao, J.; Jiang, R.; Liu, X.; Lai, F.; Tang, Y.; Xiao, H.; Jia, Y.; Bai, Q. Twice subacute MPTP administrations induced time-dependent dopaminergic neurodegeneration and inflammation in midbrain and ileum, as well as gut microbiota disorders in PD mice. Neurotoxicology 2020, 76, 200–212. [Google Scholar]
- Perry, V.H. Innate inflammation in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009373. [Google Scholar]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar]
- Youn, W.; Yun, M.; Lee, C.J.; Scholl, M. Cautions on utilizing plasma GFAP level as a biomarker for reactive astrocytes in neurodegenerative diseases. Mol. Neurodegener. 2025, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Smeyne, R.J.; Jackson-Lewis, V. The MPTP model of Parkinson’s disease. Brain Res. Mol. Brain Res. 2005, 134, 57–66. [Google Scholar]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Emerging role of S100B protein implication in Parkinson’s disease pathogenesis. Cell Mol. Life Sci. 2021, 78, 1445–1453. [Google Scholar]
- Rao, M.; Nelms, B.D.; Dong, L.; Salinas-Rios, V.; Rutlin, M.; Gershon, M.D.; Corfas, G. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 2015, 63, 2040–2057. [Google Scholar] [CrossRef]
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241. [Google Scholar]
- Bellini, G.; Benvenuti, L.; Ippolito, C.; Frosini, D.; Segnani, C.; Rettura, F.; Pancetti, A.; Bertani, L.; D’ANtongiovanni, V.; Palermo, G.; et al. Intestinal histomorphological and molecular alterations in patients with Parkinson’s disease. Eur. J. Neurol. 2023, 30, 3440–3450. [Google Scholar]
- Emmi, A.; Sandre, M.; Russo, F.P.; Tombesi, G.; Garri, F.; Campagnolo, M.; Carecchio, M.; Biundo, R.; Spolverato, G.; Macchi, V.; et al. Duodenal alpha-Synuclein Pathology and Enteric Gliosis in Advanced Parkinson’s Disease. Mov. Disord. 2023, 38, 885–894. [Google Scholar] [PubMed]
- Heng, Y.; Li, Y.Y.; Wen, L.; Yan, J.Q.; Chen, N.H.; Yuan, Y.H. Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse. Molecules 2022, 27, 7414. [Google Scholar] [CrossRef] [PubMed]
- Jarras, H.; Bourque, M.; Poirier, A.A.; Morissette, M.; Coulombe, K.; Di Paolo, T.; Soulet, D. Neuroprotection and immunomodulation of progesterone in the gut of a mouse model of Parkinson’s disease. J. Neuroendocrinol. 2020, 32, e12782. [Google Scholar] [PubMed]
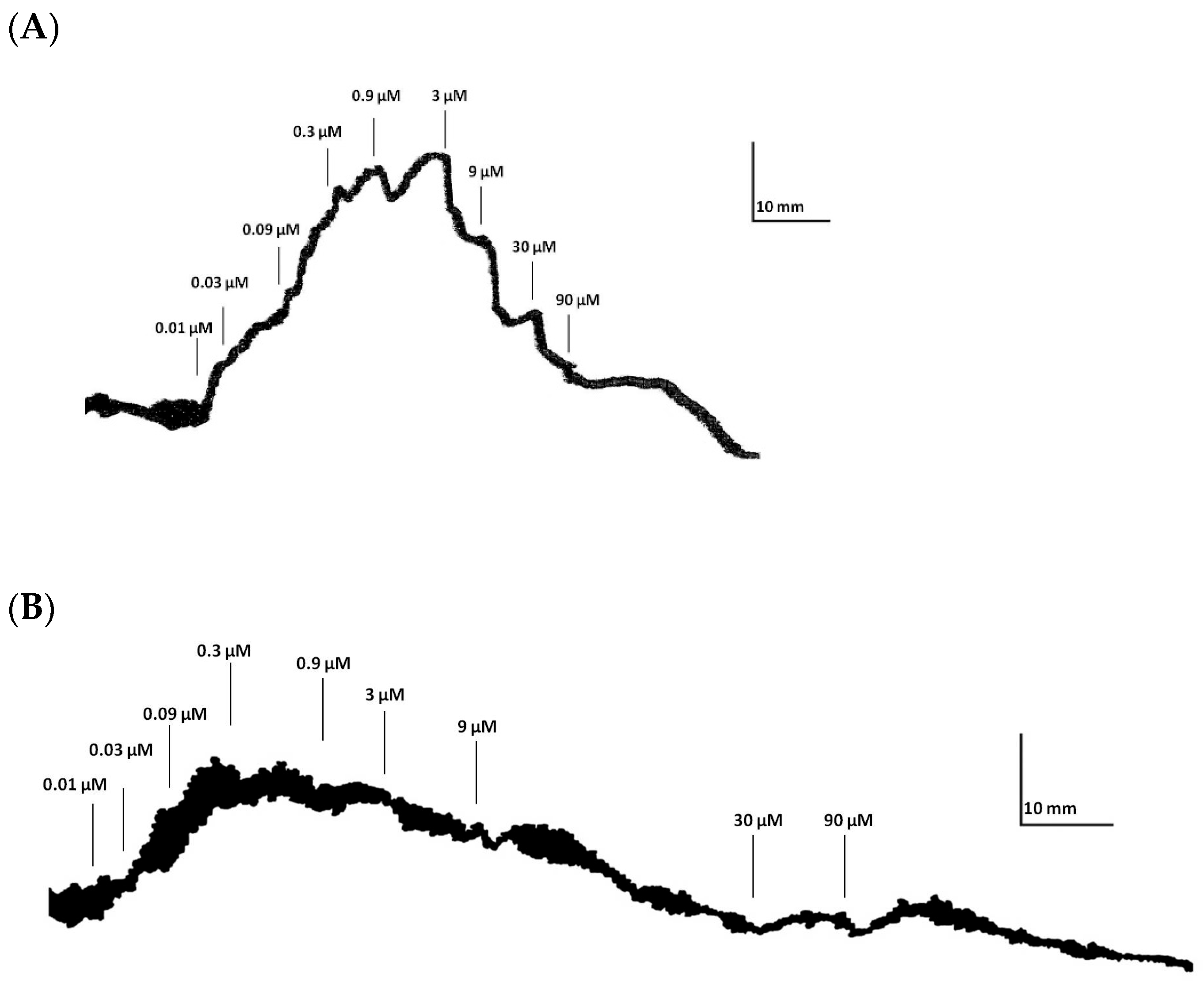

| Control | Control + Sulpiride | MPTP | MPTP + Sulpiride | |
|---|---|---|---|---|
| Emax | 46.44 ± 6.57 | 18.25 ± 5.23 * | 22.55 ± 4.35 * | 19.05 ± 5.25 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Viana, S.; Pita, I.; Lemos, C.; Matheus, F.C.; Carvalho, L.; Fontes Ribeiro, C.A.; Prediger, R.D.; Pereira, F.C.; Silva, S. Intranasal 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Administration Hampered Contractile Response of Dopamine in Isolated Rat Ileum. Biomedicines 2025, 13, 2400. https://doi.org/10.3390/biomedicines13102400
Silva A, Viana S, Pita I, Lemos C, Matheus FC, Carvalho L, Fontes Ribeiro CA, Prediger RD, Pereira FC, Silva S. Intranasal 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Administration Hampered Contractile Response of Dopamine in Isolated Rat Ileum. Biomedicines. 2025; 13(10):2400. https://doi.org/10.3390/biomedicines13102400
Chicago/Turabian StyleSilva, Ana, Sofia Viana, Inês Pita, Cristina Lemos, Filipe C. Matheus, Lina Carvalho, Carlos A. Fontes Ribeiro, Rui D. Prediger, Frederico C. Pereira, and Sónia Silva. 2025. "Intranasal 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Administration Hampered Contractile Response of Dopamine in Isolated Rat Ileum" Biomedicines 13, no. 10: 2400. https://doi.org/10.3390/biomedicines13102400
APA StyleSilva, A., Viana, S., Pita, I., Lemos, C., Matheus, F. C., Carvalho, L., Fontes Ribeiro, C. A., Prediger, R. D., Pereira, F. C., & Silva, S. (2025). Intranasal 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Administration Hampered Contractile Response of Dopamine in Isolated Rat Ileum. Biomedicines, 13(10), 2400. https://doi.org/10.3390/biomedicines13102400






