Microbiota Modulation as an Approach to Prevent the Use of Antimicrobials Associated with Canine Atopic Dermatitis
Abstract
1. Introduction
2. The Gastrointestinal Tract and Host Immune System
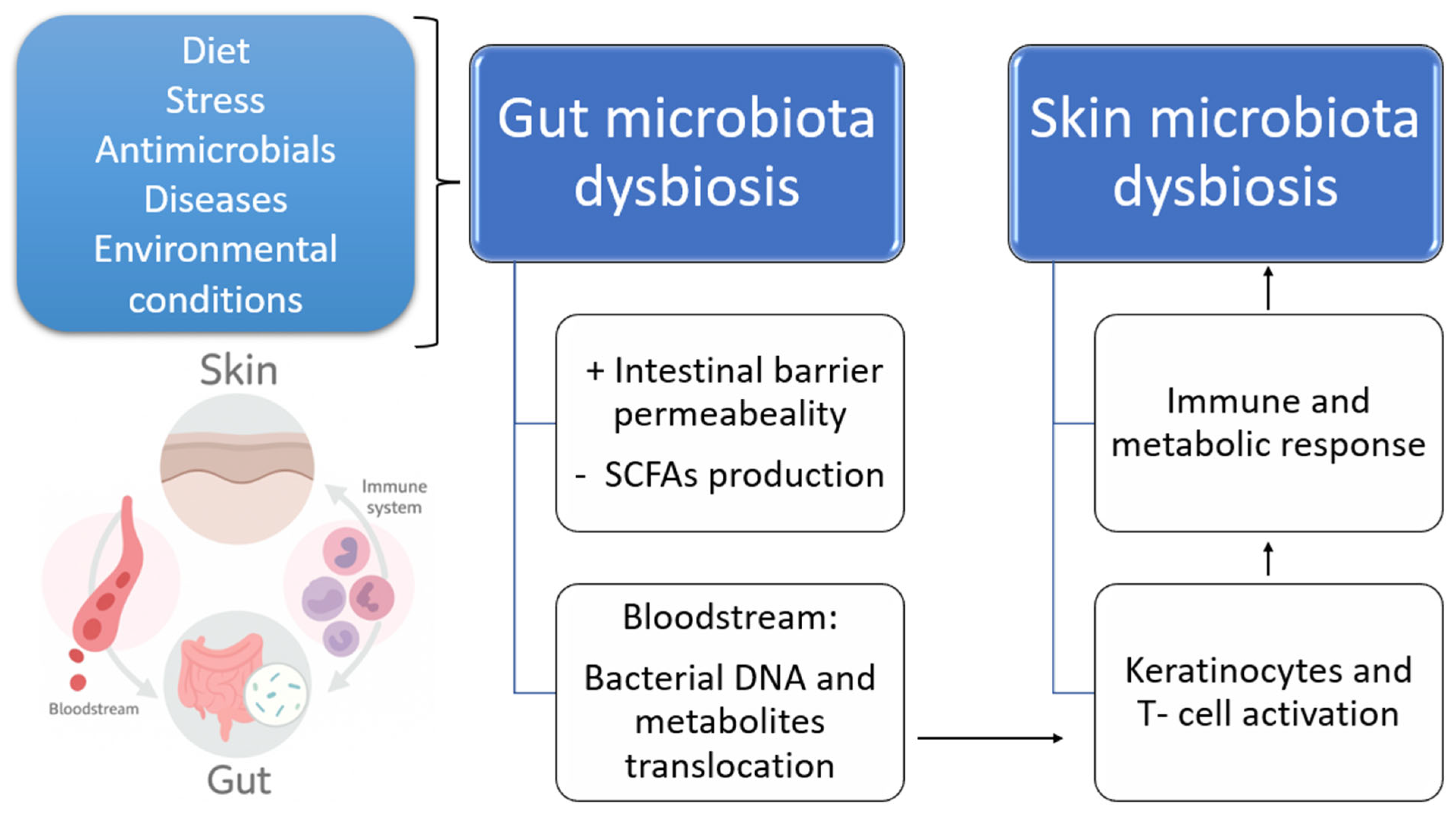
2.1. The Intestinal Microbiota and Dysbiosis
2.2. The Role of Intestinal Microbiota in Atopic Dermatitis and Other Skin Conditions
| Patient | N | Site | Microbiota | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| AD | Healthy | ||||||||
| Diversity | Increased | Decreased | Diversity | Increased | Decreased | ||||
| Adult Beagle dog | 7 | Gut | − | Conchiformibius, Catenibacterium spp. Ruminococcus gnavus group Megamonas | Lachnospira Ruminococcus torques group Anaerovocacaceae (family) Peptostreptococcaceae/Peptoclostridium UCG—005 (Oscillospiraceae) Sutterella Fusobacterium | + | Lachnospiraceae Anaerovoracaceae Oscillospiraceae genera Lachnospira Ruminococcus torques group Fusobacterium Fecalibacterium | [9] | |
| Dogs | 896 | Skin | − | P. aeruginosa E. coli S. pseudintermedius | + | [45] | |||
| Shiba Inu dogs | 40 | Gut and skin | − | Skin: Staphylococcus Escherichia/Shigella Clostridium sensu stricto | Gut: Fusobacteria Megamonas | + | Gut: Fusobacteria Megamonas Bacteroidaceae family | Skin: Staphylococcus Eschrichia/Shigella Clostridium sensu stricto | [49] |
| Dogs | 62 | Gut | − | Streptococcus spp. Fusobacterium spp. E. coli C. difficile | Lactobacillus spp. | + | L. acidophilus Lactobacillus spp. | Streptococcus spp. Fusobacterium spp. E. coli C. difficile | [48] |
| Shiba Inu Dogs | 53 | Gut and oral | Oral = Gut | Oral: Proteobacteria phylum Gut: Anaerovoracaceae | Oral: Bacteroidota phylum | + | Gut: Anaerovoracaceae | [50] | |
| Human | 132 | Gut | − | Faecalibacterium prausnitzii | Lactobacillus Bifidobacterium spp. | + | F. prausnitzii | [51] | |
| Children | 139 | Gut | − | Parabacteroides | Severe AD (vs. mild): Clostridium sensu stricto Collinsella | + | [53] | ||
| Children | 62 | Gut | − | Anaerostipes Butyricicoccus Ruminococcus Lactobacillus spp. | [56] | ||||
| Children | 70 | Gut | R. broomi | R. broomi | [57] | ||||
2.3. Antimicrobial Treatment and Its Effects on the Intestinal Microbiota
2.4. Antimicrobial Resistance
3. Microbiota Manipulation
3.1. Diet
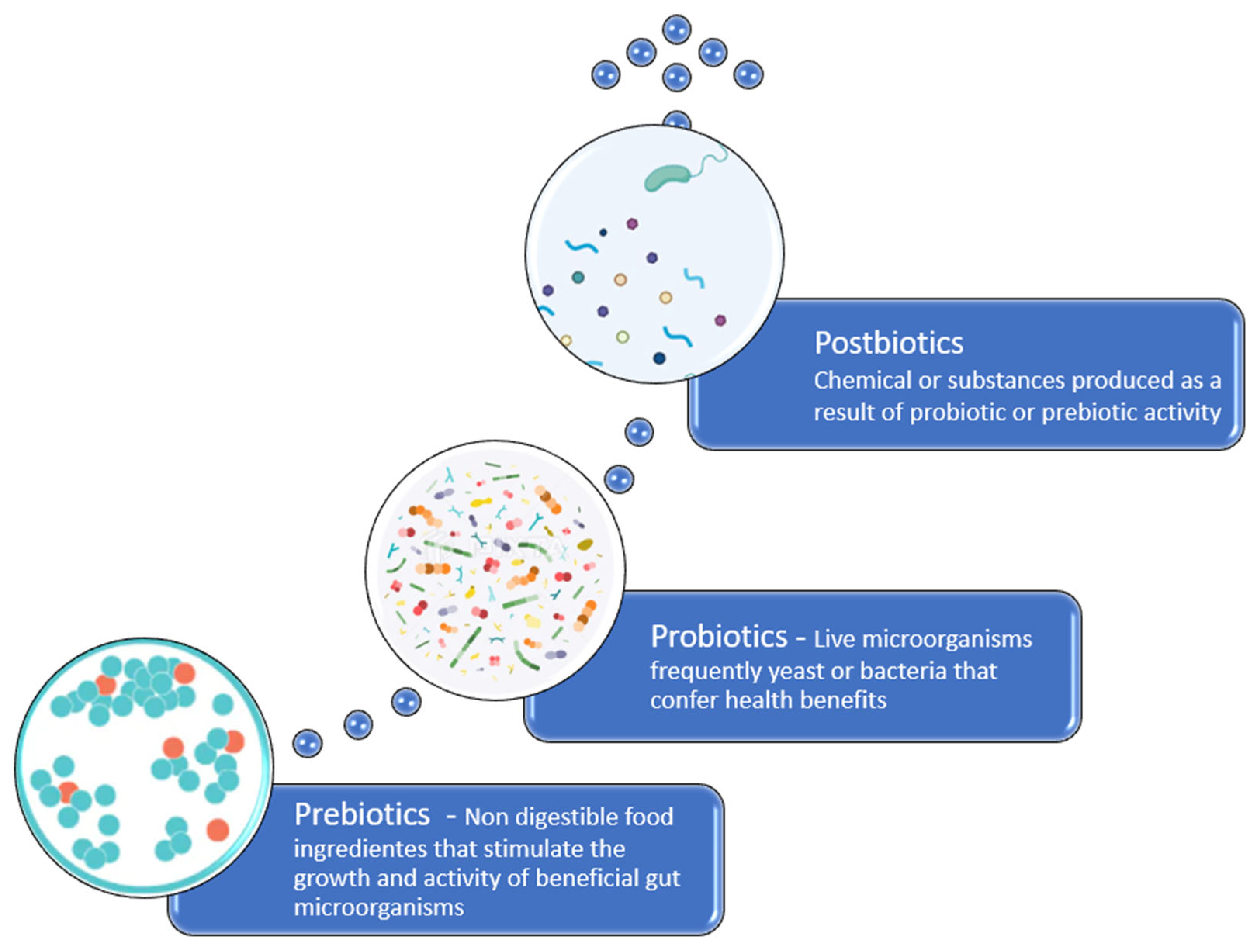
3.2. Probiotics
3.3. Prebiotics
3.4. Postbiotics
3.5. Stress Reduction
3.6. Fecal Microbial Transplantation (FMT)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junca, H.; Pieper, D.H.; Medina, E. The emerging potential of microbiome transplantation on human health interventions. Comput. Struct. Biotechnol. J. 2022, 20, 615–627. [Google Scholar] [CrossRef]
- Kamble, N.S.; Bera, S.; Bhedase, S.A.; Gaur, V.; Chowdhury, D. Review on applied applications of microbiome on human lives. Bacteria 2024, 3, 141–159. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [PubMed]
- Karahan, F. Environmental Factors Affecting the Gut Microbiota and Their Consequences. Nat. Cell Sci. 2024, 2, 133–140. [Google Scholar] [CrossRef]
- Ignacio, A.; Czyz, S.; McCoy, K.D. Early life microbiome influences on development of the mucosal innate immune system. Semin. Immunol. 2024, 73, 101885. [Google Scholar] [CrossRef] [PubMed]
- Hakanen, E.; Lehtimäki, J.; Salmela, E.; Tiira, K.; Anturaniemi, J.; Hielm-Björkman, A.; Ruokolainen, L.; Lohi, H. Urban Environment Predisposes Dogs and Their Owners to Allergic Symptoms. Sci. Rep. 2018, 8, 1585. [Google Scholar] [CrossRef]
- Lodge, C.J.; Allen, K.J.; Lowe, A.J.; Hill, D.J.; Hosking, C.S.; Abramson, M.J.; Dharmage, S.C. Perinatal cat and dog exposure and the risk of asthma and allergy in the urban environment: A systematic review of longitudinal studies. Clin. Dev. Immunol. 2012, 2012, 176484. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the gut–skin axis in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Rostaher, A.; Morsy, Y.; Favrot, C.; Unterer, S.; Schnyder, M.; Scharl, M.; Fischer, N.M. Comparison of the Gut Microbiome between Atopic and Healthy Dogs—Preliminary Data. Animals 2022, 12, 2377. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Zhai, S.; Tang, X.; Liu, C.; Li, W. Gut Microbiota and Atopic Dermatitis in Children: A Scoping Review. BMC Pediatr. 2022, 22, 323. [Google Scholar] [CrossRef]
- Eisenschenk, M.C.; Hensel, P.; Saridomichelakis, M.N.; Tamamoto-Mochizuki, C.; Pucheu-Haston, C.M.; Santoro, D. Introduction to the ICADA 2023 canine atopic dermatitis pathogenesis review articles and updated definition. Vet. Dermatol. 2024, 35, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef]
- Drechsler, Y.; Dong, C.; Clark, D.; Kaur, G. Canine Atopic Dermatitis: Prevalence, Impact, and Management Strategies. Vet. Med. Res. Rep. 2024, 15, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.M. Atopic dermatitis and the intestinal microbiota in humans and dogs. Vet. Med. Sci. 2016, 2, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lathakumari, R.H.; Vajravelu, L.K.; Satheesan, A.; Ravi, S.; Thulukanam, J. Antibiotics and the gut microbiome: Understanding the impact on human health. Med. Microecol. 2024, 20, 100106. [Google Scholar] [CrossRef]
- Munteanu, C.; Turti, S.; Marza, S.M. Unraveling the Gut–Skin Axis: The Role of Microbiota in Skin Health and Disease. Cosmetics 2025, 12, 167. [Google Scholar] [CrossRef]
- Colombino, E.; Prieto-Botella, D.; Capucchio, M.T. Gut Health in Veterinary Medicine: A Bibliometric Analysis of the Literature. Animals 2021, 11, 1997. [Google Scholar] [CrossRef]
- Raya Tonetti, F.; Eguileor, A.; Llorente, C. Goblet Cells: Guardians of Gut Immunity and Their Role in Gastrointestinal Diseases. eGastroenterology 2024, 2, e100098. [Google Scholar] [CrossRef]
- Goto, Y. Epithelial cells as a transmitter of signals from commensal bacteria and host immune cells. Front. Immunol. 2019, 10, 2057. [Google Scholar] [CrossRef]
- Wang, J.; He, M.; Yang, M.; Ai, X. Gut microbiota as a key regulator of intestinal mucosal immunity. Life Sci. 2024, 345, 122612. [Google Scholar] [CrossRef]
- Ruigrok, R.A.A.A.; Weersma, R.K.; Vich Vila, A. The emerging role of the small intestinal microbiota in human health and disease. Gut Microbes 2023, 15, 2201155. [Google Scholar] [CrossRef]
- Bemark, M.A.A.A.; Pitcher, M.J.; Dionisi, C.; Spencer, J. Gut-associated lymphoid tissue: A microbiota-driven hub of B cell immunity. Trends Immunol. 2024, 45, 211–223. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Burelbach, K.R.; McBride, M.A.; Stothers, C.L.; Owen, A.M.; Hernandez, A.; Patil, N.K.; Williams, D.L.; Bohannon, J.K. Innate Immune Memory and the Host Response to Infection. J. Immunol. 2022, 208, 785–792. [Google Scholar] [CrossRef]
- Negi, S.; Das, D.K.; Pahari, S.; Nadeem, S.; Agrewala, J.N. Potential role of gut microbiota in induction and regulation of innate immune memory. Front. Immunol. 2019, 10, 2441. [Google Scholar] [CrossRef]
- Łoś-Rycharska, E.; Gołębiewski, M.; Grzybowski, T.; Rogalla-Ładniak, U.; Krogulska, A. The microbiome and its impact on food allergy and atopic dermatitis in children. Postepy Dermatol. Alergol. 2020, 37, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Ekici, Y.E.; Ok, M. Investigation of the Relationship between Atopic Dermatitis of Dogs and Intestinal Epithelial Damage. Vet. Med. Sci. 2024, 10, e1453. [Google Scholar] [CrossRef] [PubMed]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019, 9, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50 (Suppl. 1), 6–17. [Google Scholar] [CrossRef]
- Ding, G.; Yang, X.; Li, Y.; Wang, Y.; Du, Y.; Wang, M.; Ye, R.; Wang, J.; Zhang, Y.; Chen, Y.; et al. Gut Microbiota Regulates Gut Homeostasis, Mucosal Immunity and Influences Immune-Related Diseases. Mol. Cell. Biochem. 2025, 480, 1969–1981. [Google Scholar] [CrossRef]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut Microbiota Promote Hematopoiesis to Control Bacterial Infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef]
- Andreescu, M. Molecular Insights Into the Role of Gut Microbiota in Antibiotic Therapy Selection and Resistance Mitigation. Cureus 2023, 15, e50318. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: A review. Front. Immunol. 2021, 12, 720393. [Google Scholar] [CrossRef] [PubMed]
- Schnupf, P.; Gaboriau-Routhiau, V.; Cerf-Bensussan, N. Modulation of the gut microbiota to improve innate resistance. Curr. Opin. Immunol. 2018, 54, 137–144. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota–gut–brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Kalakuntla, A.S.; Nalakonda, G.; Nalakonda, K.; Pidikiti, C.V.; Aasim, S.A. Probiotics and Clostridium Difficile: A Review of Dysbiosis and the Rehabilitation of Gut Microbiota. Cureus 2019, 11, e5063. [Google Scholar] [CrossRef]
- Hain-Saunders, N.M.R.; Knight, D.R.; Bruce, M.; Riley, T.V. Clostridioides difficile infection and One Health: An equine perspective. Environ. Microbiol. 2022, 24, 985–997. [Google Scholar] [CrossRef]
- Reijnders, D.; Goossens, G.H.; Hermes, G.D.A.; Neis, E.P.J.G.; van der Beek, C.M.; Most, J.; Holst, J.J.; Lenaerts, K.; Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016, 24, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Hülpüsch, C.; Rohayem, R.; Reiger, M.; Traidl-Hoffmann, C. Exploring the skin microbiome in atopic dermatitis pathogenesis and disease modification. J. Allergy Clin. Immunol. 2024, 154, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liegenfeld, S.C.; Stenzel, S.; Rembe, J.-D.; Dittmer, M.; Ramos, P.; Stuermer, E.K. Pathogenic and non-pathogenic microbes in the wound microbiome—How to flip the switch. Microbiol. Res. 2025, 16, 39. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Ma, S.; Jiao, Y.; Hong, H.; Wang, S.; Huang, W.; An, Q.; Song, Y.; Dang, X.; et al. Antimicrobial Resistance and Risk Factors of Canine Bacterial Skin Infections. Pathogens 2025, 14, 309. [Google Scholar] [CrossRef]
- Lee, E.; Lee, S.Y.; Kang, M.J.; Kim, K.; Won, S.; Kim, B.J.; Choi, K.Y.; Kim, B.S.; Cho, H.J.; Kim, Y.; et al. Clostridia in the Gut and Onset of Atopic Dermatitis via Eosinophilic Inflammation. Ann. Allergy Asthma Immunol. 2016, 117, 91–92.e1. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Brindisi, G.; Martinelli, I.; Bonucci, E.; D’Orsi, M.; Ialongo, S.; Nyffenegger, A.; Raso, T.; Spatuzzo, M.; De Castro, G.; et al. Probiotics function in preventing atopic dermatitis in children. Int. J. Mol. Sci. 2022, 23, 5409. [Google Scholar] [CrossRef]
- Swain, S.; Sahoo, P.; Biswal, S.; Sethy, K.; Panda, A.N.; Sahoo, N. Fecal Bacterial Microbiota Diversity Characterized for Dogs with Atopic Dermatitis: Its Alteration and Clinical Recovery after Meat-Exclusion Diet. Am. J. Vet. Res. 2025, 86, ajvr.24.09.0274. [Google Scholar] [CrossRef]
- Thomsen, M.; Künstner, A.; Wohlers, I.; Olbrich, M.; Lenfers, T.; Osumi, T.; Shimazaki, Y.; Nishifuji, K.; Ibrahim, S.M.; Watson, A.; et al. A Comprehensive Analysis of Gut and Skin Microbiota in Canine Atopic Dermatitis in Shiba Inu Dogs. Microbiome 2023, 11, 162. [Google Scholar] [CrossRef]
- Uchiyama, J.; Osumi, T.; Mizukami, K.; Fukuyama, T.; Shima, A.; Unno, A.; Takemura-Uchiyama, I.; Une, Y.; Murakami, H.; Sakaguchi, M. Characterization of the oral and faecal microbiota associated with atopic dermatitis in dogs selected from a purebred Shiba Inu colony. Lett. Appl. Microbiol. 2022, 75, 1607–1616. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium Prausnitzii Subspecies-Level Dysbiosis in the Human Gut Microbiome Underlying Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The human gut microbiome in health and disease. Integr. Med. 2014, 13, 17–22. [Google Scholar] [PubMed]
- Liu, X.; Cai, M.; Chen, M.; Chen, J.; Zhu, T.; Wu, S.; Jia, J. Alterations in gut microbiome associated with severity of atopic dermatitis in infants. Australas. J. Dermatol. 2024, 65, 82–90. [Google Scholar] [CrossRef]
- Paixão, A.; Caldeira, J.; Leocádio, J.; Martins, L. The Importance of Skin Barrier Integrity for the Prevention of Veterinary Allergy. Rev. Port. Imunoalergol. 2022, 30, 9–20. [Google Scholar] [CrossRef]
- Penders, J.; Gerhold, K.; Stobberingh, E.E.; Thijs, C.; Zimmermann, K.; Lau, S.; Hamelmann, E. Establishment of the Intestinal Microbiota and Its Role for Atopic Dermatitis in Early Childhood. J. Allergy Clin. Immunol. 2013, 132, 601–607.e8. [Google Scholar] [CrossRef]
- Patumcharoenpol, P.; Kingkaw, A.; Nakphaichit, M.; Chatchatee, P.; Suratannon, N.; Panagiotou, G.; Vongsangnak, W. Exploring Longitudinal Gut Microbiome towards Metabolic Functional Changes Associated in Atopic Dermatitis in Early Childhood. Biology 2023, 12, 1262. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Schwab, C.; Ramirez Garcia, A.; Li, Q.; Ferstl, R.; Bersuch, E.; Akdis, C.A.; Lauener, R.; Frei, R.; Roduit, C.; et al. The Abundance of Ruminococcus Bromii Is Associated with Faecal Butyrate Levels and Atopic Dermatitis in Infancy. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 3629–3640. [Google Scholar] [CrossRef]
- Xue, Q.; Li, X.; Li, Y.; Xu, J.; Wu, Z.; Wang, J. Dialogue between Gastrointestinal Tract and Skin: New Insights into the Helicobacter Pylori and Atopic Dermatitis. Helicobacter 2021, 26, e12871. [Google Scholar] [CrossRef] [PubMed]
- Bosman, E.S.; Albert, A.Y.; Lui, H.; Dutz, J.P.; Vallance, B.A. Skin Exposure to Narrow Band Ultraviolet (Uvb) Light Modulates the Human Intestinal Microbiome. Front. Microbiol. 2019, 10, 2410. [Google Scholar] [CrossRef]
- Shinno-Hashimoto, H.; Hashimoto, Y.; Wei, Y.; Chang, L.; Fujita, Y.; Ishima, T.; Matsue, H.; Hashimoto, K. Abnormal Composition of Microbiota in the Gut and Skin of Imiquimod-Treated Mice. Sci. Rep. 2021, 11, 11267. [Google Scholar] [CrossRef] [PubMed]
- Thirion, F.; Guilly, S.; Fromentin, S.; Oñate, F.P.; Alvarez, A.S.; Le Chatelier, E.; Pons, N.; Levenez, F.; Quinquis, B.; Ehrlich, S.; et al. Changes in Gut Microbiota of Patients with Atopic Dermatitis During Balneotherapy. Clin. Cosmet. Investig. Dermatol. 2022, 15, 163–176. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The Gut-Skin Axis in Health and Disease: A Paradigm with Therapeutic Implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Feng, S.; Huo, F.; Liu, H. Effects of Four Antibiotics on the Diversity of the Intestinal Microbiota. Microbiol. Spectr. 2022, 10, e0190421. [Google Scholar] [CrossRef] [PubMed]
- Mangin, I.; Suau, A.; Gotteland, M.; Brunser, O.; Pochart, P. Amoxicillin Treatment Modifies the Composition of Bifidobacterium Species in Infant Intestinal Microbiota. Anaerobe 2010, 16, 433–438. [Google Scholar] [CrossRef]
- Buffie, C.G.; Jarchum, I.; Equinda, M.; Lipuma, L.; Gobourne, A.; Viale, A.; Ubeda, C.; Xavier, J.; Pamer, E.G. Profound Alterations of Intestinal Microbiota Following a Single Dose of Clindamycin Results in Sustained Susceptibility to Clostridium Difficile-Induced Colitis. Infect. Immun. 2012, 80, 62–73. [Google Scholar] [CrossRef]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Yang, J.H.; Bhargava, P.; McCloskey, D.; Mao, N.; Palsson, B.O.; Collins, J.J. Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host Microbe 2017, 22, 757–765.e3. [Google Scholar] [CrossRef]
- Ribeiro, C.F.A.; Silveira, G.G.D.O.S.; Cândido, E.D.S.; Cardoso, M.H.; Espínola Carvalho, C.M.; Franco, O.L. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health. ACS Infect. Dis. 2020, 6, 2544–2559. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Martinez-Garcia, E.; Xavier, J.; Durham, W.M.; Kolter, R.; Kim, W.; Foster, K.R. Biofilm Formation as a Response to Ecological Competition. PLoS Biol. 2015, 13, e1002191. [Google Scholar] [CrossRef]
- Stiefel, U.; Tima, M.A.; Nerzic, M.M. Metallo-β-Lactamase-Producing Bacteroides Species Can Shield Other Members of the Gut Microbiota from Antibiotics. Antimicrob. Agents Chemother. 2015, 59, 650–653. [Google Scholar] [CrossRef]
- Parker, J.L.; Page, A.; Jacob, O.; Stanton, V.; Davis, B.; Flythe, M.; Adam, E.N. Equine Fecal Microbiota Response to Short Term Antibiotic Administration. J. Equine Vet. Sci. 2024, 133, 104993. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Potes, J.; Queiroga, M.C.; Castro, J.L.; Pereira, A.F.; Rehman, S.; Dalgarno, K.; Ramos, A.; Vitale-Brovarone, C.; Reis, J.C. Percutaneous Vertebroplasty: A New Animal Model. Spine J. 2016, 16, 1253–1262. [Google Scholar] [CrossRef]
- Lagoa, T.; Queiroga, M.C.; Martins, L. An overview of wound dressing materials. Pharmaceuticals 2024, 17, 1110. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut–skin axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Sutanto, H.; Elisa, E.; Rachma, B.; Fetarayani, D. Gut microbiome modulation in allergy treatment: The role of fecal microbiota transplantation. Am. J. Med. 2025, 138, 769–777.e3. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Perelman, D.; Topf, M.; Fragiadakis, G.K.; Robinson, J.L.; Sonnenburg, J.L.; Gardner, C.D.; Sonnenburg, E.D. Randomized Controlled Trial Demonstrates Response to a Probiotic Intervention for Metabolic Syndrome That May Correspond to Diet. Gut Microbes 2023, 15, 2178794. [Google Scholar] [CrossRef]
- Lewis, S.; Nash, A.; Li, Q.; Ahn, T.H. Comparison of 16S and Whole Genome Dog Microbiomes Using Machine Learning. BioData Min. 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chae, Y.; Cho, J.H.; Song, M.; Kwak, J.; Doo, H.; Choi, Y.; Kang, J.; Yang, H.; Lee, S.; et al. Understanding the Diversity and Roles of the Canine Gut Microbiome. J. Anim. Sci. Biotechnol. 2025, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the Dog and Human Gut Microbiomes in Gene Content and Response to Diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism: An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Konturek, P.C.; Sliwowski, Z.; Koziel, J.; Ptak-Belowska, A.; Burnat, G.; Brzozowski, T.; Konturek, S.J. Probiotic bacteria Escherichia coli strain Nissle 1917 attenuates acute gastric lesions induced by stress. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 41–48. [Google Scholar] [PubMed]
- Shen, N.T.; Maw, A.; Tmanova, L.L.; Pino, A.; Ancy, K.; Crawford, C.V.; Simon, M.S.; Evans, A.T. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium Difficile Infection: A Systematic Review with Meta-Regression Analysis. Gastroenterology 2017, 152, 1889–1900.e9. [Google Scholar] [CrossRef]
- Kang, A.; Kwak, M.-J.; Lee, D.J.; Lee, J.J.; Kim, M.K.; Song, M.; Lee, M.; Yang, J.; Oh, S.; Kim, Y. Dietary Supplementation with Probiotics Promotes Weight Loss by Reshaping the Gut Microbiome and Energy Metabolism in Obese Dogs. Microbiol. Spectr. 2024, 12, e02552-23. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Jang, Y.J.; Park, S.J.; Min, S.G.; Kwon, H.; Jo, M.J.; Ko, G.P. Lactobacillus Acidophilus KBL409 Ameliorates Atopic Dermatitis in a Mouse Model. J. Microbiol. 2024, 62, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, V.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Sánchez-Pellicer, P.; Agüera-Santos, J.; Navarro-Moratalla, L. Probiotics in the therapeutic arsenal of dermatologists. Microorganisms 2021, 9, 1513. [Google Scholar] [CrossRef]
- Eguren, C.; Navarro-Blasco, A.; Corral-Forteza, M.; Reolid-Pérez, A.; Setó-Torrent, N.; García-Navarro, A.; Prieto-Merino, D.; Núñez-Delegido, E.; Sánchez-Pellicer, P.; Navarro-López, V. A Randomized Clinical Trial to Evaluate the Efficacy of an Oral Probiotic in Acne Vulgaris. Acta Derm. Venereol. 2024, 104, adv33206. [Google Scholar] [CrossRef]
- Bae, H.; Hwang, T.S.; Lee, H.C.; Jung, D.I.; Kim, S.H.; Yu, D.H. Successful Treatment of Canine Infective Endocarditis Caused by Bacillus amyloliquefaciens. Vet. Q. 2022, 42, 41–47. [Google Scholar] [CrossRef]
- Alves, A.C.; Martins, S.M.d.S.B.; Belo, J.V.T.; Lemos, M.V.C.; Lima, C.E.d.M.C.; Silva, C.D.d.; Zagmignan, A.; Nascimento da Silva, L.C. Global trends and scientific impact of topical probiotics in dermatological treatment and skincare. Microorganisms 2024, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Varian, B.J.; Poutahidis, T.; DiBenedictis, B.T.; Levkovich, T.; Ibrahim, Y.; Didyk, E.; Shikhman, L.; Cheung, H.K.; Hardas, A.; Ricciardi, C.E.; et al. Microbial Lysate Upregulates Host Oxytocin. Brain Behav. Immun. 2017, 61, 36–49. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Kamble, N.S.; Thomas, S.; Madaan, T.; Ehsani, N.; Sange, S.; Tucker, K.; Muhumure, A.; Kunkler, S.; Kotagiri, N. Engineered Bacteria as an Orally Administered Anti-Viral Treatment and Immunization System. Gut Microbes 2025, 17. [Google Scholar] [CrossRef]
- Jiang, W.; Ni, B.; Liu, Z.; Liu, X.; Xie, W.; Wu, I.X.Y.; Li, X. The role of probiotics in the prevention and treatment of atopic dermatitis in children: An updated systematic review and meta-analysis of randomized controlled trials. Paediatr. Drugs 2020, 22, 535–549. [Google Scholar] [CrossRef]
- Roslan, M.A.M.; Omar, M.N.; Sharif, N.A.M.; Raston, N.H.A.; Arzmi, M.H.; Neoh, H.M.; Ramzi, A.B. Recent advances in single-cell engineered live biotherapeutic products research for skin repair and disease treatment. npj Biofilms Microbiomes 2023, 9, 95. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Selle, A.; Palmer, D.J.; Prescott, S.L.; Barbarot, S.; Bodinier, M. Prebiotics: Mechanisms and preventive effects in allergy. Nutrients 2019, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The role of prebiotics in modulating gut microbiota: Implications for human health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.; Yi, S.; Kim, W.-H.; Kim, Y.; Namgoong, B.; Choe, A.; Cho, G.; Shin, J.; Park, Y.; et al. Red Ginseng Dietary Fiber Shows Prebiotic Potential by Modulating Gut Microbiota in Dogs. Microbiol. Spectr. 2023, 11, e00949-23. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Liu, Y.; Wei, F.; Li, X.; Feng, Y.; Jin, X.; Liu, D.; Guo, Y.; Hu, Y. Inulin-Enriched Megamonas funiformis Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease by Producing Propionic Acid. NPJ Biofilms Microbiomes 2023, 9, 73. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and postbiotics in skin health. Biomedicines 2025, 13, 791. [Google Scholar] [CrossRef]
- AL-Smadi, K.; Leite-Silva, V.R.; Filho, N.A.; Lopes, P.S.; Mohammed, Y. Innovative approaches for maintaining and enhancing skin health and managing skin diseases through microbiome-targeted strategies. Antibiotics 2023, 12, 1698. [Google Scholar] [CrossRef]
- Da, M.; Sun, J.; Ma, C.; Li, D.; Dong, L.; Wang, L.-S.; Chen, F. Postbiotics: Enhancing human health with a novel concept. eFood 2024, 5, e180. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Ma, Y.; Yang, Y.; Cheng, Y.; Ma, H.; Ren, D.; Chen, P. Regulation of Viable/Inactivated/Lysed Probiotic Lactobacillus plantarum H6 on Intestinal Microbiota and Metabolites in Hypercholesterolemic Mice. npj Sci. Food 2022, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Cho, M.; Kang, D.J. Anti-Inflammatory Response of New Postbiotics in TNF-α/IFN-γ-Induced Atopic Dermatitis-like HaCaT Keratinocytes. Curr. Issues Mol. Biol. 2024, 46, 6100–6111. [Google Scholar] [CrossRef]
- Nicholas-Haizelden, K.; Murphy, B.; Hoptroff, M.; Horsburgh, M.J. Bioprospecting the skin microbiome: Advances in therapeutics and personal care products. Microorganisms 2023, 11, 1899. [Google Scholar] [CrossRef]
- Gurunathan, S.; Thangaraj, P.; Kim, J.-H. Postbiotics: Functional food materials and therapeutic agents for cancer, diabetes, and inflammatory diseases. Foods 2024, 13, 89. [Google Scholar] [CrossRef]
- Paz, M.; Lio, P. Postbiotics and atopic dermatitis: Aiming to modulate the gut-skin axis. J. Integr. Dermatol. 2024, 9, 22073. [Google Scholar]
- Beurel, E. Stress in the microbiome-immune crosstalk. Gut Microbes 2024, 16, 2327409. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Delgadillo, D.R.; Borelli, J.L.; Mayer, E.A.; Labus, J.S.; Cross, M.P.; Pressman, S.D. Biological, Environmental, and Psychological Stress and the Human Gut Microbiome in Healthy Adults. Sci. Rep. 2025, 15, 8351. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-H.; Woo, Y.-S.; Lee, S.-Y.; Bahk, W.-M. The brain–gut–microbiome axis in psychiatry. Int. J. Mol. Sci. 2020, 21, 7122. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Pasquini, L.; Visconti, E.; Vaccaro, M.; Rossi-Espagnet, M.C.; Napolitano, A. Gut-Brain Axis and Neuroplasticity in Health and Disease: A Systematic Review. Radiol. Med. 2025, 130, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Oroojzadeh, P.; Valivand, N.; Sambrani, R.; Lotfi, H. Immunological aspects of probiotics for improving skin diseases: Influence on the gut-brain-skin axis. Biochem. Biophys. Res. Commun. 2024, 702, 149632. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Therap. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical Efficacy of Fecal Microbial Transplantation Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. Immunol. Infect. Dis. 2022, 10, e570. [Google Scholar] [CrossRef]
- Deng, W.Y.; Chen, W.J.; Zhong, H.J.; Wu, L.H.; He, X.X. Washed Microbiota Transplantation: A Case Report of Clinical Success with Skin and Gut Microbiota Improvement in an Adolescent Boy with Atopic Dermatitis. Front. Immunol. 2023, 14, 1275427. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, W. Gut Microbiota Restoration through Fecal Microbiota Transplantation: A New Atopic Dermatitis Therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef]
- Wu, M.; Chen, X.; Lu, Q.; Yao, X. Fecal microbiota transplantation for the treatment of chronic inflammatory skin diseases. Heliyon 2024, 10, e37432. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagoa, T.; Martins, L.; Queiroga, M.C. Microbiota Modulation as an Approach to Prevent the Use of Antimicrobials Associated with Canine Atopic Dermatitis. Biomedicines 2025, 13, 2372. https://doi.org/10.3390/biomedicines13102372
Lagoa T, Martins L, Queiroga MC. Microbiota Modulation as an Approach to Prevent the Use of Antimicrobials Associated with Canine Atopic Dermatitis. Biomedicines. 2025; 13(10):2372. https://doi.org/10.3390/biomedicines13102372
Chicago/Turabian StyleLagoa, Tânia, Luís Martins, and Maria Cristina Queiroga. 2025. "Microbiota Modulation as an Approach to Prevent the Use of Antimicrobials Associated with Canine Atopic Dermatitis" Biomedicines 13, no. 10: 2372. https://doi.org/10.3390/biomedicines13102372
APA StyleLagoa, T., Martins, L., & Queiroga, M. C. (2025). Microbiota Modulation as an Approach to Prevent the Use of Antimicrobials Associated with Canine Atopic Dermatitis. Biomedicines, 13(10), 2372. https://doi.org/10.3390/biomedicines13102372








