Impact of KMT2A Rearrangement on Peripheral T-Cell Lymphoma, Not Otherwise Specified, and Angioimmunoblastic T-Cell Lymphoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. NGS
2.3. Therapy and Response Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Cohort, Baseline Features, and Survival Outcomes
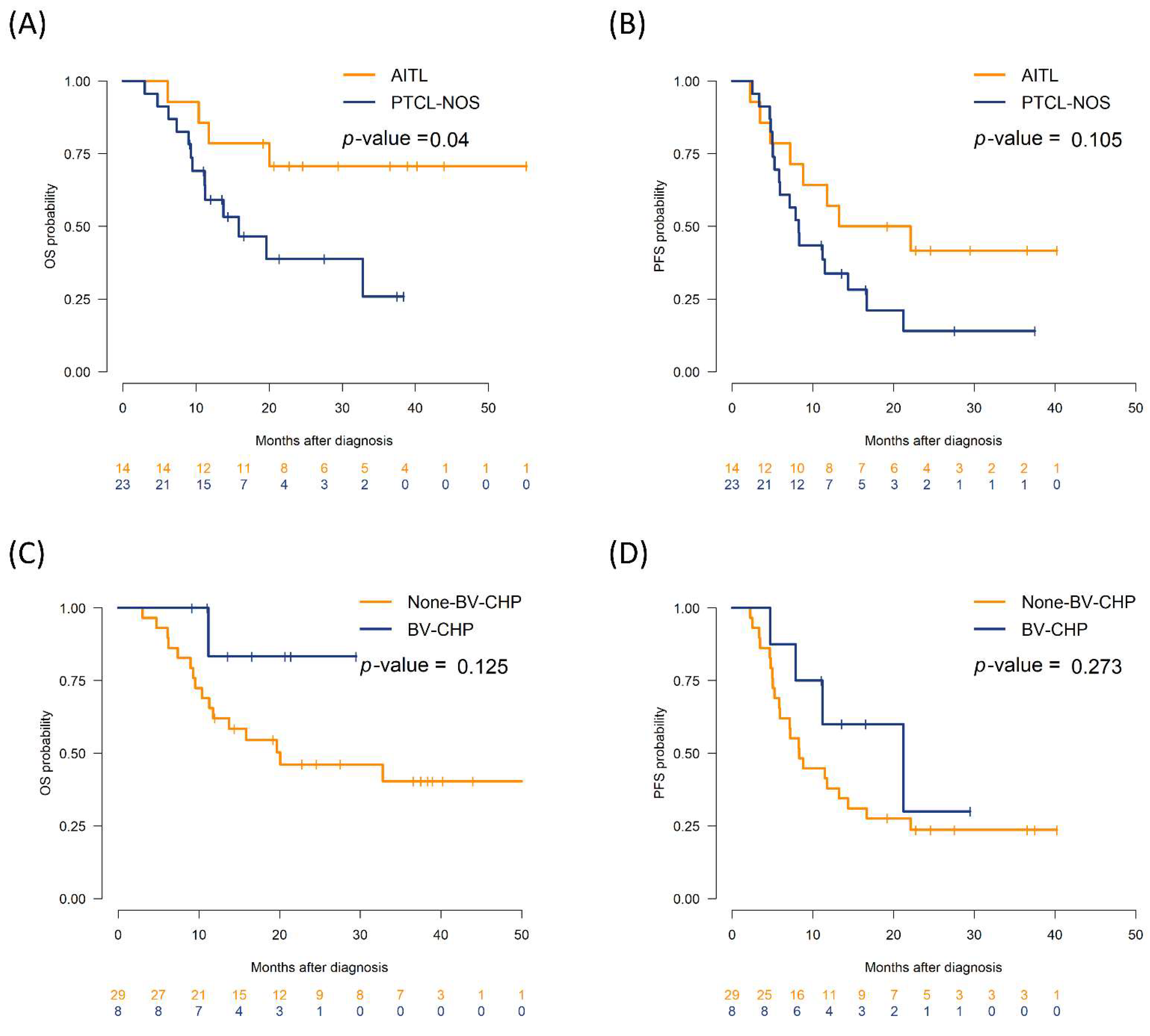
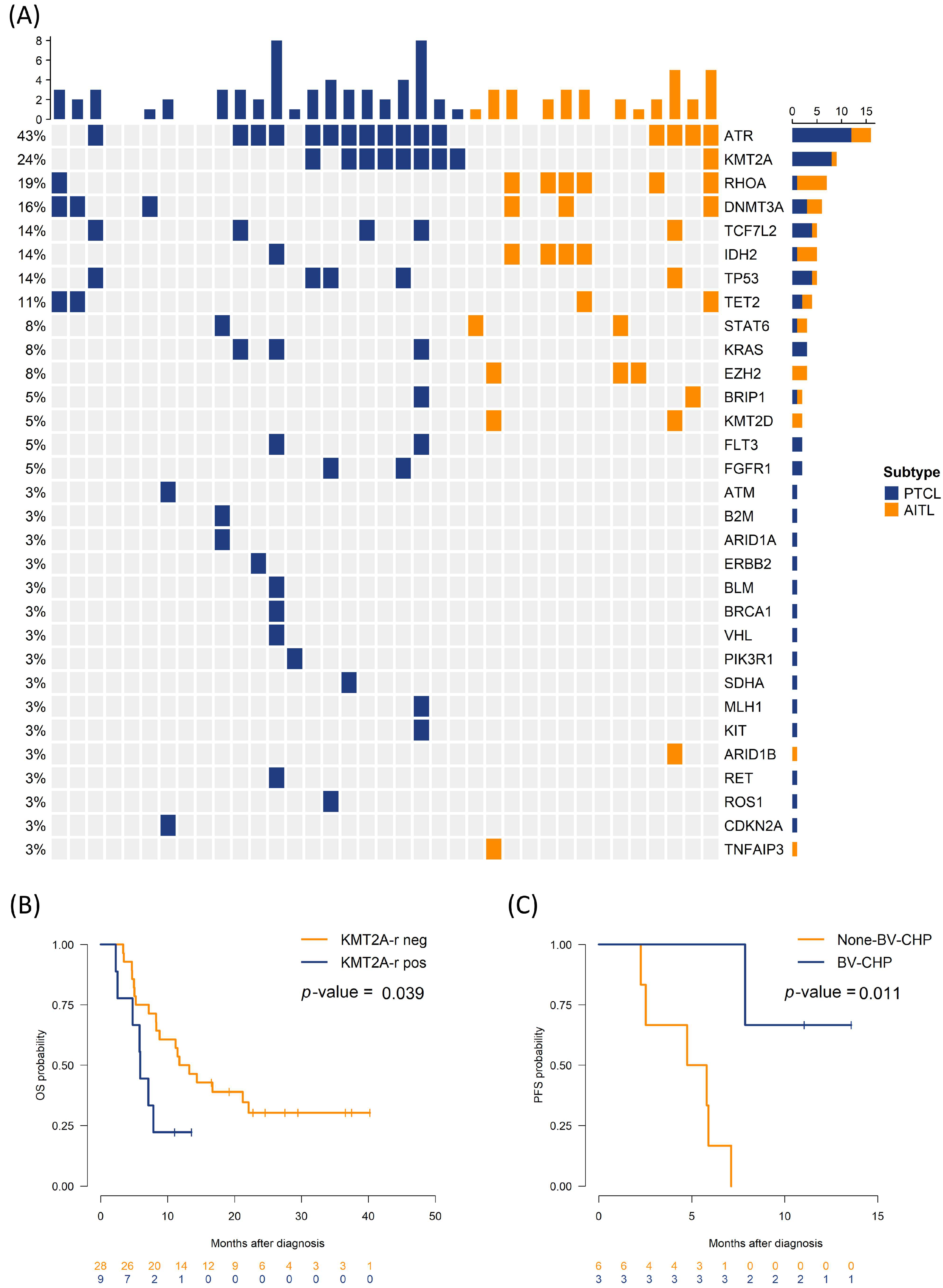
3.2. Impact of Genetic Alteration on Survival Outcomes
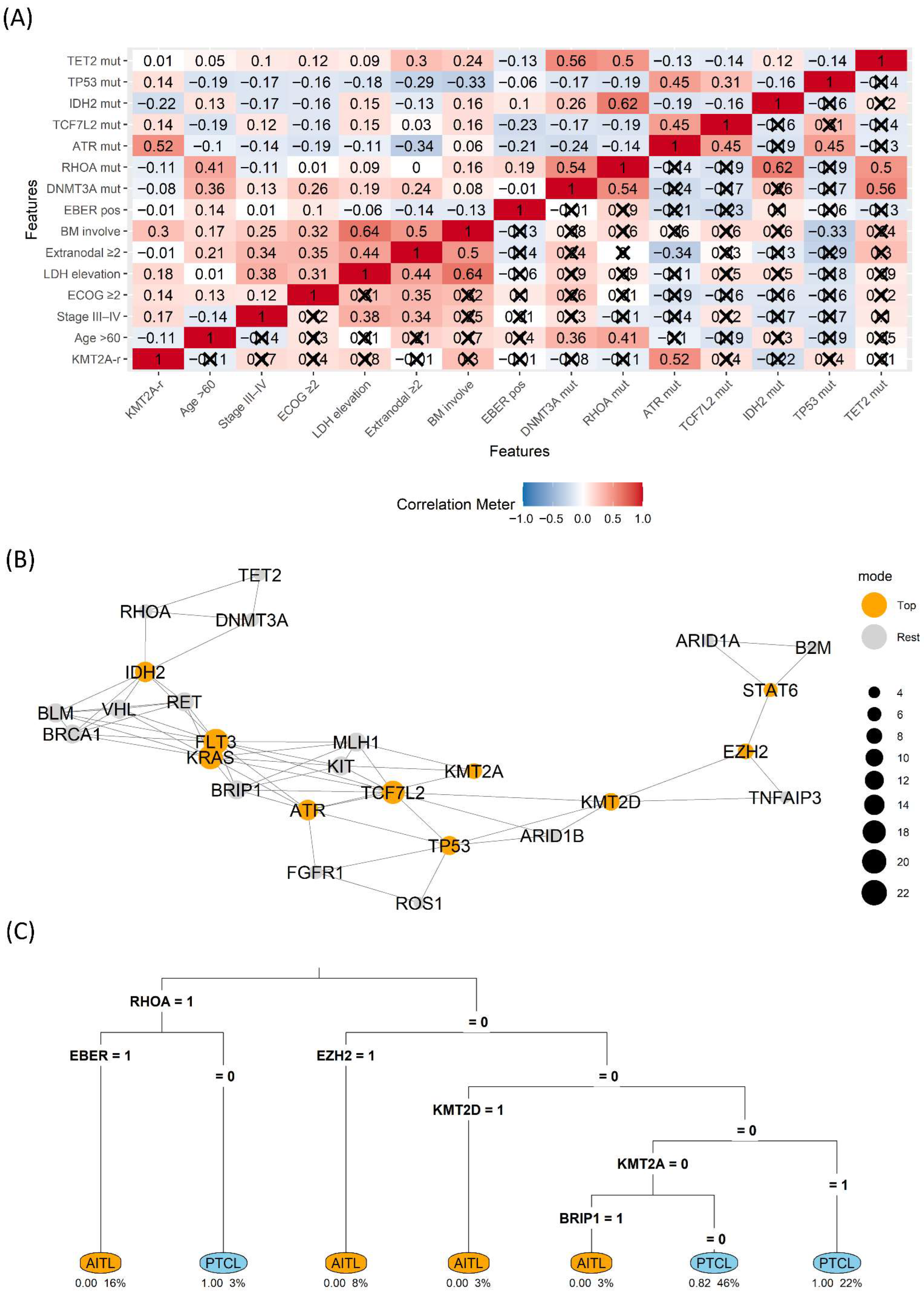
3.3. Interrelationship Among the Molecular Landscape
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AITL | Angioimmunoblastic T-cell lymphoma |
| PTCL-NOS | Peripheral T-cell lymphoma, not otherwise specified |
| RHOA | Ras homolog family member A |
| NGS | Next-generation sequencing |
| FFPE | Formalin-fixed, paraffin-embedded |
| PCR | Polymerase chain reaction |
| DDR | DNA damage response |
| BV | Brentuximab vedotin |
| BV-CHP | Brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone |
| ASCT | Autologous stem cell transplantation |
| TFH | T-follicular helper (cell) |
| LDH | Lactate dehydrogenase |
| ECOG PS | Eastern Cooperative Oncology Group performance status |
| ALCL | Anaplastic large cell lymphoma |
| EBER | EBV-encoded RNA in situ hybridization |
| IPI | International Prognostic Index |
| HR | Hazard ratio |
| CI | Confidence interval |
| PFS | Progression-free survival |
| OS | Overall survival |
| KMT2A-r | KMT2A rearrangement |
| CHP | Cyclophosphamide, doxorubicin, and prednisone |
| CR | Complete remission |
References
- Bellei, M.; Chiattone, C.S.; Luminari, S.; Pesce, E.A.; Cabrera, M.E.; de Souza, C.A.; Gabús, R.; Zoppegno, L.; Zoppegno, L.; Milone, J.; et al. T-cell lymphomas in South America and Europe. Rev. Bras. Hematol. Hemoter. 2012, 34, 42–47. [Google Scholar] [CrossRef]
- Vega, F.; Medeiros, L.J. A suggested immunohistochemical algorithm for the classification of T-cell lymphomas involving lymph nodes. Hum. Pathol. 2020, 102, 104–116. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Paik, J.H.; Koh, J.; Han, B.; Kim, S.; Lee, K.R.; Lee, S.; Lee, J.O.; Kim, T.M.; Kim, W.Y.; Jeon, Y.K. Distinct and overlapping features of nodal peripheral T-cell lymphomas exhibiting a follicular helper T-cell phenotype: A multicenter study emphasizing the clinicopathological significance of follicular helper T-cell marker expression. Hum. Pathol. 2023, 131, 47–60. [Google Scholar] [CrossRef]
- Dobson, R.; Du, P.Y.; Rásó-Barnett, L.; Yao, W.Q.; Chen, Z.; Casa, C.; Ei-Daly, H.; Farkas, L.; Soilleux, E.; Wright, P.; et al. Early detection of T-cell lymphoma with T follicular helper phenotype by RHOA mutation analysis. Haematologica 2022, 107, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Leca, J.; Lemonnier, F.; Meydan, C.; Foox, J.; El Ghamrasni, S.; Mboumba, D.L.; Duncan, G.S.; Fortin, J.; Sakamoto, T.; Tobin, C.; et al. IDH2 and TET2 mutations synergize to modulate T Follicular Helper cell functional interaction with the AITL microenvironment. Cancer Cell 2023, 41, 323–339.e10. [Google Scholar] [CrossRef]
- Couronné, L.; Bastard, C.; Bernard, O.A. TET2 and DNMT3A mutations in human T-cell lymphoma. N. Engl. J. Med. 2012, 366, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.R.; Ambesi-Impiombato, A.; Couronné, L.; Quinn, S.A.; Kim, C.S.; da Silva Almeida, A.C.; West, Z.; Belver, L.; Martin, M.S.; Scourzic, L.; et al. RHOA G17V induces t follicular helper cell specification and promotes lymphomagenesis. Cancer Cell 2018, 33, 259–273.e7. [Google Scholar] [CrossRef]
- Lemonnier, F.; Dupuis, J.; Sujobert, P.; Tournillhac, O.; Cheminant, M.; Sarkozy, C.; Pelletier, L.; Marçais, A.; Robe, C.; Fataccioli, V.; et al. Treatment with 5-azacytidine induces a sustained response in patients with angioimmunoblastic T-cell lymphoma. Blood 2018, 132, 2305–2309. [Google Scholar] [CrossRef]
- Vallois, D.; Dobay, M.P.; Morin, R.D.; Lemonnier, F.; Missiaglia, E.; Juilland, M.; Iwaszkiewicz, J.; Fataccioli, V.; Bisig, B.; Roberti, A.; et al. Activating mutations in genes related to TCR signaling in angioimmunoblastic and other follicular helper T-cell-derived lymphomas. Blood 2016, 128, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.C.; Dou, Y. Hijacked in cancer: The KMT2 (MLL) family of methyltransferases. Nat. Rev. Cancer 2015, 15, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, E.; García-Conde, J.; Viñolas, N.; López-Guillermo, A.; Hernández-Nieto, L.; Zubizarreta, A.; Maldonado, J.; Alcalá, A.; Faura, M.V.; Llorente, A.; et al. CHOP vs. ProMACE-CytaBOM in the treatment of aggressive non-Hodgkin’s lymphomas: Long-term results of a multicenter randomized trial. (PETHEMA: Spanish Cooperative Group for the Study of Hematological Malignancies Treatment, Spanish Society of Hematology). Eur. J. Haematol. 1996, 57, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef]
- Sibon, D. Peripheral T-Cell Lymphomas: Therapeutic Approaches. Cancers 2022, 14, 2332. [Google Scholar] [CrossRef]
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447. [Google Scholar] [CrossRef]
- Yoon, J.H.; Min, G.J.; Park, S.S.; Jeon, Y.W.; Lee, S.E.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Lee, S.; Kim, H.J.; et al. Autologous hematopoietic cell transplantation using dose-reduced intravenous busulfan, melphalan, and thiotepa for high-risk or relapsed lymphomas. Bone Marrow Transpl. 2019, 54, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T.; Traag, V.; Horvát, S.; Zanini, F.; Noom, D.; Müller, K. igraph: Network Analysis and Visualization in R; R Package Version 2. 2024. Available online: https://CRAN.R-project.org/package=igraph (accessed on 1 August 2025).
- Therneau, T.M.; Atkinson, B. rpart: Recursive Partitioning and Regression Trees; Version 4. 2024. Available online: https://cran.r-project.org/package=rpart (accessed on 1 August 2025).
- Timmins, M.A.; Wagner, S.D.; Ahearne, M.J. The new biology of PTCL-NOS and AITL: Current status and future clinical impact. Br. J. Haematol. 2020, 189, 54–66. [Google Scholar] [CrossRef]
- Kim, T.Y.; Min, G.J.; Jeon, Y.W.; Park, S.S.; Park, S.; Shin, S.H.; Yahng, S.A.; Yoon, J.H.; Lee, S.E.; Cho, B.S.; et al. Impact of Epstein-Barr virus on peripheral T-cell lymphoma not otherwise specified and angioimmunoblastic T-cell lymphoma. Front. Oncol. 2021, 11, 797028. [Google Scholar] [CrossRef]
- Park, S.I.; Horwitz, S.M.; Foss, F.M.; Pinter-Brown, L.C.; Carson, K.R.; Rosen, S.T.; Pro, B.; Hsi, E.D.; Federico, M.; Gisselbrecht, C.; et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer 2019, 125, 1507–1517. [Google Scholar] [CrossRef]
- Odejide, O.; Weigert, O.; Lane, A.A.; Toscano, D.; Lunning, M.A.; Kopp, N.; Kim, S.; van Bodegom, D.; Bolla, S.; Schatz, J.H.; et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 2014, 123, 1293–1296. [Google Scholar] [CrossRef]
- Martín García-Sancho, A.; Rodríguez-Pinilla, S.M.; Domingo-Domenech, E.; Climent, F.; Sánchez-Garcia, J.; López Jiménez, J.; García-Cosío Piqueras, M.; Castellvi, J.; González, A.J.; González de Villambrosia, S.; et al. Peripheral T-cell lymphoma with a T follicular-helper phenotype: A different entity? Results of the Spanish Real-T study. Br. J. Haematol. 2023, 203, 182–193. [Google Scholar] [CrossRef]
- Chen, W.; Kumar, A.R.; Hudson, W.A.; Li, Q.; Wu, B.; Staggs, R.A.; Lund, E.A.; Sam, T.N.; Kersey, J.H. Malignant transformation initiated by Mll-AF9: Gene dosage and critical target cells. Cancer Cell 2008, 13, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, D.; Horwitz, S.; Bartlett, N.L.; Kim, Y.; Jacobsen, E.; Duvic, M.; Little, M.; Trepicchio, W.; Fenton, K.; Onsum, M.; et al. Response to brentuximab vedotin by CD30 expression in non-Hodgkin lymphoma. Oncologist 2022, 27, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104. [Google Scholar] [CrossRef]
- Issa, G.C.; Aldoss, I.; Thirman, M.J.; DiPersio, J.; Arellano, M.; Blachly, J.S.; Mannis, G.N.; Perl, A.; Dickens, D.S.; McMahon, C.M.; et al. Menin inhibition with revumenib for KMT2A-rearranged relapsed or refractory acute leukemia (AUGMENT-101). J. Clin. Oncol. 2025, 43, 75–84. [Google Scholar] [CrossRef]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.; Jacob, A.T.; et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: A multicenter phase 2 study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Falchi, L.; Lue, J.K.; Marchi, E.; Kinahan, C.; Sawas, A.; Deng, C.; Montanari, F.; Amengual, J.E.; Kim, H.A.; et al. Oral 5-azacytidine and romidepsin exhibit marked activity in patients with PTCL: A multicenter phase 1 study. Blood 2019, 134, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 37) | AITL (n = 14) | PTCL (n = 23) | p-Value |
|---|---|---|---|---|
| Sex, n (%) | 0.64 | |||
| Female | 18 (48.6) | 8 (57.1) | 10 (43.5) | |
| Male | 19 (51.4) | 6 (42.9) | 13 (56.5) | |
| Diagnosed Age | 0.76 | |||
| ≤60 years | 21 (56.8) | 7 (50.0) | 14 (60.9) | |
| >60 years | 16 (43.2) | 7 (50.0) | 9 (39.1) | |
| Ann Arbor stage | 0.65 | |||
| I–II | 3 (8.1) | 2 (14.3) | 1 (4.3) | |
| III–IV | 34 (91.9) | 12 (85.7) | 22 (95.7) | |
| ECOG2 | 0.698 | |||
| <2 | 32 (86.5) | 13 (92.9) | 19 (82.6) | |
| ≥2 | 5 (13.5) | 1 (7.1) | 4 (17.4) | |
| Lactate dehydrogenase | 0.124 | |||
| Normal | 14 (37.8) | 8 (57.1) | 6 (26.1) | |
| Elevated | 23 (62.2) | 6 (42.9) | 17 (73.9) | |
| Extranodal site involvement | 0.76 | |||
| <2 | 16 (43.2) | 7 (50.0) | 9 (39.1) | |
| ≥2 | 21 (56.8) | 7 (50.0) | 14 (60.9) | |
| Bone marrow involvement | 0.417 | |||
| Negative | 22 (59.5) | 10 (71.4) | 12 (52.2) | |
| Positive | 15 (40.5) | 4 (28.6) | 11 (47.8) | |
| The International Prognostic Index for Non-Hodgkin’s lymphoma | 0.526 | |||
| Low or Low-Intermediate risk | 20 (54.1) | 9 (64.3) | 11 (47.8) | |
| High-Intermediate or High risk | 17 (45.9) | 5 (35.7) | 12 (52.2) | |
| T-follicular helper type | 0.168 | |||
| Negative | 32 (86.5) | 14 (100.0) | 18 (78.3) | |
| Positive | 5 (13.5) | 0 (0.0) | 5 (21.7) | |
| Frontline regimen | 0.699 | |||
| BV-CHP | 8 (21.6) | 2 (14.3) | 6 (26.1) | |
| CHOP/CHOEP | 17 (45.9) | 7 (50.0) | 10 (43.5) | |
| ProMACE-CytaBOM | 12 (32.4) | 5 (35.7) | 7 (30.4) | |
| ASCT | 0.083 | |||
| No | 26 (70.3%) | 7 (50.0%) | 19 (82.6%) | |
| Yes | 11 (29.7%) | 7 (50.0%) | 4 (17.4%) | |
| Immunophenotype | ||||
| Epstein–Barr virus-encoded RNAs | 0.14 | |||
| Negative | 12 (32.4) | 2 (14.3) | 10 (43.5) | |
| Positive | 25 (67.6) | 12 (85.7) | 13 (56.5) | |
| CD30 | 0.394 | |||
| Negative | 29 (80.6) | 9 (69.2) | 20 (87.0) | |
| Positive | 7 (19.4) | 4 (30.8) | 3 (13.0) | |
| CD10 | 0.001 | |||
| Negative | 26 (74.3) | 5 (38.5) | 21 (95.5) | |
| Positive | 9 (25.7) | 8 (61.5) | 1 (4.5) | |
| CD21 | 0.001 | |||
| Negative | 19 (54.3) | 1 (7.1) | 18 (85.7) | |
| Positive | 16 (45.7) | 13 (92.9) | 3 (14.3) | |
| CD23 | 0.011 | |||
| Negative | 10 (45.5) | 3 (21.4) | 7 (87.5) | |
| Positive | 12 (54.5) | 11 (78.6) | 1 (12.5) | |
| BCL6 | 0.004 | |||
| Negative | 13 (39.4) | 1 (7.1) | 12 (63.2) | |
| Positive | 20 (60.6) | 13 (92.9) | 7 (36.8) | |
| PD1 | 0.014 | |||
| Negative | 9 (25.7) | 0 (0.0) | 9 (42.9) | |
| Positive | 26 (74.3) | 14 (100.0) | 12 (57.1) | |
| Ki-67 proliferation index | 64.9 ± 21.8 | 65.4 ± 22.9 | 64.6 ± 21.7 | 0.917 |
| Next-generation sequencing | ||||
| ATR | 0.288 | |||
| Unmutated | 21 (56.8) | 10 (71.4) | 11 (47.8) | |
| Mutated | 16 (43.2) | 4 (28.6) | 12 (52.2) | |
| KMT2A rearrangement | 0.132 | |||
| Negative | 28 (75.7) | 13 (92.9) | 15 (65.2) | |
| Positive | 9 (24.3) | 1 (7.1) | 8 (34.8) | |
| RHOA | 0.014 | |||
| Unmutated | 30 (81.1) | 8 (57.1) | 22 (95.7) | |
| Mutated | 7 (18.9) | 6 (42.9) | 1 (4.3) | |
| DNMT3A | 0.833 | |||
| Unmutated | 31 (83.8) | 11 (78.6) | 20 (87.0) | |
| Mutated | 6 (16.2) | 3 (21.4) | 3 (13.0) | |
| TCF7L2 | 0.698 | |||
| Unmutated | 32 (86.5) | 13 (92.9) | 19 (82.6) | |
| Mutated | 5 (13.5) | 1 (7.1) | 4 (17.4) | |
| IDH2 | 0.111 | |||
| Unmutated | 32 (86.5) | 10 (71.4) | 22 (95.7) | |
| Mutated | 5 (13.5) | 4 (28.6) | 1 (4.3) | |
| TP53 | 0.698 | |||
| Unmutated | 32 (86.5) | 13 (92.9) | 19 (82.6) | |
| Mutated | 5 (13.5) | 1 (7.1) | 4 (17.4) | |
| TET2 | >0.999 | |||
| Unmutated | 33 (89.2) | 12 (85.7) | 21 (91.3) | |
| Mutated | 4 (10.8) | 2 (14.3) | 2 (8.7) | |
| Variables | HR (95% CI) | p-Value | BH-FDR q-Value |
|---|---|---|---|
| PTCL-NOS vs. AITL | 2 (0.85, 4.68) | 0.111 | 0.317 |
| Female vs. Male | 0.52 (0.23, 1.14) | 0.102 | 0.317 |
| Age > 60 years vs. ≤60 | 1.01 (0.47, 2.19) | 0.979 | 0.979 |
| Ann Arbor stage, III–IV vs. I–II | 3.14 (0.42, 23.4) | 0.263 | 0.386 |
| ECOG PS ≥ 2 vs. <2 | 2.62 (0.86, 8.03) | 0.092 | 0.317 |
| LDH elevation vs. normal | 3.1 (1.27, 7.58) | 0.013 | 0.136 |
| Extranodal site involvement, ≥2 vs. <2 | 1.57 (0.71, 3.49) | 0.266 | 0.386 |
| Bone marrow involvement, positive vs. negative | 2.98 (1.25, 7.08) | 0.014 | 0.136 |
| IPI score ≥3 vs. <3 | 1.74 (0.79, 3.82) | 0.167 | 0.339 |
| TFH phenotype, yes or no | 1.74 (0.65, 4.68) | 0.269 | 0.386 |
| Frontline regimen: BV-CHP vs. others | 0.56 (0.19, 1.61) | 0.28 | 0.386 |
| EBER positive vs. negative | 0.56 (0.25, 1.28) | 0.169 | 0.339 |
| ATR mutation vs. unmutated | 1.48 (0.68, 3.22) | 0.327 | 0.409 |
| KMT2A rearranged, yes vs. no | 2.56 (1.02, 6.45) | 0.046 | 0.306 |
| RHOA mutation vs. unmutated | 1.33 (0.53, 3.33) | 0.542 | 0.603 |
| DNMT3A mutation vs. unmutated | 2.28 (0.90, 5.81) | 0.083 | 0.317 |
| TCF7L2 mutation vs. unmutated | 1.23 (0.42, 3.61) | 0.705 | 0.742 |
| IDH2 mutation vs. unmutated | 2.05 (0.75, 5.56) | 0.16 | 0.339 |
| TP53 mutation vs. unmutated | 1.58 (0.54, 4.65) | 0.402 | 0.473 |
| TET2 mutation vs. unmutated | 1.79 (0.61, 5.30) | 0.29 | 0.386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Kim, T.-J.; Han, E.J.; Min, G.-J.; Cho, S.-G.; Jeon, Y. Impact of KMT2A Rearrangement on Peripheral T-Cell Lymphoma, Not Otherwise Specified, and Angioimmunoblastic T-Cell Lymphoma. Biomedicines 2025, 13, 2347. https://doi.org/10.3390/biomedicines13102347
Kim T-Y, Kim T-J, Han EJ, Min G-J, Cho S-G, Jeon Y. Impact of KMT2A Rearrangement on Peripheral T-Cell Lymphoma, Not Otherwise Specified, and Angioimmunoblastic T-Cell Lymphoma. Biomedicines. 2025; 13(10):2347. https://doi.org/10.3390/biomedicines13102347
Chicago/Turabian StyleKim, Tong-Yoon, Tae-Jung Kim, Eun Ji Han, Gi-June Min, Seok-Goo Cho, and Youngwoo Jeon. 2025. "Impact of KMT2A Rearrangement on Peripheral T-Cell Lymphoma, Not Otherwise Specified, and Angioimmunoblastic T-Cell Lymphoma" Biomedicines 13, no. 10: 2347. https://doi.org/10.3390/biomedicines13102347
APA StyleKim, T.-Y., Kim, T.-J., Han, E. J., Min, G.-J., Cho, S.-G., & Jeon, Y. (2025). Impact of KMT2A Rearrangement on Peripheral T-Cell Lymphoma, Not Otherwise Specified, and Angioimmunoblastic T-Cell Lymphoma. Biomedicines, 13(10), 2347. https://doi.org/10.3390/biomedicines13102347







