Psoriasis as a Potential Risk Factor for Inflammatory Bowel Disease: Findings from a Nationally Representative Korean Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Data Source
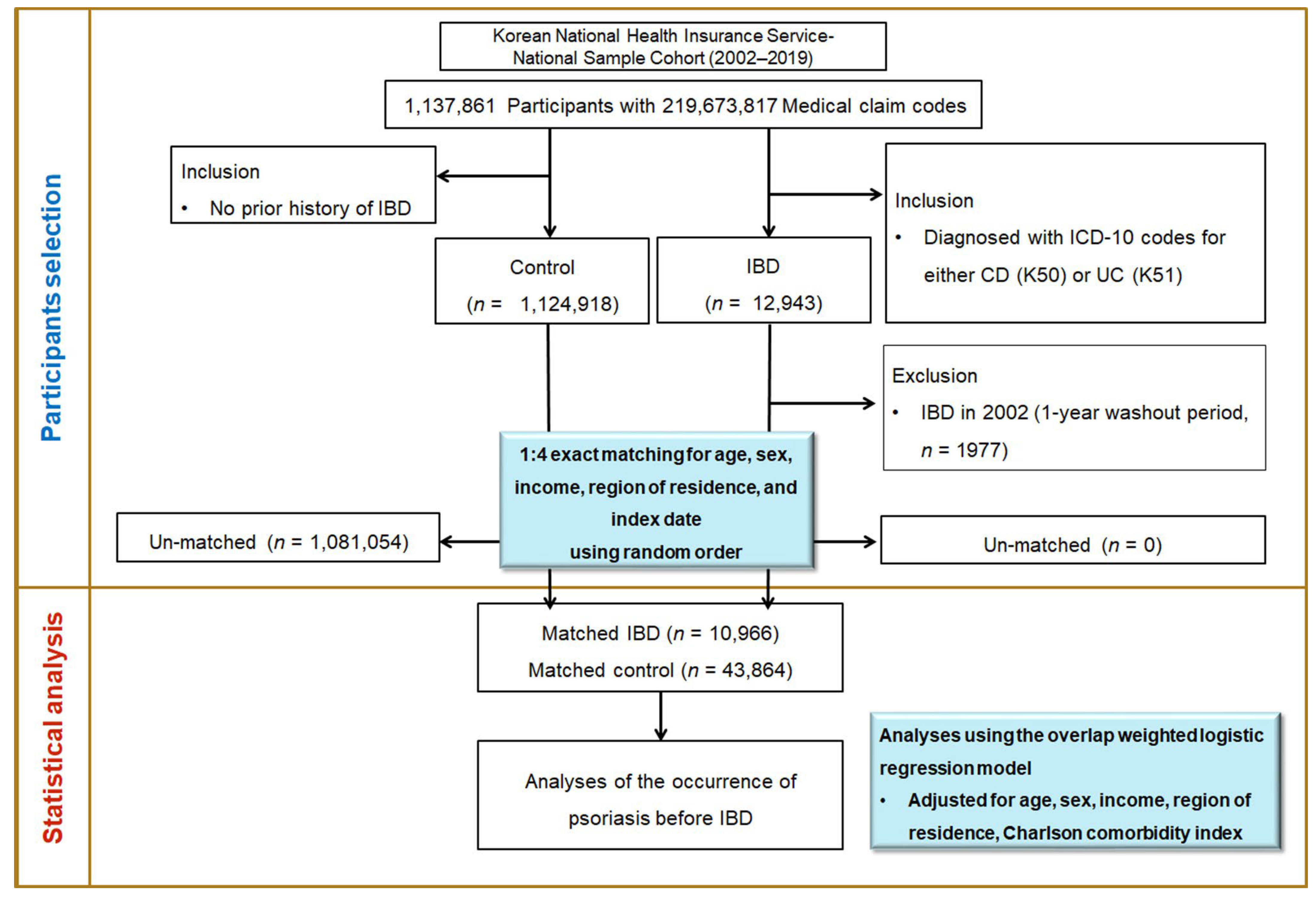
2.2. Study Population and Case Definition
2.3. Exposure Assessment
2.4. Final Cohort
2.5. Covariates and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Oragnization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Parisi, R.; Iskandar, I.Y.K.; Kontopantelis, E.; Augustin, M.; Griffiths, C.E.M.; Ashcroft, D.M. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ 2020, 369, m1590. [Google Scholar] [CrossRef]
- Kubota, K.; Kamijima, Y.; Sato, T.; Ooba, N.; Koide, D.; Iizuka, H.; Nakagawa, H. Epidemiology of psoriasis and palmoplantar pustulosis: A nationwide study using the Japanese national claims database. BMJ Open 2015, 5, e006450. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, S.; Park, J.S.; Jo, S.J. Prevalence of Psoriasis in Korea: A Population-Based Epidemiological Study Using the Korean National Health Insurance Database. Ann. Dermatol. 2017, 29, 761–767. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef]
- Zachariae, H. Prevalence of joint disease in patients with psoriasis: Implications for therapy. Am. J. Clin. Dermatol. 2003, 4, 441–447. [Google Scholar] [CrossRef]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar] [CrossRef]
- Park, J.; Cheon, J.H. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med. J. 2021, 62, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Mallbris, L.; Warren, R.B.; Bachelez, H.; Gislason, G.H.; Hansen, P.R.; Skov, L. Association between psoriasis and inflammatory bowel disease: A Danish nationwide cohort study. Br. J. Dermatol. 2016, 175, 487–492. [Google Scholar] [CrossRef]
- Skroza, N.; Proietti, I.; Pampena, R.; La Viola, G.; Bernardini, N.; Nicolucci, F.; Tolino, E.; Zuber, S.; Soccodato, V.; Potenza, C. Correlations between psoriasis and inflammatory bowel diseases. Biomed. Res. Int. 2013, 2013, 983902. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008, 8, 458–466. [Google Scholar] [CrossRef]
- Fu, Y.; Lee, C.H.; Chi, C.C. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1417–1423. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Ubeda, C.; Artacho, A.; Attur, M.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Bui, A.; Lee, K.; Truong, A.; Milush, J.M.; Somsouk, M.; Liao, W. Multiomic Analysis of the Gut Microbiome in Psoriasis Reveals Distinct Host–Microbe Associations. JID Innov. 2022, 2, 100115. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.J.; Rhee, K.H.; Kim, Y.H.; Hong, S.N.; Kim, K.H.; Seo, S.I.; Cha, J.M.; Park, S.Y.; Jeong, S.K.; et al. A 30-year Trend Analysis in the Epidemiology of Inflammatory Bowel Disease in the Songpa-Kangdong District of Seoul, Korea in 1986–2015. J. Crohns Colitis 2019, 13, 1410–1417. [Google Scholar] [CrossRef]
- Zhiqin, W.; Palaniappan, S.; Raja Ali, R.A. Inflammatory Bowel Disease-related Colorectal Cancer in the Asia-Pacific Region: Past, Present, and Future. Intest. Res. 2014, 12, 194–204. [Google Scholar] [CrossRef]
- Kochhar, R.; Goenka, M.K.; Kaushik, S.P.; Gupta, N.M.; Nagi, B.; Mehta, S.K. Colorectal carcinoma in Indian patients with idiopathic ulcerative colitis. Eur. J. Cancer Prev. 1992, 1, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Konishi, T.; Kishimoto, J.; Kotake, K.; Muto, T.; Sugihara, K. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: A nationwide Japanese study. Inflamm. Bowel Dis. 2011, 17, 802–808. [Google Scholar] [CrossRef]
- Lee, J.W.; Eun, C.S. Inflammatory bowel disease in Korea: Epidemiology and pathophysiology. Korean J. Intern. Med. 2022, 37, 885–894. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.-B.; von Känel, R.; Anderegg, C.; Bauerfeind, P.; Beglinger, C.; Begré, S.; Belli, D.; Bengoa, J.M.; et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835.e821. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Dreiher, J.; Birkenfeld, S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-F.; Wang, T.-S.; Hung, S.-T.; Tsai, P.I.C.; Schenkel, B.; Zhang, M.; Tang, C.-H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 2011, 63, 40–46. [Google Scholar] [CrossRef]
- Alinaghi, F.; Tekin, H.G.; Burisch, J.; Wu, J.J.; Thyssen, J.P.; Egeberg, A. Global Prevalence and Bidirectional Association Between Psoriasis and Inflammatory Bowel Disease-A Systematic Review and Meta-analysis. J. Crohns Colitis 2020, 14, 351–360. [Google Scholar] [CrossRef]
- Masaki, S.; Bayaraa, B.; Imafuku, S. Prevalence of inflammatory bowel disease in Japanese psoriatic patients. J. Dermatol. 2019, 46, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Ham, S.P.; Oh, J.H.; Park, H.J.; Kim, J.U.; Kim, H.Y.; Jung, S.Y.; Choi, S.Y.; Seol, J.E.; Kim, H.; Kim, M.S.; et al. Validity of Diagnostic Codes for Identification of Psoriasis Patients in Korea. Ann. Dermatol. 2020, 32, 115–121. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Makredes, M.; Robinson, D.; Bala, M.; Kimball, A.B. The burden of autoimmune disease: A comparison of prevalence ratios in patients with psoriatic arthritis and psoriasis. J. Am. Acad. Dermatol. 2009, 61, 405–410. [Google Scholar] [CrossRef]
- Thrash, B.; Patel, M.; Shah, K.R.; Boland, C.R.; Menter, A. Cutaneous manifestations of gastrointestinal disease: Part II. J. Am. Acad. Dermatol. 2013, 68, 211.e1–211.e33. [Google Scholar] [CrossRef]
- Zákostelská, Z.; Málková, J.; Klimešová, K.; Rossmann, P.; Hornová, M.; Novosádová, I.; Stehlíková, Z.; Kostovčík, M.; Hudcovic, T.; Štepánková, R.; et al. Intestinal Microbiota Promotes Psoriasis-Like Skin Inflammation by Enhancing Th17 Response. PLoS ONE 2016, 11, e0159539. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, Y.-J.; Lee, W.J.; Won, C.H.; Lee, M.W.; Chang, S.E. Risk of incident autoimmune diseases in patients with newly diagnosed psoriatic disease: A nationwide population-based study. Sci. Rep. 2023, 13, 16738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, W.; Deng, J.; Zhai, B.; Zhu, G.; Gao, R.; Zeng, Q.; Qiu, J.; Bian, Z.; Xiao, H.; et al. Multi-ancestry genome-wide meta-analysis with 472,819 individuals identifies 32 novel risk loci for psoriasis. J. Transl. Med. 2025, 23, 133. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Ellinghaus, E.; Nair, R.P.; Stuart, P.E.; Esko, T.; Metspalu, A.; Debrus, S.; Raelson, J.V.; Tejasvi, T.; Belouchi, M.; et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am. J. Hum. Genet. 2012, 90, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lv, Y.; Wang, L.; Chang, X.; Zhao, K.; Liu, X. Mendelian randomization analysis of psoriasis and psoriatic arthritis associated with risks of ulcerative colitis. Skin. Res. Technol. 2024, 30, e13795. [Google Scholar] [CrossRef]
- Kyoung, D.S.; Kim, H.S. Understanding and Utilizing Claim Data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) Database for Research. J. Lipid Atheroscler. 2022, 11, 103–110. [Google Scholar] [CrossRef]
- Li, F.; Morgan, K.L.; Zaslavsky, A.M. Balancing covariates via propensity score weighting. J. Am. Stat. Assoc. 2017, 113, 390–400. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Verstockt, B.; Bressler, B.; Martinez-Lozano, H.; McGovern, D.; Silverberg, M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology 2022, 162, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Before Overlap PS Weighting Adjustment | After Overlap PS Weighting Adjustment | ||||
|---|---|---|---|---|---|---|
| IBD | Control | Standardized Difference | IBD | Control | Standardized Difference | |
| Age (n, %) | 0.00 | 0.00 | ||||
| 0–4 | 355 (3.24) | 1420 (3.24) | 284 (3.24) | 284 (3.24) | ||
| 5–9 | 306 (2.79) | 1224 (2.79) | 245 (2.79) | 245 (2.79) | ||
| 10–14 | 361 (3.29) | 1444 (3.29) | 289 (3.30) | 289 (3.30) | ||
| 15–19 | 551 (5.02) | 2204 (5.02) | 440 (5.03) | 440 (5.03) | ||
| 20–24 | 655 (5.97) | 2620 (5.97) | 524 (5.98) | 524 (5.98) | ||
| 25–29 | 734 (6.69) | 2936 (6.69) | 587 (6.70) | 587 (6.70) | ||
| 30–34 | 806 (7.35) | 3224 (7.35) | 644 (7.36) | 644 (7.36) | ||
| 35–39 | 769 (7.01) | 3076 (7.01) | 614 (7.01) | 614 (7.01) | ||
| 40–44 | 901 (8.22) | 3604 (8.22) | 720 (8.22) | 720 (8.22) | ||
| 45–49 | 925 (8.44) | 3700 (8.44) | 739 (8.44) | 739 (8.44) | ||
| 50–54 | 994 (9.06) | 3976 (9.06) | 796 (9.08) | 796 (9.08) | ||
| 55–59 | 884 (8.06) | 3536 (8.06) | 705 (8.05) | 705 (8.05) | ||
| 60–64 | 770 (7.02) | 3080 (7.02) | 614 (7.01) | 614 (7.01) | ||
| 65–69 | 765 (6.98) | 3060 (6.98) | 610 (6.97) | 610 (6.97) | ||
| 70–74 | 572 (5.22) | 2288 (5.22) | 455 (5.20) | 455 (5.20) | ||
| 75–79 | 348 (3.17) | 1392 (3.17) | 277 (3.17) | 277 (3.17) | ||
| 80–84 | 184 (1.68) | 736 (1.68) | 147 (1.67) | 147 (1.67) | ||
| 85+ | 86 (0.78) | 344 (0.78) | 68 (0.78) | 68 (0.78) | ||
| Sex (n, %) | 0.00 | 0.00 | ||||
| Male | 5747 (52.41) | 22,988 (52.41) | 4590 (52.41) | 4590 (52.41) | ||
| Female | 5219 (47.59) | 20,876 (47.59) | 4167 (47.59) | 4167 (47.59) | ||
| Income (n, %) | 0.00 | 0.00 | ||||
| 1 (lowest) | 1833 (16.72) | 7332 (16.72) | 1462 (16.70) | 1462 (16.70) | ||
| 2 | 1554 (14.17) | 6216 (14.17) | 1241 (14.17) | 1241 (14.17) | ||
| 3 | 1992 (18.17) | 7968 (18.17) | 1590 (18.16) | 1591 (18.16) | ||
| 4 | 2533 (23.10) | 10,132 (23.10) | 2024 (23.11) | 2024 (23.11) | ||
| 5 (highest) | 3054 (27.85) | 12,216 (27.85) | 2440 (27.86) | 2440 (27.86) | ||
| Region of residence (n, %) | 0.00 | 0.00 | ||||
| Urban | 4882 (44.52) | 19,528 (44.52) | 3901 (44.55) | 3901 (44.55) | ||
| Rural | 6084 (55.48) | 24,336 (55.48) | 4856 (55.45) | 4856 (55.45) | ||
| CCI score (Mean, SD) | 0.72 (1.48) | 0.58 (1.35) | 0.10 | 0.69 (1.28) | 0.69 (0.68) | 0.00 |
| Psoriasis (n, %) | 141 (1.29) | 349 (0.80) | 0.05 | 113 (1.28) | 69 (0.79) | 0.05 |
| Characteristics |
N of
Event |
N of
Control | Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p |
Overlap PS Weighted
Model † | p | |
| Odds ratio of IBD (n = 54,830) | ||||||
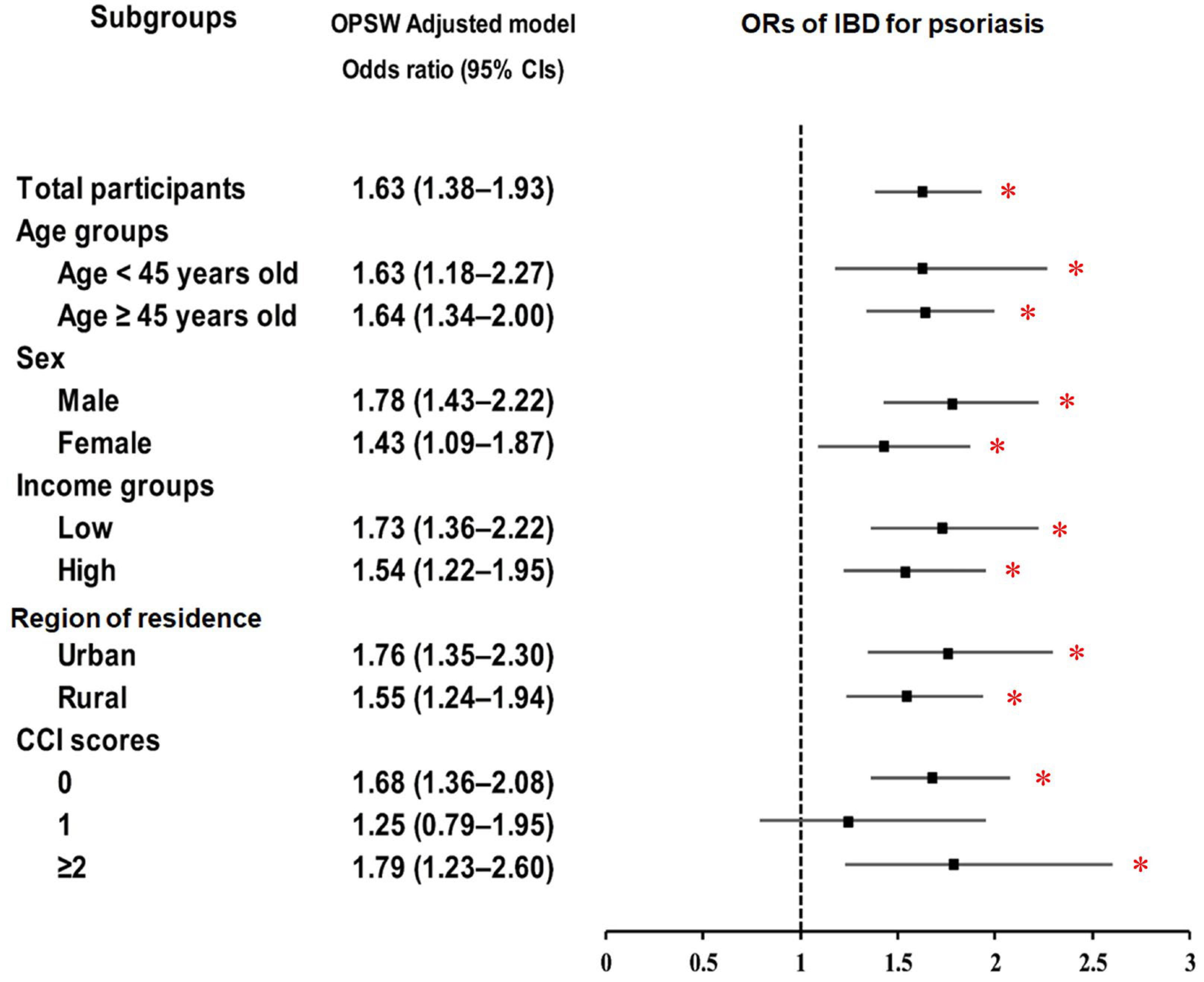
| Psoriasis | 141/10,966 (1.3) | 349/43,864 (0.8) | 1.63 (1.34–1.98) | <0.001 * | 1.63 (1.38–1.93) | <0.001 * |
| Control | 10,825/10,966 (98.7) | 43,515/43,864 (99.2) | 1 | 1 | ||
| Odds ratio of CD (n = 23,185) | ||||||
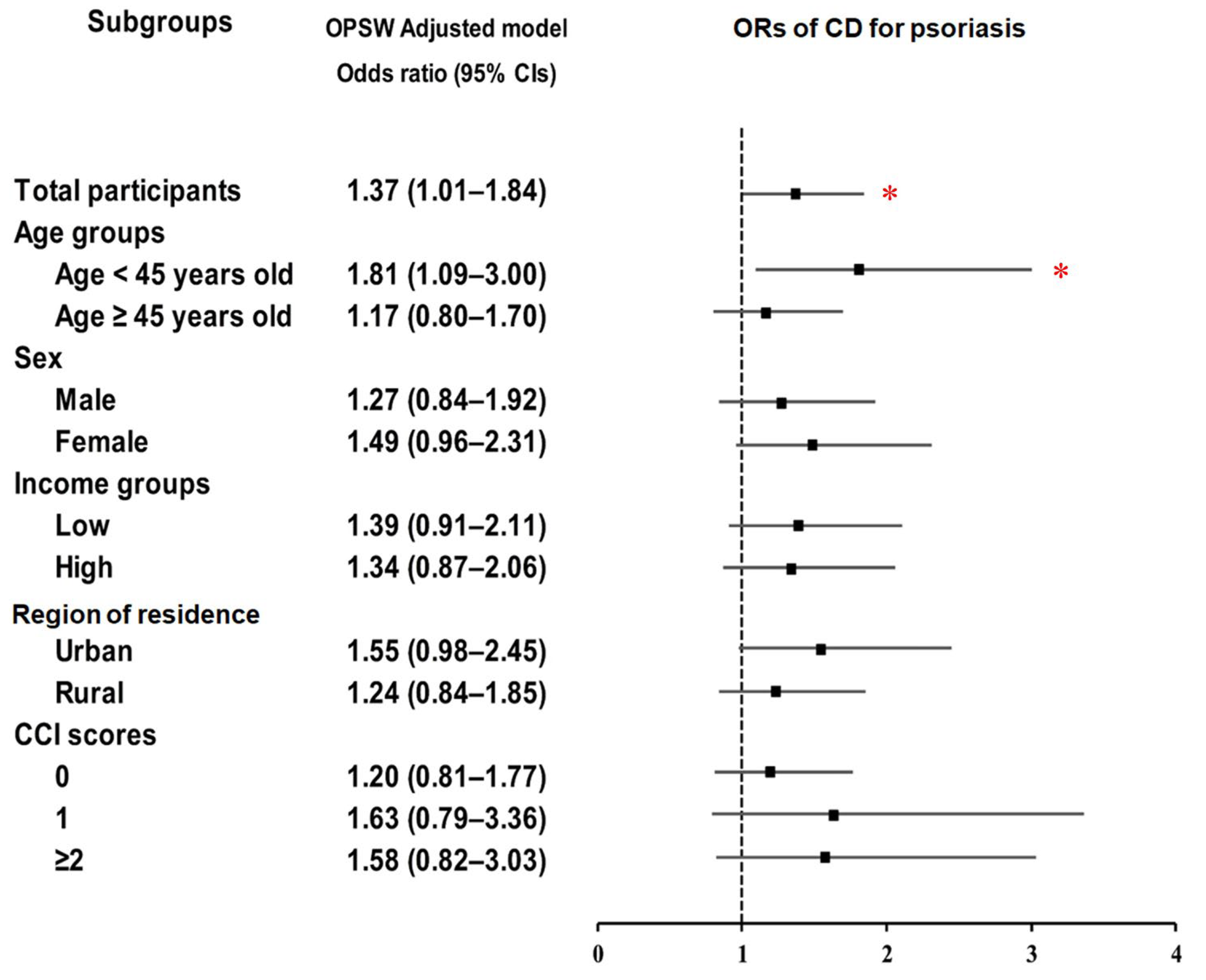
| Psoriasis | 41/4637 (0.9) | 120/18,548 (0.6) | 1.37 (0.96–1.96) | 0.083 | 1.37 (1.01–1.84) | 0.041 * |
| Control | 4596/4637 (99.1) | 18,428/18,548 (99.4) | 1 | 1 | ||
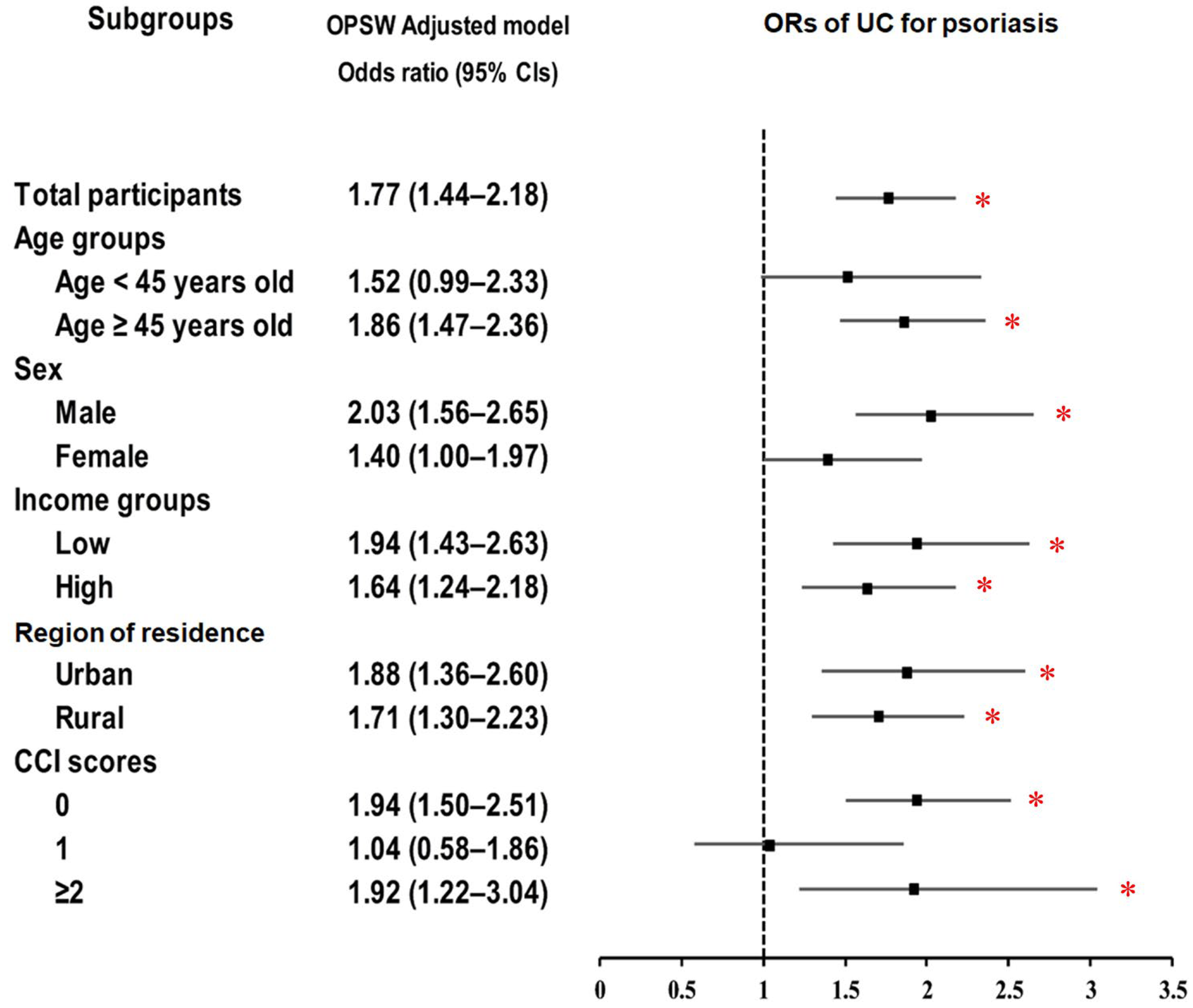
| Odds ratio of UC (n = 31,645) | ||||||
| Psoriasis | 100/6329 (1.6) | 229/25,316 (0.9) | 1.76 (1.39–2.23) | <0.001 * | 1.77 (1.44–2.18) | <0.001 * |
| Control | 6229/6329 (98.4) | 25,087/25,316 (99.1) | 1 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Han, K.M.; Kim, J.-H.; Yoo, D.M.; Choi, H.G.; Kim, N.Y.; Min, K.-W.; Kwon, M.J. Psoriasis as a Potential Risk Factor for Inflammatory Bowel Disease: Findings from a Nationally Representative Korean Population. Biomedicines 2025, 13, 2334. https://doi.org/10.3390/biomedicines13102334
Kang HS, Han KM, Kim J-H, Yoo DM, Choi HG, Kim NY, Min K-W, Kwon MJ. Psoriasis as a Potential Risk Factor for Inflammatory Bowel Disease: Findings from a Nationally Representative Korean Population. Biomedicines. 2025; 13(10):2334. https://doi.org/10.3390/biomedicines13102334
Chicago/Turabian StyleKang, Ho Suk, Kyeong Min Han, Joo-Hee Kim, Dae Myoung Yoo, Hyo Geun Choi, Nan Young Kim, Kyueng-Whan Min, and Mi Jung Kwon. 2025. "Psoriasis as a Potential Risk Factor for Inflammatory Bowel Disease: Findings from a Nationally Representative Korean Population" Biomedicines 13, no. 10: 2334. https://doi.org/10.3390/biomedicines13102334
APA StyleKang, H. S., Han, K. M., Kim, J.-H., Yoo, D. M., Choi, H. G., Kim, N. Y., Min, K.-W., & Kwon, M. J. (2025). Psoriasis as a Potential Risk Factor for Inflammatory Bowel Disease: Findings from a Nationally Representative Korean Population. Biomedicines, 13(10), 2334. https://doi.org/10.3390/biomedicines13102334







