Comparison of Radio- and Phototoxicity in Association with an Enhancing Effect of the Photosensitizers Psoralen, Trioxsalen and Ortho-Iodo-Hoechst33258 on FaDu, PC-3, 4T1 and B16-F10 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Characterization of Cells
2.3. PS and Irradiation
2.4. Colony Formation Assay
2.5. Detection of the DSB Using the H2A.X Assay
2.6. DNA Extraction and ELISA Assay
2.7. Statistics
3. Results
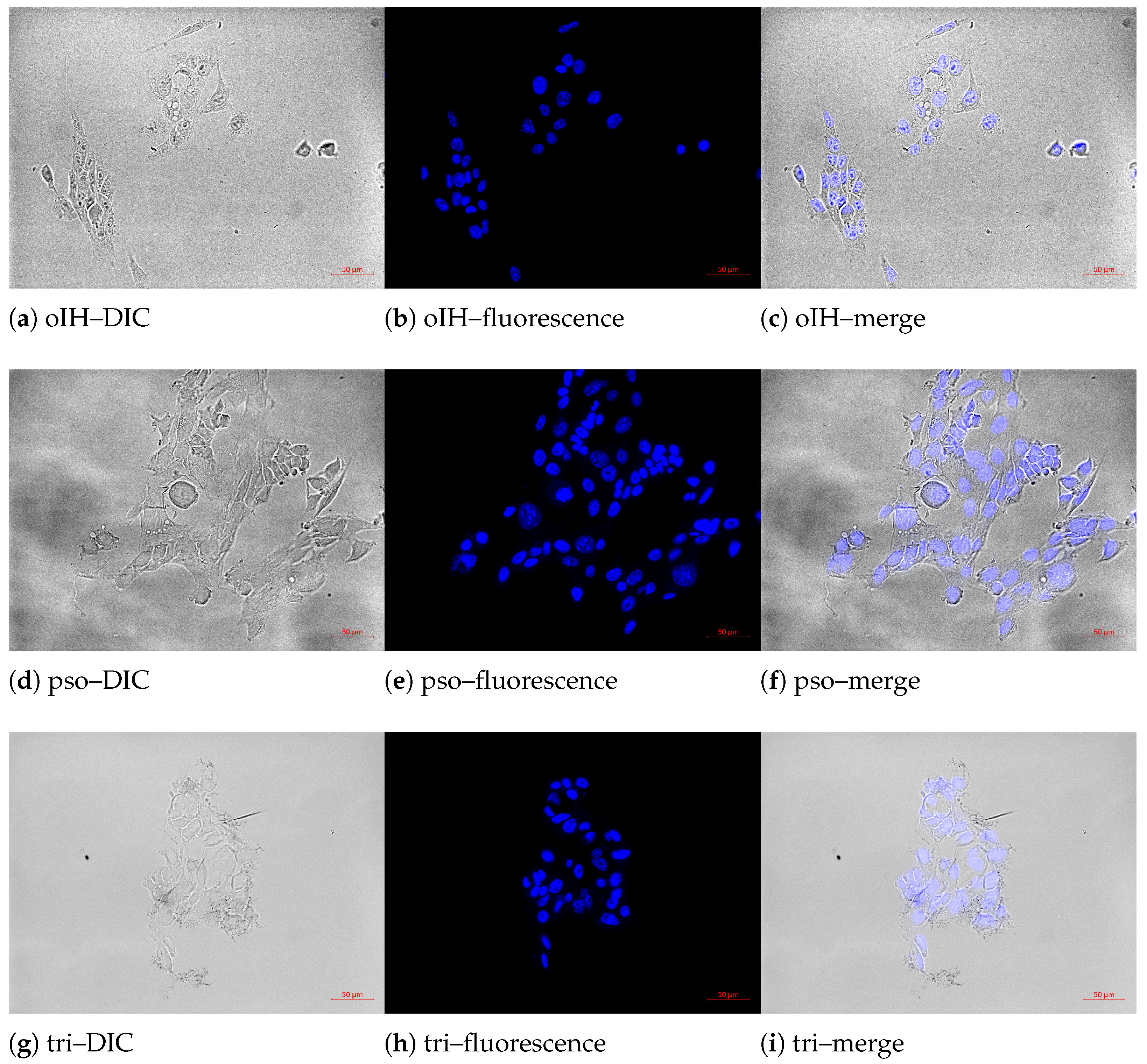
3.1. Cell and Nuclear Size, Nuclear–Plasma Ratio, Mean DNA Content and the Doubling Time of the Cell Lines
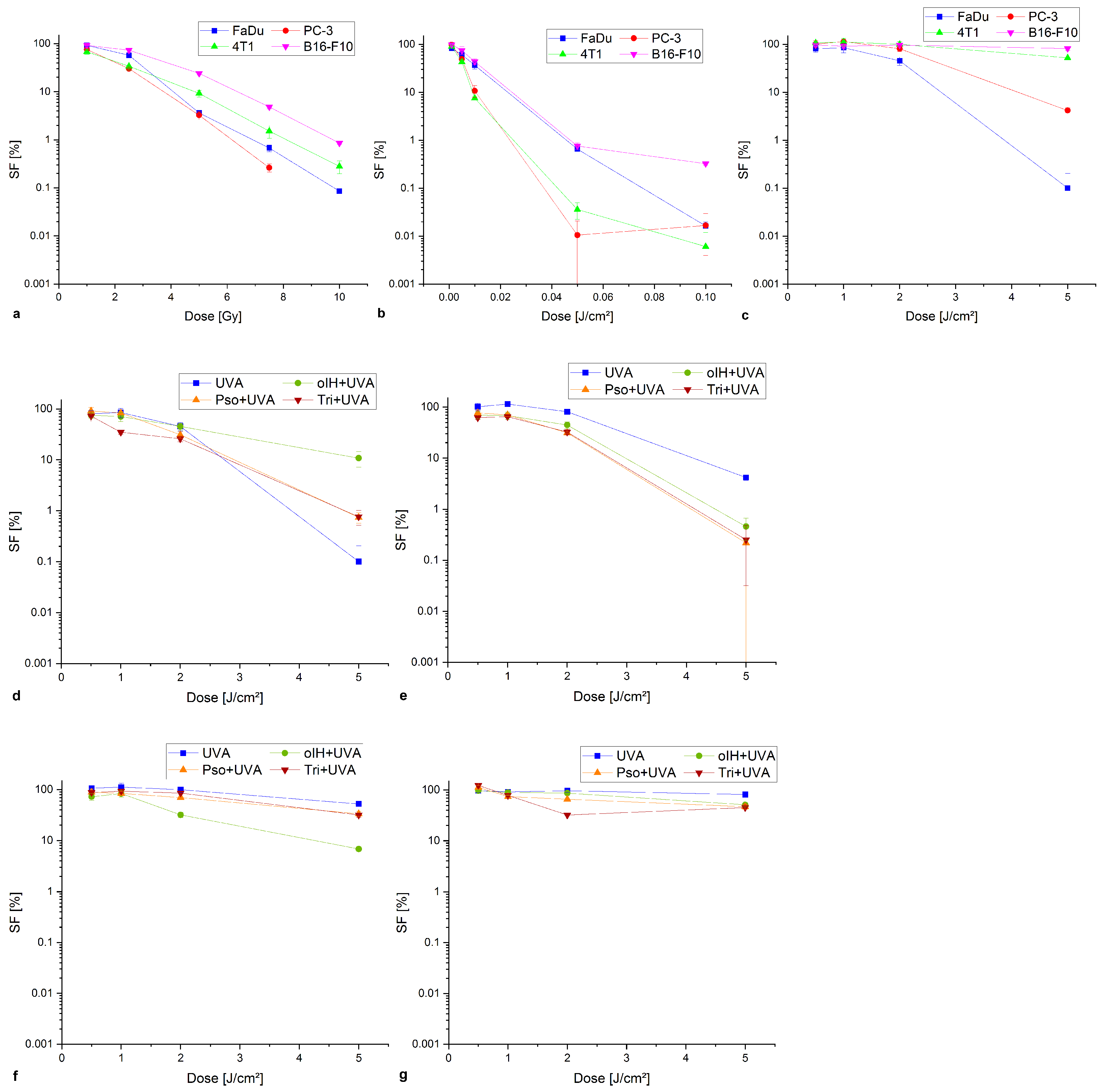
3.2. Determination of the Survival Fraction
3.3. Damage Analysis on the Example of PC-3 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPD | cyclobutane-pyrimidine dimer |
| DSB | double-strand break |
| MTP | multititer plate |
| NER | nucleotide excision repair |
| oIH | ortho-iodo-Hoechst33258 |
| PS | photosensitizer |
| pso | psoralen |
| ROS | reactive oxygen species |
| SF | survival fraction |
| tri | trioxsalen |
| UV | ultraviolet light |
| X-rays | external ionizing radiation |
Appendix A
Appendix A.1. Colony Formation Assay Compared to Damage Analysis of Double-Stand Breaks and Cyclobutane Pyrimidine Dimers
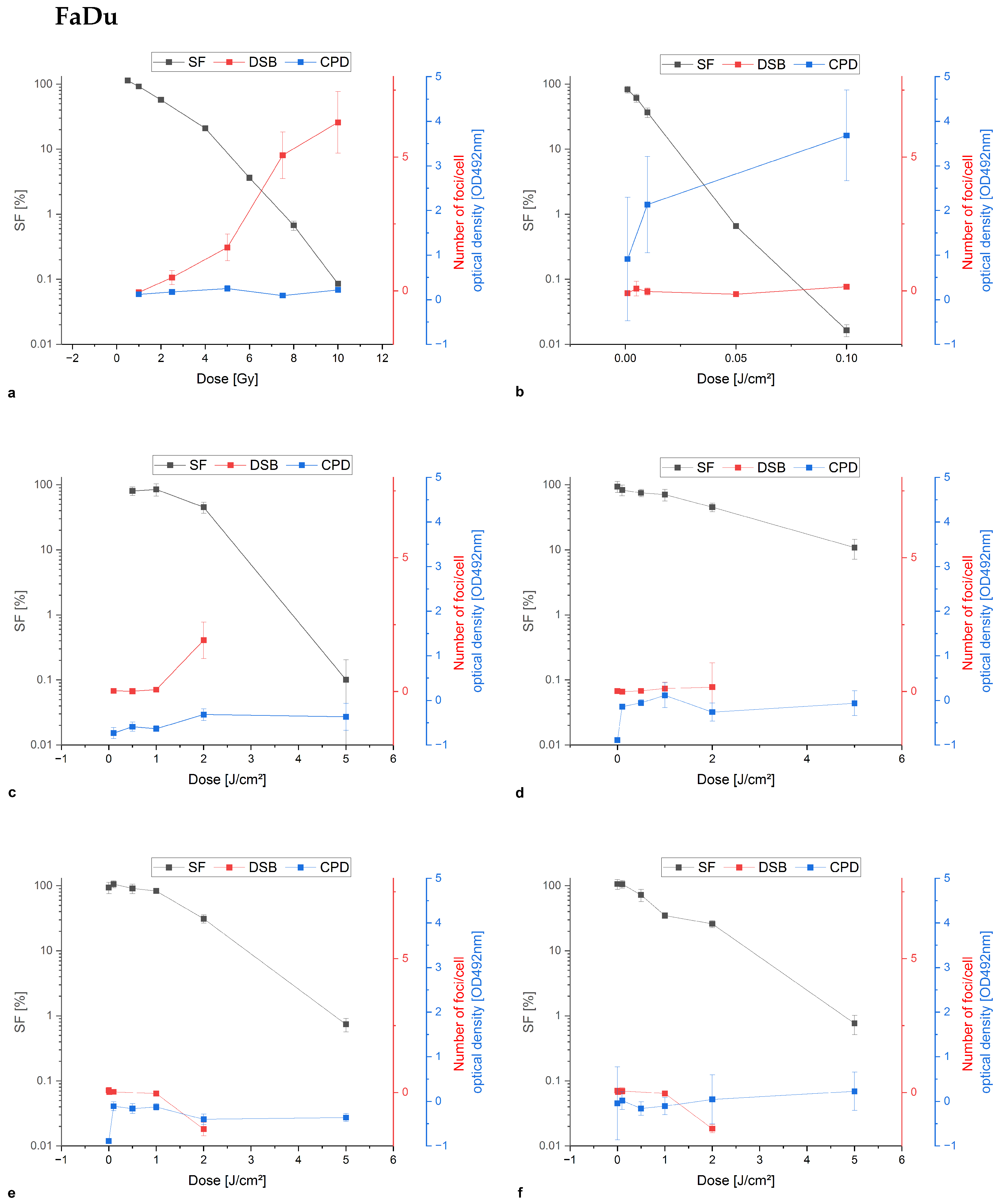

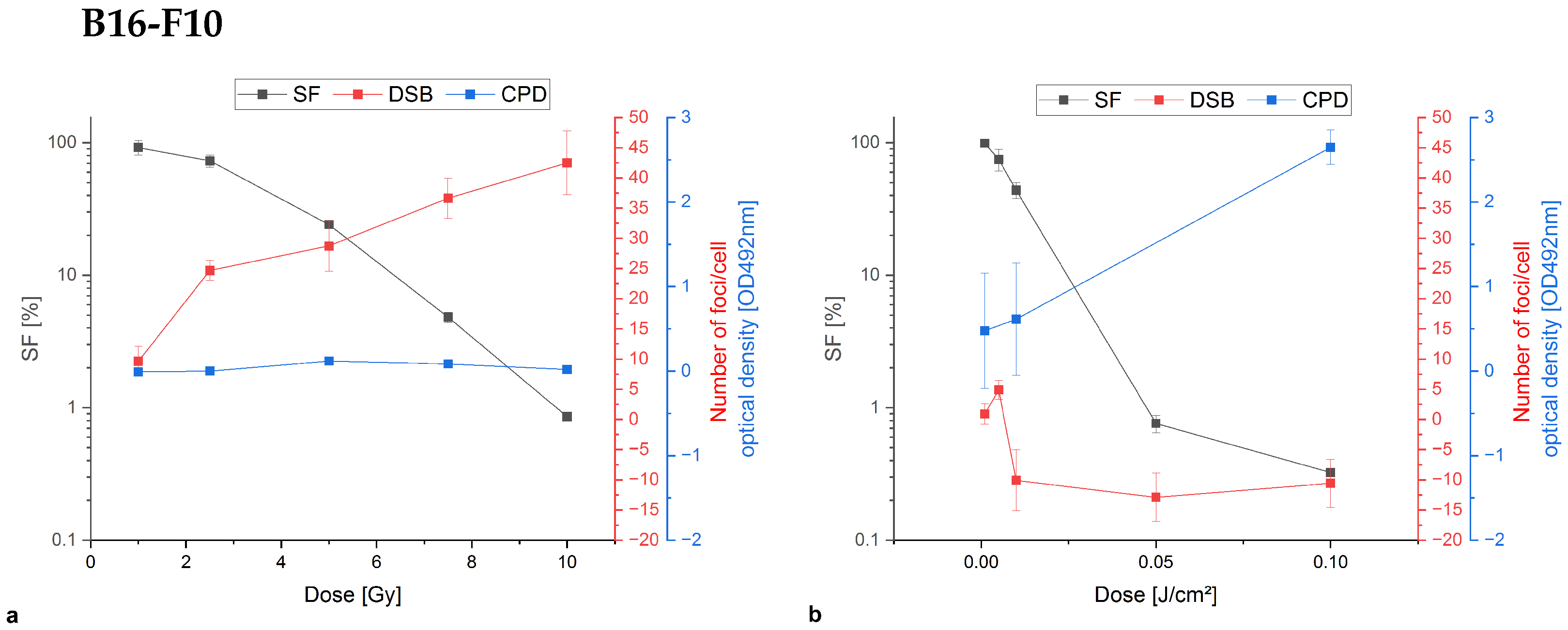
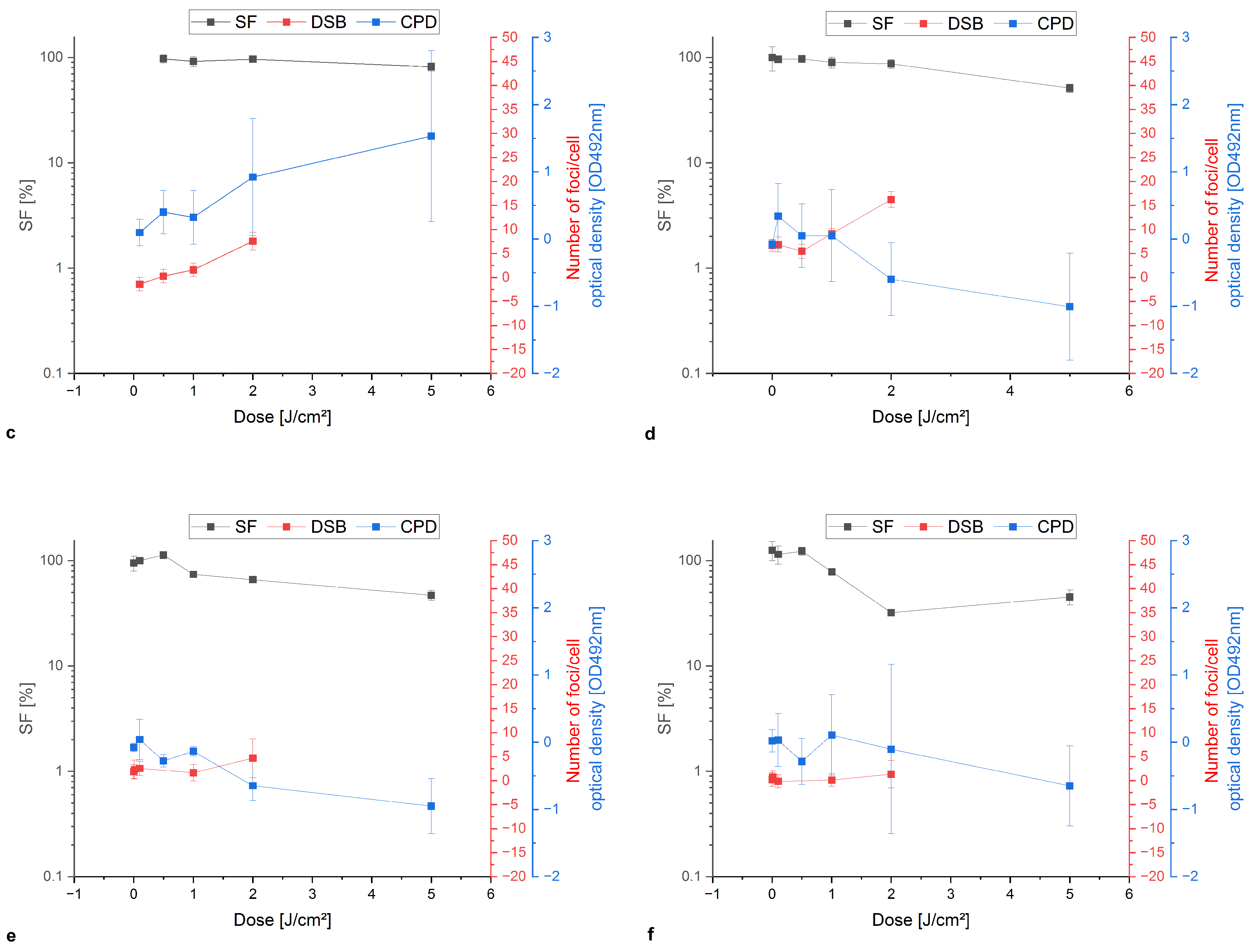
References
- Mouret, S.; Baudouin, C.; Charveron, M.; Favier, A.; Cadet, J.; Douki, T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc. Natl. Acad. Sci. USA 2006, 103, 13765–13770. [Google Scholar] [CrossRef]
- Cortat, B.; Garcia, C.C.M.; Quinet, A.; Schuch, A.P.; de Lima-Bessa, K.M.; Menck, C.F.M. The relative roles of DNA damage induced by UVA irradiation in human cells. Photochem. Photobiol. Sci. 2013, 12, 1483–1495. [Google Scholar] [CrossRef]
- You, Y.H.; Lee, D.H.; Yoon, J.H.; Nakajima, S.; Yasui, A.; Pfeifer, G. Cyclobutane Pyrimidine Dimers Are Responsible for the Vast Majority of Mutations Induced by UVB Irradiation in Mammalian Cells. J. Biol. Chem. 2001, 276, 44688–44694. [Google Scholar] [CrossRef]
- Lee, W.; Matsika, S. Mechanistic Aspects of the Effect of Flanking Nucleotide Sequence on CPD Formation and CPD Self-Repair in DNA. J. Phys. Chem. B 2023, 127, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.K.; Datta, A.K.; Worobo, R.W. Ultraviolet Light. J. Food Sci. 2000, 65, 90–92. [Google Scholar] [CrossRef]
- Peter, G.; Rajewsky, B. Die indirekte Wirkung von Röntgenstrahlen auf Aminosäuren II. Bestrahlung von Tryptophan. Z. Naturforschung B 1963, 18, 110–114. [Google Scholar] [CrossRef]
- Chilakamathi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Meunier, J.R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Pathak, M.A.; Mohn, G.R. Molecular and genetic basis of furocoumarin reactions. Mutat. Res. Genet. Toxicol. 1976, 39, 29–74. [Google Scholar] [CrossRef]
- Mouret, S.; Philippe, C.; Gracia-Chantegrel, J.; Banyasz, A.; Karpati, S.; Markovitsi, D.; Douki, T. UVA-induced cyclobutane pyrimidine dimers in DNA: A direct photochemical mechanism? Org. Biomol. Chem. 2010, 8, 1706–1711. [Google Scholar] [CrossRef]
- Aboul-Enein, H.; Kladna, A.; Kruk, I.; Lichszteld, K.; Michalska, T. Effect of psoralens on Fenton-like reaction generating reactive oxygen species. Biopolymers 2003, 72, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Murray, V.; D’Cunha, G.; Pardee, M.; Kampouris, E.; Haigh, A.; Kelly, D.; Hodgson, G. Rapid communication: Radiation Sensitization by an Iodine-labelled DNA Ligand. Int. J. Radiat. Biol. 1990, 57, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Rodd, A.; Ververis, K.; Karagiannis, T. Combination Phototherapy with a Histone Deacetylase Inhibitor and a Potent DNA-Binding Bibenzimidazole: Effects in Haematological Cell Lines. Lymphoma 2012, 2012, 405327. [Google Scholar] [CrossRef]
- Sung, J.; Ververis, K.; Karagiannis, T. Histone deacetylase inhibitors potentiate photochemotherapy in cutaneous T-cell lymphoma MyLa cells. J. Photochem. Photobiol. B Biol. 2014, 131, 104–112. [Google Scholar] [CrossRef]
- Podhorecka, M.; Skldanowski, A.; Bozko, P. H2A.X Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef] [PubMed]
- Eicheler, W.; Zips, D.; Dörfler, A.; Grénman, R.; Baumann, M. Splicing Mutations in TP53 in Human Squamous Cell Carcinoma Lines Influence Immunohistochemical Detection. J. Histochem. Cytochem. 2002, 50, 197–204. [Google Scholar] [CrossRef]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- McMillan, T.; Leatherman, E.; Ridley, A.; Shorrocks, J.; Tobi, S.; Whiteside, J. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 2008, 60, 969–976. [Google Scholar] [CrossRef]
- Huang, R.; Fan, Y.; deBelle, I.; Ni, Z.; Matheny, W.; Adamson, E. Egr-1 inhibits apoptosis during the UV response: Correlation of cell survival with Egr-1 phosphorylation. Cell Death Differ. 1998, 1, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, T.; Lobachevsky, P.; Martin, R. DNA targeted UVA photosensitization: Characterization of an extremely photopotent iodinated minor groove binding DNA ligand. J. Photochem. Photobiol. B Biol. 2006, 83, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hearst, J.E. Psoralen Photochemistry and Nucleic Acid Structure. J. Investig. Dermatol. 1981, 77, 39–44. [Google Scholar] [CrossRef]
- Jiang, J.; Bellani, M.; Li, J.; Wang, P.; Seidman, M.M.; Wang, Y. Arsenite Binds to the RING Finger Domain of FANCL E3 Ubiquitin Ligase and Inhibits DNA Interstrand Crosslink Repair. ACS Chem. Biol. 2017, 12, 1858–1866. [Google Scholar] [CrossRef]
- van Oorschot, B.; Hovingh, S.E.; Rodermond, H.; Güçlü, A.; Losekoot, N.; Geldof, A.A.; Barendsen, G.W.; Stalpers, L.J.; Franken, N.A. Decay of γ-H2AX foci correlates with potentially lethal damage repair in prostate cancer cells. Oncol. Rep. 2013, 29, 2175–2180. [Google Scholar] [CrossRef]
- Eichler, H.J.; Kronfeldt, H.D.; Sahm, J. Chapter 46—Röntgenstrahlung. In Das Neue Physikalische Grundpraktikum; Springer: Berlin/Heidelberg, Germany, 2001; pp. 477–487. [Google Scholar] [CrossRef]
- Staszewski, O.; Nikolova, T.; Kaina, B. Kinetics of gamma-H2AX focus formation upon treatment of cells with UV light and alkylating agents. Environ. Mol. Mutagen. 2008, 49, 734–740. [Google Scholar] [CrossRef]
- Gyenge, E.B.; Hiestand, S.; Graefe, S.; Walt, H.; Maake, C. Cellular and molecular effects of the liposomal mTHPC derivative Foslipos in prostate carcinoma cells in vitro. Photodiagn. Photodyn. Ther. 2011, 8, 86–96. [Google Scholar] [CrossRef]
- Briggs, B.; Ververis, K.; Rodd, A.L.; Foong, L.J.L.; Da Silva, F.M.; Karagiannis, T.C. Photosensitization by iodinated DNA minor groove binding ligands: Evaluation of DNA double-strand break induction and repair. J. Photochem. Photobiol. B Biol. 2011, 103, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Aekrungrueangkit, C.; Wangngae, S.; Kamkaew, A.; Ardkhean, R.; Thongnest, S.; Boonsombat, J.; Ruchirawat, S.; Khotavivattana, T. Novel psoralen derivatives as anti-breast cancer agents and their light-activated cytotoxicity against HER2 positive breast cancer cells. Sci. Rep. 2022, 12, 13487. [Google Scholar] [CrossRef]
- Cole, R.S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim. Biophys. Acta (BBA) Nucleic Acids Protein Synth. 1971, 254, 30–39. [Google Scholar] [CrossRef]
- Maor-Shoshani, A.; Meira, L.B.; Yang, X.; Samson, L.D. 3-Methyladenine DNA glycosylase is important for cellular resistance to psoralen interstrand cross-links. DNA Repair 2008, 7, 1399–1406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Besaratinia, A.; Yoon, J.I.; Schroeder, C.; Bradforth, S.E.; Cockburn, M.; Pfeifer, G.P. Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011, 25, 3079–3091. [Google Scholar] [CrossRef]
- Chinna Ayya Swamy, P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Maiti, R.; Patel, B.; Patel, N.; Patel, M.; Patel, A.; Dhanesha, N. Antibody drug conjugates as targeted cancer therapy: Past development, present challenges and future opportunities. Arch. Pharmacal Res. 2023, 46, 361–388. [Google Scholar] [CrossRef]
- Gordon, P.; Diffey, B.; Matthews, J.; Farr, P. A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis. J. Am. Acad. Dermatol. 1999, 41, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Kanwar, A.; Parsad, D.; De, D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: An open prospective study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 638–642. [Google Scholar] [CrossRef] [PubMed]

| Cell Lines | Sizes of Cells | Nuclear Size | Nuclear–Plasma Ratio | DNA Content | Doubling Time |
|---|---|---|---|---|---|
| [m] | [m] | [x% nuclei] | [AU] | [h] | |
| FaDu | 14.75 | 8.22 | 55.7 | 193.3 | 35 |
| PC-3 | 16.25 | 8.83 | 54.3 | 187.2 | 37 |
| 4T1 | 12.58 | 8.72 | 69.3 | 184.1 | 22 |
| B16-F10 | 10.83 | 7.90 | 72.9 | 184.0 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tietze, K.; Brandt, F.; Wetzig, K.; Hübinger, L.; Pretze, M.; Bundschuh, R.A.; Kotzerke, J. Comparison of Radio- and Phototoxicity in Association with an Enhancing Effect of the Photosensitizers Psoralen, Trioxsalen and Ortho-Iodo-Hoechst33258 on FaDu, PC-3, 4T1 and B16-F10 Cells. Biomedicines 2025, 13, 73. https://doi.org/10.3390/biomedicines13010073
Tietze K, Brandt F, Wetzig K, Hübinger L, Pretze M, Bundschuh RA, Kotzerke J. Comparison of Radio- and Phototoxicity in Association with an Enhancing Effect of the Photosensitizers Psoralen, Trioxsalen and Ortho-Iodo-Hoechst33258 on FaDu, PC-3, 4T1 and B16-F10 Cells. Biomedicines. 2025; 13(1):73. https://doi.org/10.3390/biomedicines13010073
Chicago/Turabian StyleTietze, Katja, Florian Brandt, Kerstin Wetzig, Lisa Hübinger, Marc Pretze, Ralph Alexander Bundschuh, and Jörg Kotzerke. 2025. "Comparison of Radio- and Phototoxicity in Association with an Enhancing Effect of the Photosensitizers Psoralen, Trioxsalen and Ortho-Iodo-Hoechst33258 on FaDu, PC-3, 4T1 and B16-F10 Cells" Biomedicines 13, no. 1: 73. https://doi.org/10.3390/biomedicines13010073
APA StyleTietze, K., Brandt, F., Wetzig, K., Hübinger, L., Pretze, M., Bundschuh, R. A., & Kotzerke, J. (2025). Comparison of Radio- and Phototoxicity in Association with an Enhancing Effect of the Photosensitizers Psoralen, Trioxsalen and Ortho-Iodo-Hoechst33258 on FaDu, PC-3, 4T1 and B16-F10 Cells. Biomedicines, 13(1), 73. https://doi.org/10.3390/biomedicines13010073







