Inferior Outcomes of Fludarabine–Cyclophosphamide–Rituximab Chemotherapy in Korean Chronic Lymphocytic Leukemia Patients with Concurrent Thrombocytopenia and Anemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment, Response, and MRD Assessment
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Patient Characteristics
3.2. Efficacy of FCR and Cumulative Incidence of Secondary Malignancies
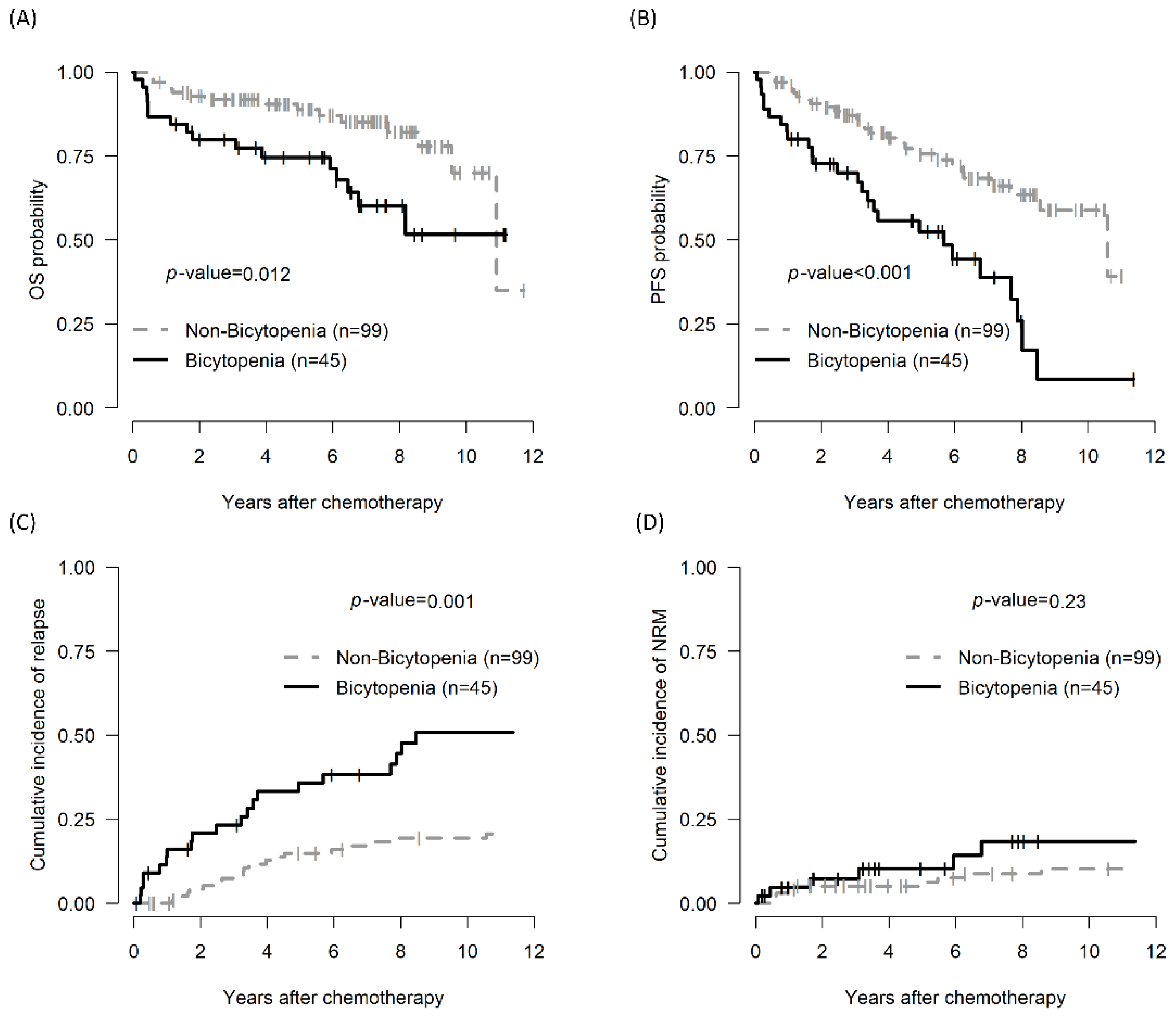
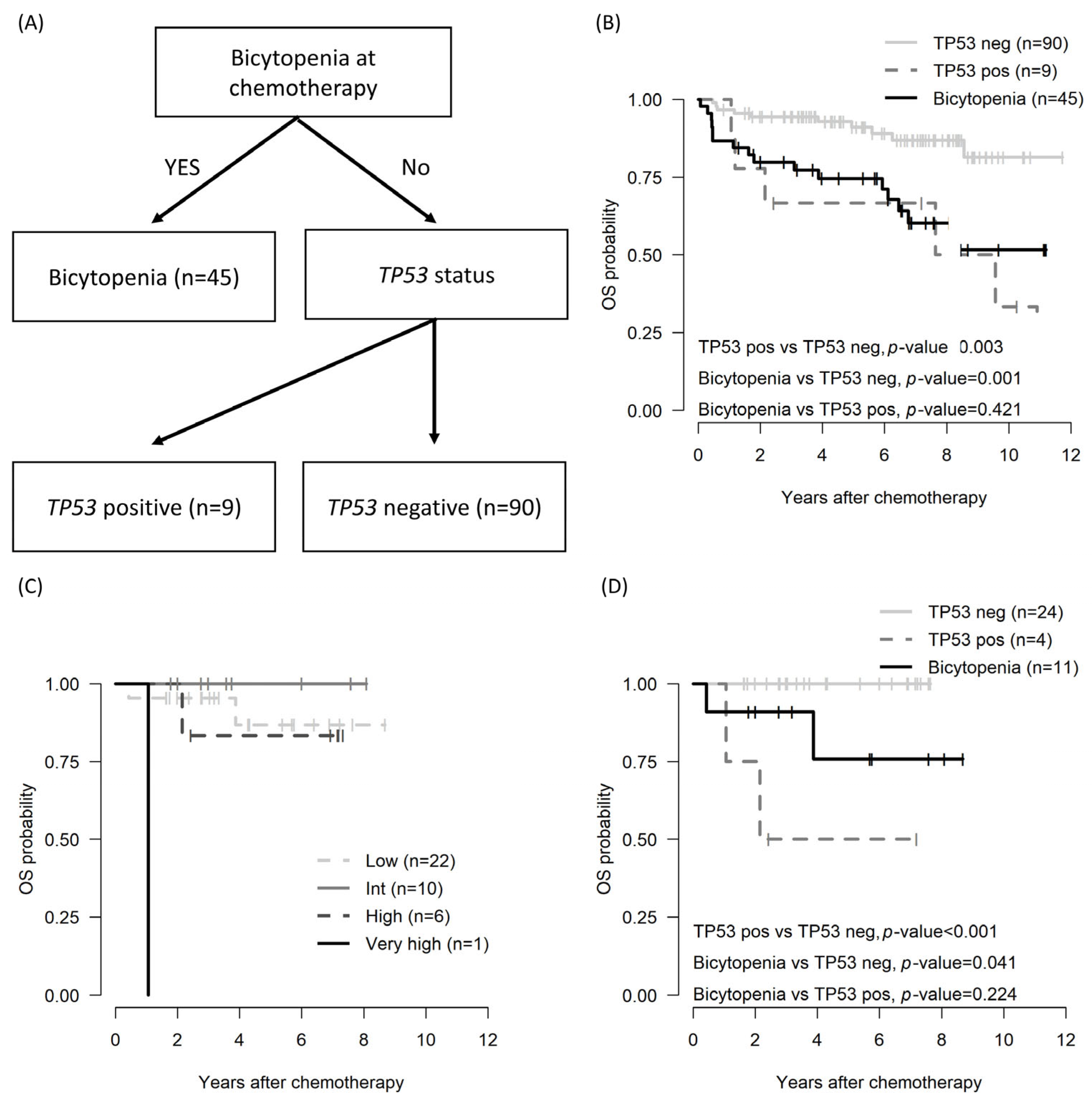
3.3. Impact of the Bicytopenia Group: OS, PFS, CIR, TRM, and Prognostic Factors
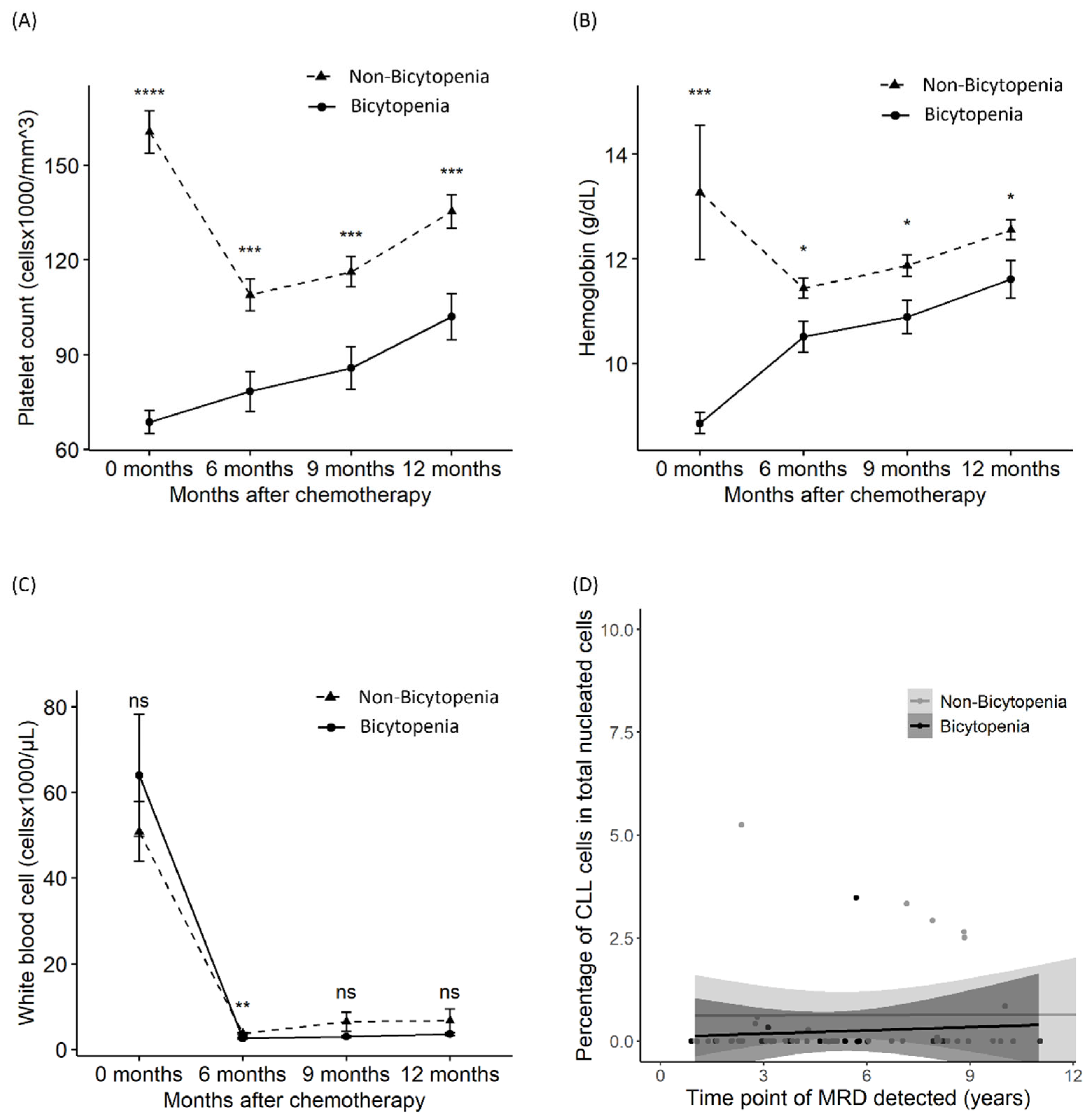
3.4. Suppression in Platelets, Hemoglobin, WBCs, and MRD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, S.O.; Suh, C.; Lee, D.H.; Chi, H.S.; Park, C.J.; Jang, S.S.; Shin, H.R.; Park, B.H.; Huh, J. Distribution of lymphoid neoplasms in the Republic of Korea: Analysis of 5318 cases according to the World Health Organization classification. Am. J. Hematol. 2010, 85, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Ito, H.; Matsuda, T.; Shibata, A.; Katsumi, A.; Nakamura, S.; Tomotaka, S.; Morton, L.M.; Weisenburger, D.D.; Matsuo, K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br. J. Haematol. 2014, 164, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Fink, A.M.; Bahlo, J.; Busch, R.; Kovacs, G.; Maurer, C.; Lange, E.; Köppler, H.; Kiehl, M.; Sökler, M.; et al. First-line chemoimmunotherapy with Bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016, 17, 928–942. [Google Scholar] [CrossRef]
- Benjamini, O.; Jain, P.; Trinh, L.; Qiao, W.; Strom, S.S.; Lerner, S.; Wang, X.; Burger, J.; Ferrajoli, A.; Kantarjian, H.; et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: Distribution and clinical outcomes. Leuk. Lymphoma 2015, 56, 1643–1650. [Google Scholar] [CrossRef]
- Kutsch, N.; Bahlo, J.; Robrecht, S.; Franklin, J.; Zhang, C.; Maurer, C.; De Silva, N.; Lange, E.; Weide, R.; Kiehl, M.G.; et al. Long term follow-up data and health-related quality of life in Frontline Therapy of Fit Patients Treated with FCR versus BR (CLL10 Trial of the GCLLSG). Hemasphere 2020, 4, e336. [Google Scholar] [CrossRef]
- Ahn, I.E.; Farooqui, M.Z.H.; Tian, X.; Valdez, J.; Sun, C.; Soto, S.; Lotter, J.; Housel, S.; Stetler-Stevenson, M.; Yuan, C.M.; et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 2018, 131, 2357–2366. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib–rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef]
- Munir, T.; Cairns, D.A.; Bloor, A.; Allsup, D.; Cwynarski, K.; Pettitt, A.; Paneesha, S.; Fox, C.P.; Eyre, T.A.; Forconi, F.; et al. Chronic lymphocytic leukemia therapy guided by measurable residual disease. N. Engl. J. Med. 2024, 390, 326–337. [Google Scholar] [CrossRef]
- Eichhorst, B.; Niemann, C.U.; Kater, A.P.; Fürstenau, M.; von Tresckow, J.; Zhang, C.; Robrecht, S.; Gregor, M.; Juliusson, G.; Thornton, P.; et al. First-line venetoclax combinations in chronic lymphocytic leukemia. N. Engl. J. Med. 2023, 388, 1739–1754. [Google Scholar] [CrossRef]
- Huber, H.; Edenhofer, S.; von Tresckow, J.; Robrecht, S.; Zhang, C.; Tausch, E.; Schneider, C.; Bloehdorn, J.; Fürstenau, M.; Dreger, P.; et al. Obinutuzumab (GA-101), ibrutinib, and venetoclax (GIVe) frontline treatment for high-risk chronic lymphocytic leukemia. Blood 2022, 139, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Hillmen, P.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): Interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Bazinet, A.; Wierda, W.G.; Tam, C.S.; O’Brien, S.M.; Saha, S.; Peterson, C.B.; Plunkett, W.; Keating, M.J. Sustained remissions in CLL after frontline FCR treatment with very-long-term follow-up. Blood 2023, 142, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, T.M.; Kang, M.J.; Koh, S.A.; Park, H.; Nam, S.H.; Han, J.J.; Lee, G.W.; Yuh, Y.J.; Lee, H.J.; et al. Treatment pattern of chronic lymphocytic leukemia/small lymphocytic lymphoma in Korea: A multicenter retrospective study (KCSG LY20-06). Korean J. Intern. Med. 2023, 38, 747–757. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Wierda, W.G.; Rawstron, A.; Cymbalista, F.; Badoux, X.; Rossi, D.; Brown, J.R.; Egle, A.; Abello, V.; Cervera Ceballos, E.; Herishanu, Y.; et al. Measurable residual disease in chronic lymphocytic leukemia: Expert review and consensus recommendations. Leukemia 2021, 35, 3059–3072. [Google Scholar] [CrossRef]
- Kwok, M.; Rawstron, A.C.; Varghese, A.; Evans, P.A.S.; O’Connor, S.J.M.; Doughty, C.; Newton, D.J.; Moreton, P.; Hillmen, P. Minimal residual disease is an independent predictor for 10-year survival in CLL. Blood 2016, 128, 2770–2773. [Google Scholar] [CrossRef]
- Keating, M.J.; O’Brien, S.; Albitar, M.; Lerner, S.; Plunkett, W.; Giles, F.; Andreeff, M.; Cortes, J.; Faderl, S.; Thomas, D.; et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J. Clin. Oncol. 2005, 23, 4079–4088. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Böttcher, S.; Letestu, R.; Villamor, N.; Fazi, C.; Kartsios, H.; de Tute, R.M.; Shingles, J.; Ritgen, M.; Moreno, C.; et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 2013, 27, 142–149. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Fazi, C.; Agathangelidis, A.; Villamor, N.; Letestu, R.; Nomdedeu, J.; Palacio, C.; Stehlikova, O.; Kreuzer, K.A.; Liptrot, S.; et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: An European Research Initiative on CLL study. Leukemia 2016, 30, 929–936. [Google Scholar] [CrossRef]
- Ahn, A.; Kim, H.S.; Kim, T.Y.; Lee, J.M.; Kang, D.; Yu, H.; Lee, C.Y.; Kim, Y.; Eom, K.S.; Kim, M. Genetic and clinical characteristics of Korean chronic lymphocytic leukemia patients with high frequencies of MYD88 mutations. Int. J. Mol. Sci. 2023, 24, 3177. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.G.; Ghosh, S. Survival trees based on heterogeneity in time-to-event and censoring distributions using parameter instability test. Stat. Anal. Data Min. ASA Data Sci. J. 2021, 14, 466–483. [Google Scholar] [CrossRef]
- Nabhan, C.; Rosen, S.T. Chronic lymphocytic leukemia: A clinical review. JAMA 2014, 312, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): A meta-analysis of individual patient data. Lancet Oncol. 2016, 17, 779–790. [Google Scholar] [CrossRef]
- Jang, M.A.; Yoo, E.H.; Kim, K.; Kim, W.S.; Jung, C.W.; Kim, S.H.; Kim, H.J. Chronic lymphocytic leukemia in Korean patients: Frequent atypical immunophenotype and relatively aggressive clinical behavior. Int. J. Hematol. 2013, 97, 403–408. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.H.; Jung, C.W.; Jo, J.C.; Kim, J.S.; Kim, I.; Park, S.; Cheong, J.W.; Park, S.H.; Kim, S.Y.; et al. Treatment outcome and prognostic factors of Korean patients with chronic lymphocytic leukemia: A multicenter retrospective study. Korean J. Intern. Med. 2021, 36, 194–204. [Google Scholar] [CrossRef]
- Herling, C.D.; Cymbalista, F.; Groß-Ophoff-Müller, C.; Bahlo, J.; Robrecht, S.; Langerbeins, P.; Fink, A.M.; Al-Sawaf, O.; Busch, R.; Porcher, R.; et al. Early treatment with FCR versus watch and wait in patients with stage Binet A high-risk chronic lymphocytic leukemia (CLL): A randomized phase 3 trial. Leukemia 2020, 34, 2038–2050. [Google Scholar] [CrossRef]
- Gill, S.; Carney, D.; Ritchie, D.; Wolf, M.; Westerman, D.; Prince, H.M.; Januszewicz, H.; Seymour, J.F. The frequency, manifestations, and duration of prolonged cytopenias after first-line fludarabine combination chemotherapy. Ann. Oncol. 2010, 21, 331–334. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, G.; Medeiros, L.J.; McDonnell, T.J.; Keating, M.J.; Wierda, W.G.; Wang, S.A. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod. Pathol. 2012, 25, 237–245. [Google Scholar] [CrossRef]
- Shatzel, J.J.; Olson, S.R.; Tao, D.L.; McCarty, O.J.T.; Danilov, A.V.; DeLoughery, T.G. Ibrutinib-associated bleeding: Pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. 2017, 15, 835–847. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, C.; Austen, B.; Powell, J.E.; Fegan, C.; Wandroo, F.; Jacobs, A.; Pratt, G.; Moss, P. South Asian chronic lymphocytic leukaemia patients have more rapid disease progression in comparison to White patients. Br. J. Haematol. 2008, 142, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Yang, D.H.; Oh, S.J.; Park, J.H.; Kim, J.A.; Kim, S.Y.; Choi, C.W.; Lee, W.S.; Kim, I.H.; Mun, Y.C.; et al. Effectiveness of pegfilgrastim prophylaxis in preventing febrile neutropenia during R-FC chemoimmunotherapy for chronic lymphocytic leukemia: A multicenter prospective phase II study. Front. Oncol. 2023, 13, 998014. [Google Scholar] [CrossRef] [PubMed]
| Total | Non-Bicytopenia (n = 99) | Bicytopenia (n = 45) | p-Value | |
|---|---|---|---|---|
| Age > 65 years at chemotherapy, N (%) | 44 (30.6) | 23 (23.2) | 21 (46.7) | 0.008 |
| Sex, female, N (%) | 53 (36.8) | 37 (37.4) | 16 (35.6) | 0.981 |
| Binet stage, N (%) | <0.001 | |||
| −A | 14 (9.7) | 14 (14.1) | 0 (0.0) | |
| −B | 33 (22.9) | 33 (33.3) | 0 (0.0) | |
| −C | 97 (67.4) | 52 (52.5) | 45 (100.0) | |
| Rai points, N (%) | <0.001 | |||
| 0 | 11 (7.6) | 11 (11.1) | 0 (0.0) | |
| −1 | 23 (16.0) | 23 (23.2) | 0 (0.0) | |
| −2 | 13 (9.0) | 13 (13.1) | 0 (0.0) | |
| −3 | 33 (22.9) | 33 (33.3) | 0 (0.0) | |
| −4 | 64 (44.4) | 19 (19.2) | 45 (100.0) | |
| Binet B–C or Rai I–IV, N (%) | 97 (67.4) | 52 (52.5) | 45 (100.0) | <0.001 |
| Serum β2 microglobulin, N (%) | 0.001 | |||
| >3.5 mg/L | 20 (19.6) | 8 (11.0) | 12 (41.4) | |
| ≤3.5 mg/L | 82 (80.4) | 65 (89.0) | 17 (58.6) | |
| IGHV mutational status, N (%) | 0.23 | |||
| Unmutated | 10 (21.7) | 9 (28.1) | 1 (7.1) | |
| Mutated | 36 (78.3) | 23 (71.9) | 13 (92.9) | |
| TP53 status positivity, N (%) | 11 (7.6) | 9 (9.1) | 2 (4.4) | 0.526 |
| Deletion 11 positivity, N (%) | 15 (10.4) | 8 (8.1) | 7 (15.6) | 0.286 |
| Complex karyotype, N (%) | 22 (15.3) | 16 (16.2) | 6 (13.3) | 0.851 |
| Failure to complete six cycles of chemotherapy, N (%) | 32 (22.2) | 16 (16.2) | 16 (35.6) | 0.017 |
| Overall Survival | Progression-Free Survival | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| Age > 65 years vs. ≤65 years | 1.38 (0.66, 2.87) | 0.389 | 1.17 (0.66, 2.09) | 0.586 |
| Sex, female vs. male | 0.84 (0.40, 1.75) | 0.641 | 0.84 (0.48, 1.47) | 0.531 |
| Bicytopenia vs. non-bicytopenia | 2.38 (1.19, 4.79) | 0.015 | 2.81 (1.64, 4.83) | <0.001 |
| Binet B–C or Rai I–IV vs the other | 2.46 (1.01, 5.99) | 0.047 | 1.56 (0.86, 2.83) | 0.147 |
| β2 microglobulin, >3.5 vs. ≤3.5 | 3.49 (1.51, 8.05) | 0.003 | 2.47 (1.28, 4.76) | 0.007 |
| IGHV, unmutated vs. mutated | 1.04 (0.11, 10.1) | 0.97 | 0.82 (0.21, 3.15) | 0.775 |
| TP53 status, positive vs. negative | 3.03 (1.30, 7.08) | 0.01 | 2.71 (1.32, 5.59) | 0.007 |
| Deletion 11, positive vs. negative | 1.11 (0.39, 3.18) | 0.846 | 2.23 (1.08, 4.60) | 0.031 |
| Complex karyotype vs. the other | 0.86 (0.30, 2.45) | 0.775 | 2.16 (1.13, 4.14) | 0.02 |
| Cumulative incidence of relapse | Cumulative incidence of non-relapse | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| Age > 65 years vs. ≤65 | 3.06 (1.48, 6.36) | 0.77 | 0.39 (0.05, 2.99) | 0.71 |
| Sex, female vs. male | 1.02 (0.53, 1.95) | 0.96 | 0.58 (0.19, 1.79) | 0.34 |
| Bicytopenia vs. non-bicytopenia | 2.87 (1.55, 5.30) | 0.001 | 1.52(0.54, 4.23) | 0.43 |
| Binet B–C or Rai I–IV vs. the other | 1.43 (0.72, 2.82) | 0.31 | 1.45 (0.48, 4.41) | 0.52 |
| β2 microglobulin, >3.5 vs. ≤3.5 | 1.3 (0.56, 3.02) | 0.55 | 4.13 (1.47, 11.57) | 0.007 |
| IGHV, unmutated vs. mutated | 0.49 (0.11, 2.10) | 0.33 | NA | NA |
| TP53 status, positive vs. negative | 2.64 (1.33, 5.25) | 0.006 | 1.73 (0.37, 8.01) | 0.48 |
| Deletion 11, positive vs. negative | 3.71 (1.93, 7.16) | <0.001 | NA | NA |
| Complex karyotype vs. the other | 3.06 (1.48, 6.35) | 0.003 | 0.39 (0.05, 2.99) | 0.37 |
| Overall Survival | Progression-Free Survival | Cumulative Incidence of Relapse | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Bicytopenia vs. non-bicytopenia | 2.43 (0.98, 6.05) | 0.056 | 3.67 (1.70, 7.90) | <0.001 | 3.41 (1.76, 6.61) | <0.001 |
| β2 microglobulin, >3.5 vs. ≤3.5 | 2.92 (1.15, 7.43) | 0.025 | 1.69 (0.82, 3.49) | 0.158 | ||
| TP53 status, positive vs. negative | 3.50 (1.11, 11.1) | 0.033 | 2.61 (1.03, 6.60) | 0.042 | 3.07 (1.37, 6.85) | 0.006 |
| Deletion 11, positive vs. negative | 1.07 (0.41, 2.80) | 0.89 | 2.42 (1.26, 4.67) | 0.008 | ||
| Complex karyotype vs. the other | 3.02 (1.09, 8.37) | 0.034 | 2.38 (1.04, 5.44) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Min, G.-J.; Jeon, Y.-W.; Yahng, S.-A.; Cho, S.-G.; Lee, J.-M.; Kim, M.; Eom, K.-S. Inferior Outcomes of Fludarabine–Cyclophosphamide–Rituximab Chemotherapy in Korean Chronic Lymphocytic Leukemia Patients with Concurrent Thrombocytopenia and Anemia. Biomedicines 2025, 13, 194. https://doi.org/10.3390/biomedicines13010194
Kim T-Y, Min G-J, Jeon Y-W, Yahng S-A, Cho S-G, Lee J-M, Kim M, Eom K-S. Inferior Outcomes of Fludarabine–Cyclophosphamide–Rituximab Chemotherapy in Korean Chronic Lymphocytic Leukemia Patients with Concurrent Thrombocytopenia and Anemia. Biomedicines. 2025; 13(1):194. https://doi.org/10.3390/biomedicines13010194
Chicago/Turabian StyleKim, Tong-Yoon, Gi-June Min, Young-Woo Jeon, Seung-Ah Yahng, Seok-Goo Cho, Jong-Mi Lee, Myungshin Kim, and Ki-Seong Eom. 2025. "Inferior Outcomes of Fludarabine–Cyclophosphamide–Rituximab Chemotherapy in Korean Chronic Lymphocytic Leukemia Patients with Concurrent Thrombocytopenia and Anemia" Biomedicines 13, no. 1: 194. https://doi.org/10.3390/biomedicines13010194
APA StyleKim, T.-Y., Min, G.-J., Jeon, Y.-W., Yahng, S.-A., Cho, S.-G., Lee, J.-M., Kim, M., & Eom, K.-S. (2025). Inferior Outcomes of Fludarabine–Cyclophosphamide–Rituximab Chemotherapy in Korean Chronic Lymphocytic Leukemia Patients with Concurrent Thrombocytopenia and Anemia. Biomedicines, 13(1), 194. https://doi.org/10.3390/biomedicines13010194





