Nanoparticle-Based Therapies for Management of Subarachnoid Hemorrhage, Neurotrauma, and Stroke
Abstract
1. Introduction
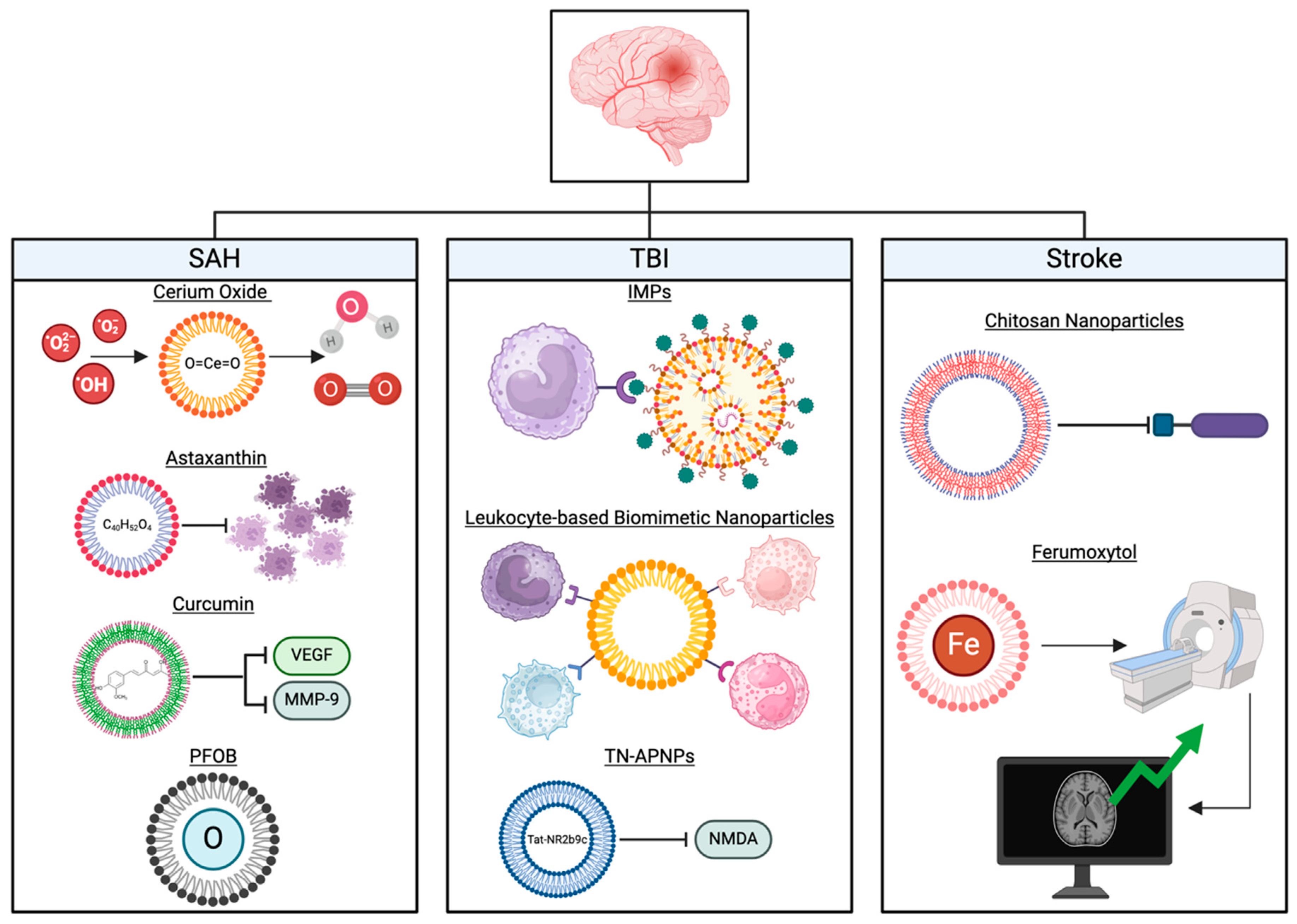
2. Subarachnoid Hemorrhage
2.1. Cerium Oxide Nanoparticles in the Scavenging of Reactive Oxygenated Species
2.2. Antioxidant and Anti-Inflammatory Properties of Astaxanthin
2.3. Neuroprotective Effects of Curcumin After BBB Disruption
2.4. Early Brain Injury Mitigation Effects of Perfluorooctyl-Bromide (PFOB)
3. Neurotrauma
3.1. PLGA Nanoparticles
3.2. Immunomodulatory Nanoparticles
3.3. Leukocyte-Based Biomimetic Nanoparticles
3.4. TN-APNPs Carrying Tat-NR2b9c
4. Stroke
4.1. Current Management
4.2. Nanoparticles as a Drug Delivery System
4.3. Nanoparticles as Agents of Neurotherapy and Enhanced Imaging
5. Translational Potential of Nanoparticle-Based Therapeutics
6. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| ATX | astaxanthin |
| BBB | blood–brain barrier |
| C-NPs | curcumin nanoparticles |
| CeNPs | cerium oxide nanoparticles |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine |
| EBI | early brain injury |
| GSH | glutathione |
| HIF-1a | Hypoxia-Inducible Factor-1 alpha |
| IMPs | immunomodulatory nanoparticles |
| NMDA | N-methyl-D-aspartate |
| NP | nanoparticle |
| Nrf2 | nuclear factor-erythroid 2 p45-related factor 2 |
| PFC | perfluorocarbon |
| PFOB | Perfluorooctyl-Bromide |
| PGAs | polyglycolides |
| PLAs | polylactides |
| PLGAs | poly(lactide-co-glycolides) |
| ROS | reactive oxygenated species |
| SAH | subarachnoid hemorrhage |
| TBI | traumatic brain injury |
| VEGF | vascular endothelial growth factor |
| Z-DEVD-FMK | N-benzyloxycarbonylAsp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethyl ketone |
References
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Petkar, K.C.; Chavhan, S.S.; Agatonovik-Kustrin, S.; Sawant, K.K. Nanostructured Materials in Drug and Gene Delivery: A Review of the State of the Art. Crit. Rev. Ther. Drug Carrier Syst. 2011, 28, 101–164. [Google Scholar] [CrossRef]
- Kanwar, J.; Sriramoju, B.; Kanwar, R.K. Neurological disorders and therapeutics targeted to surmount the blood–brain barrier. Int. J. Nanomed. 2012, 7, 3259–3278. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Budai, M.; Szógyi, M. Liposomes as drug carrier systems. Preparation, classification and therapeutic advantages of liposomes. Acta Pharm. Hung. 2001, 71, 114–118. [Google Scholar] [PubMed]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview / Nanonosači na bazi čvrstih lipida: Pregled. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef]
- Md, S.; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A.; Ali, J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2014, 40, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.-Y. Subarachnoid Hemorrhage. Contin. Lifelong Learn. Neurol. 2021, 27, 1201–1245. [Google Scholar] [CrossRef] [PubMed]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef]
- Dority, J.S.; Oldham, J.S. Subarachnoid Hemorrhage. Anesthesiol. Clin. 2016, 34, 577–600. [Google Scholar] [CrossRef] [PubMed]
- Baranoski, J.F.; Ducruet, A.F. Nanoparticle-Facilitated Delivery of Antioxidant Therapy following Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2019, 85, E174–E175. [Google Scholar] [CrossRef]
- Sehba, F.A.; Bederson, J.B. Mechanisms of acute brain injury after subarachnoid hemorrhage. Neurol. Res. 2006, 28, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Rifkind, J.M.; Mohanty, J.G.; Nagababu, E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 2015, 5, 500. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Berret, J.F.; Singh, S.; Vinu, A.; Karakoti, A.S. Redox Active Cerium Oxide Nanoparticles: Current Status and Burning Issues. Small Weinh. Bergstr. Ger. 2021, 17, e2102342. [Google Scholar] [CrossRef]
- Naz, S.; Beach, J.; Heckert, B.; Tummala, T.; Pashchenko, O.; Banerjee, T.; Santra, S. Cerium oxide nanoparticles: A “radical” approach to neurodegenerative disease treatment. Nanomedicine 2017, 12, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Pritchard, S.; Harper, K.; Aston, J.W.; Lynch, A.; Lucky, J.J.; Ludington, J.S.; Chatani, P.; Mosenthal, W.P.; Leiter, J.C.; et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.-Y.; Soh, M.; Kim, D.; Kim, Y.-J.; Jang, H.; Yang, H.-S.; Kim, J.Y.; Park, H.-K.; et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. Engl. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-G.; Cha, B.G.; Kang, D.-W.; Kim, D.Y.; Ki, S.K.; Kim, S.I.; Han, J.H.; Yang, W.; Kim, C.K.; Kim, J.; et al. Ceria Nanoparticles Synthesized With Aminocaproic Acid for the Treatment of Subarachnoid Hemorrhage. Stroke 2018, 49, 3030–3038. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Zhu, W.-W.; Zhou, D. Activation of nuclear factor-erythroid 2-related factor 2 (Nrf2) in the basilar artery after subarachnoid hemorrhage in rats. Ann. Clin. Lab. Sci. 2010, 40, 233–239. [Google Scholar]
- Li, T.; Wang, H.; Ding, Y.; Zhou, M.; Zhou, X.; Zhang, X.; Ding, K.; He, J.; Lu, X.; Xu, J.; et al. Genetic elimination of Nrf2 aggravates secondary complications except for vasospasm after experimental subarachnoid hemorrhage in mice. Brain Res. 2014, 1558, 90–99. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Li, G.; Zhou, L.; Huang, K.; Wu, Z.; Zhan, R.; Shen, J. Inflammation and Oxidative Stress: Potential Targets for Improving Prognosis After Subarachnoid Hemorrhage. Front. Cell. Neurosci. 2021, 15, 739506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lu, J.; Mei, S.; Wu, H.; Sun, Z.; Fang, Y.; Xu, S.; Wang, X.; Shi, L.; Xu, W.; et al. Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: Microglia-astrocyte involvement in remyelination. J. Neuroinflamm. 2021, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-S.; Zhang, X.; Zhou, M.-L.; Zhou, X.-M.; Li, N.; Li, W.; Cong, Z.-X.; Sun, Q.; Zhuang, Z.; Wang, C.-X.; et al. Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage: Laboratory investigation. J. Neurosurg. 2014, 121, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kuo, C.-C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wu, Q.; Yan, Z.Z.; He, W.-Z.; Zhou, X.-M.; Zhou, L.-J.; Zhang, J.-Y.; Zhang, X. Neuroprotective Effect of Ultrasound Triggered Astaxanthin Release Nanoparticles on Early Brain Injury After Subarachnoid Hemorrhage. Front. Chem. 2021, 9, 775274. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Wu, Q.; Zhou, X.; Zhang, X.; Yuan, B.; Wen, L.; Xu, W.; Cui, S.; Tang, X.; Zhang, X. Receptor-Mediated Delivery of Astaxanthin-Loaded Nanoparticles to Neurons: An Enhanced Potential for Subarachnoid Hemorrhage Treatment. Front. Neurosci. 2019, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar] [PubMed]
- Rapoport, N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int. J. Pharm. 2004, 277, 155–162. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, D.M.; Ortiz, D.; Alvarez, O.A.; Briceño, J.C.; Cabrales, P. Hemorheological implications of perfluorocarbon based oxygen carrier interaction with colloid plasma expanders and blood. Biotechnol. Prog. 2013, 29, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zheng, Y.; Yu, J.; Wang, Z.; Yang, Y.; Wu, W.; Guo, D.; Ran, H. Superparamagnetic perfluorooctylbromide nanoparticles as a multimodal contrast agent for US, MR, and CT imaging. Acta Radiol. 2013, 54, 278–283. [Google Scholar] [CrossRef]
- Feiler, S.; Plesnila, N.; Thal, S.C.; Zausinger, S.; Schöller, K. Contribution of Matrix Metalloproteinase-9 to Cerebral Edema and Functional Outcome following Experimental Subarachnoid Hemorrhage. Cerebrovasc. Dis. 2011, 32, 289–295. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Zhang, X.; Zhang, Q.-R.; Wu, Q.; Li, W.; Jiang, T.-W.; Hang, C.-H. Astaxanthin reduces matrix metalloproteinase-9 expression and activity in the brain after experimental subarachnoid hemorrhage in rats. Brain Res. 2015, 1624, 113–124. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, W.; Zhu, H.; Zhang, X.; Feng, Y.; Chen, Y.; Feng, H.; Lin, J. Curcumin attenuates blood-brain barrier disruption after subarachnoid hemorrhage in mice. J. Surg. Res. 2017, 207, 85–91. [Google Scholar] [CrossRef]
- Chen, J.; Pan, H.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy. Adv. Chronic Kidney Dis. 2013, 20, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain. Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Woods, S.D.; Skinner, R.D.; Ricca, A.M.; Brown, A.T.; Lowery, J.D.; Borrelli, M.J.; Lay, J.O.; Culp, W.C. Progress in Dodecafluoropentane Emulsion as a Neuroprotective Agent in a Rabbit Stroke Model. Mol. Neurobiol. 2013, 48, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, R.; Xie, F.; Xu, W.; Zeng, M.-F.; Wang, X.; Zhu, J. Protective effects of perfluorooctyl-bromide nanoparticles on early brain injuries following subarachnoid hemorrhage in rats. Am. J. Transl. Res. 2015, 7, 1404–1416. [Google Scholar]
- Fortes, G.B.; Alves, L.S.; De Oliveira, R.; Dutra, F.F.; Rodrigues, D.; Fernandez, P.L.; Souto-Padron, T.; De Rosa, M.J.; Kelliher, M.; Golenbock, D.; et al. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood 2012, 119, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, R.; Li, X.; Zhang, H.; Wang, X.; Zhu, J. Downregulating hypoxia-inducible factor-1α expression with perfluorooctyl-bromide nanoparticles reduces early brain injury following experimental subarachnoid hemorrhage in rats. Am. J. Transl. Res. 2016, 8, 2114–2126. [Google Scholar]
- Semenza, G.L.; Agani, F.; Feldser, D.; Iyer, N.; Kotch, L.; Laughner, E.; Yu, A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv. Exp. Med. Biol. 2000, 475, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Maas, A.I.R.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D. Long-Term Consequences of Traumatic Brain Injury: Current Status of Potential Mechanisms of Injury and Neurological Outcomes. J. Neurotrauma 2015, 32, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.J.; Carlos, T.M.; Kochanek, P.M.; Clark, R.S.B.; Heineman, S.; Schiding, J.K.; Franicola, D.; Memarzadeh, F.; Lo, W.; Marion, D.W.; et al. Neutrophils Do Not Mediate Blood-Brain Barrier Permeability Early After Controlled Cortical Impact in Rats. J. Neurotrauma 1999, 16, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Aertker, B.M.; Kumar, A.; Prabhakara, K.S.; Smith, P.; Furman, N.E.T.; Hasen, X.; Cox, C.S.; Bedi, S.S. Pre-injury monocyte/macrophage depletion results in increased blood–brain barrier permeability after traumatic brain injury. J. Neurosci. Res. 2019, 97, 698–707. [Google Scholar] [CrossRef]
- Lotocki, G.; De Rivero Vaccari, J.P.; Perez, E.R.; Sanchez-Molano, J.; Furones-Alonso, O.; Bramlett, H.M.; Dietrich, W.D. Alterations in Blood-Brain Barrier Permeability to Large and Small Molecules and Leukocyte Accumulation after Traumatic Brain Injury: Effects of Post-Traumatic Hypothermia. J. Neurotrauma 2009, 26, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Zinger, A.; Soriano, S.; Baudo, G.; De Rosa, E.; Taraballi, F.; Villapol, S. Biomimetic Nanoparticles as a Theranostic Tool for Traumatic Brain Injury. Adv. Funct. Mater. 2021, 31, 2100722. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhao, H.; Gou, X.; Wu, X.; Zhang, S.; Deng, G.; Chen, Q. Targeted delivery of polypeptide nanoparticle for treatment of traumatic brain injury. Int. J. Nanomed. 2019, 14, 4059–4069. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Fugaccia, I.; Brown, D.R.; Brown, L.V.; Scheff, S.W. Blood-brain barrier breach following cortical contusion in the rat. J. Neurosurg. 1996, 85, 476–481. [Google Scholar] [CrossRef]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Nguyen, T.D.; Linehan, A.R.; Yang, D.; Behrens, A.M.; Rose, S.; Tochka, Z.L.; Tzeng, S.Y.; Norman, J.J.; Anselmo, A.C.; et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 2017, 357, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.X.; Householder, K.T.; Ceton, R.; Kovalik, T.; Heffernan, J.M.; Shankar, R.V.; Bowser, R.P.; Wechsler-Reya, R.J.; Sirianni, R.W. Optical barcoding of PLGA for multispectral analysis of nanoparticle fate in vivo. J. Control. Release 2017, 253, 172–182. [Google Scholar] [CrossRef]
- Carron, S.F.; Alwis, D.S.; Rajan, R. Traumatic Brain Injury and Neuronal Functionality Changes in Sensory Cortex. Front. Syst. Neurosci. 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J. Microencapsul. 2013, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.V.; Roney, C.A.; Antich, P.P.; Bonte, F.J.; Raghu, A.V.; Aminabhavi, T.M. Quinoline- n -butylcyanoacrylate-based nanoparticles for brain targeting for the diagnosis of Alzheimer’s disease. WIREs Nanomed. Nanobiotechnol. 2010, 2, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P.; Omelyanenko, V.G.; Papisov, M.I.; Bogdanov, A.A.; Trubetskoy, V.S.; Herron, J.N.; Gentry, C.A. Poly(ethylene glycol) on the liposome surface: On the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta BBA—Biomembr. 1994, 1195, 11–20. [Google Scholar] [CrossRef]
- Levchenko, T.S.; Rammohan, R.; Lukyanov, A.N.; Whiteman, K.R.; Torchilin, V.P. Liposome clearance in mice: The effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002, 240, 95–102. [Google Scholar] [CrossRef]
- Maussang, D.; Rip, J.; Van Kregten, J.; Van Den Heuvel, A.; Van Der Pol, S.; Van Der Boom, B.; Reijerkerk, A.; Chen, L.; De Boer, M.; Gaillard, P.; et al. Glutathione conjugation dose-dependently increases brain-specific liposomal drug delivery in vitro and in vivo. Drug Discov. Today Technol. 2016, 20, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Mannix, R.; Meehan, W.P.; Mandeville, J.; Grant, P.E.; Gray, T.; Berglass, J.; Zhang, J.; Bryant, J.; Rezaie, S.; Chung, J.Y.; et al. Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 2013, 74, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ifergan, I.; Kurz, J.E.; Linsenmeier, R.A.; Xu, D.; Cooper, J.G.; Miller, S.D.; Kessler, J.A. Intravenous Immunomodulatory Nanoparticle Treatment for Traumatic Brain Injury. Ann. Neurol. 2020, 87, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Getts, D.R.; Terry, R.L.; Getts, M.T.; Deffrasnes, C.; Müller, M.; Van Vreden, C.; Ashhurst, T.M.; Chami, B.; McCarthy, D.; Wu, H.; et al. Therapeutic Inflammatory Monocyte Modulation Using Immune-Modifying Microparticles. Sci. Transl. Med. 2014, 6, 219ra7. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Fung, Y.K.; Liu, X.; Pahan, K. Up-regulation of Microglial CD11b Expression by Nitric Oxide. J. Biol. Chem. 2006, 281, 14971–14980. [Google Scholar] [CrossRef]
- Martinez, J.O.; Molinaro, R.; Hartman, K.A.; Boada, C.; Sukhovershin, R.; De Rosa, E.; Kuri, D.; Zhang, S.; Evangelopoulos, M.; Carter, A.M.; et al. Biomimetic nanoparticles with enhanced affinity towards activated endothelium as versatile tools for theranostic drug delivery. Theranostics 2018, 8, 1131–1145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alvarez-Croda, D.-M.; Stoica, B.A.; Faden, A.I.; Loane, D.J. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1732–1750. [Google Scholar] [CrossRef] [PubMed]
- Haberny, K.A. Ontogeny of the N-Methyl-D-Aspartate (NMDA) Receptor System and Susceptibility to Neurotoxicity. Toxicol. Sci. 2002, 68, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Hayashi, A.; Sun, H.-S.; Belmares, M.P.; Cobey, C.; Phan, T.; Schweizer, J.; Salter, M.W.; Wang, Y.T.; Tasker, R.A.; et al. PDZ Protein Interactions Underlying NMDA Receptor-Mediated Excitotoxicity and Neuroprotection by PSD-95 Inhibitors. J. Neurosci. 2007, 27, 9901–9915. [Google Scholar] [CrossRef]
- Cook, D.J.; Teves, L.; Tymianski, M. A Translational Paradigm for the Preclinical Evaluation of the Stroke Neuroprotectant Tat-NR2B9c in Gyrencephalic Nonhuman Primates. Sci. Transl. Med. 2012, 4, 154ra133. [Google Scholar] [CrossRef]
- Mann, A.P.; Scodeller, P.; Hussain, S.; Joo, J.; Kwon, E.; Braun, G.B.; Mölder, T.; She, Z.-G.; Kotamraju, V.R.; Ranscht, B.; et al. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat. Commun. 2016, 7, 11980. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.B.; Leite-Morris, K.A.; Wang, L.; Rumbika, K.K.; Heinrichs, S.C.; Zeng, X.; Wu, L.; Arena, D.T.; Teng, Y.D. Pathophysiological Bases of Comorbidity: Traumatic Brain Injury and Post-Traumatic Stress Disorder. J. Neurotrauma 2018, 35, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Kyle, S.; Saha, S. Nanotechnology for the Detection and Therapy of Stroke. Adv. Healthc. Mater. 2014, 3, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, S.; Saha, S. Therapeutic benefits of nanoparticles in stroke. Front. Neurosci. 2015, 9, 182. [Google Scholar] [CrossRef]
- Blanco, S.; Martínez-Lara, E.; Siles, E.; Peinado, M.Á. New Strategies for Stroke Therapy: Nanoencapsulated Neuroglobin. Pharmaceutics 2022, 14, 1737. [Google Scholar] [CrossRef]
- Mc Carthy, D.J.; Malhotra, M.; O’Mahony, A.M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticles and the Blood-Brain Barrier: Advancing from In-Vitro Models Towards Therapeutic Significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Sharma, S.; Hassan, K.M. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010, 17, 197–218. [Google Scholar] [CrossRef]
- Sarami Foroshani, M.; Sobhani, Z.S.; Mohammadi, M.T.; Aryafar, M. Fullerenol Nanoparticles Decrease Blood-Brain Barrier Interruption and Brain Edema during Cerebral Ischemia-Reperfusion Injury Probably by Reduction of Interleukin-6 and Matrix Metalloproteinase-9 Transcription. J. Stroke Cerebrovasc. Dis. 2018, 27, 3053–3065. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Dave, K.M.; Stetler, R.A.; Manickam, D.S. Targeting the blood-brain barrier for the delivery of stroke therapies. Adv. Drug Deliv. Rev. 2021, 171, 332–351. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Sha, X. Nanoparticles-mediated emerging approaches for effective treatment of ischemic stroke. Biomaterials 2021, 277, 121111. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wang, H.-B.; Jin, J.-B.; Yang, C.-L.; Hu, J.-B.; Li, J. Advances in the research of nano delivery systems in ischemic stroke. Front. Bioeng. Biotechnol. 2022, 10, 984424. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Su, Y.; Wang, Z. Nanomedicine for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7600. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; De La Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The Mechanism of Uptake of Biodegradable Microparticles in Caco-2 Cells Is Size Dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Landowski, L.M.; Niego, B.; Sutherland, B.A.; Hagemeyer, C.E.; Howells, D.W. Applications of Nanotechnology in the Diagnosis and Therapy of Stroke. Semin. Thromb. Hemost. 2020, 46, 592–605. [Google Scholar] [CrossRef]
- Hurtado, O.; Lizasoain, I.; Moro, M.Á. Neuroprotection and Recovery: Recent Data at the Bench on Citicoline. Stroke 2011, 42 (Suppl. 1), S33–S35. [Google Scholar] [CrossRef]
- Poellmann, M.J.; Bu, J.; Hong, S. Would antioxidant-loaded nanoparticles present an effective treatment for ischemic stroke? Nanomedicine 2018, 13, 2327–2340. [Google Scholar] [CrossRef]
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Aktas, Y.; Gursoy-Ozdemir, Y.; Bodur, E.; Yemisci, M.; Caban, S.; Vural, A.; Pinarbasli, O.; Capan, Y.; Fernandez-Megia, E.; et al. A Nanomedicine Transports a Peptide Caspase-3 Inhibitor across the Blood–Brain Barrier and Provides Neuroprotection. J. Neurosci. 2009, 29, 13761–13769. [Google Scholar] [CrossRef]
- Takamiya, M.; Miyamoto, Y.; Yamashita, T.; Deguchi, K.; Ohta, Y.; Abe, K. Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience 2012, 221, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, M.; Leypoldt, F.; Steinbach, K.; Behrens, D.; Choe, C.-U.; Siler, D.A.; Arumugam, T.V.; Orthey, E.; Gerloff, C.; Tolosa, E.; et al. Temporal and Spatial Dynamics of Cerebral Immune Cell Accumulation in Stroke. Stroke 2009, 40, 1849–1857. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic Resonance Imaging and Iron-oxide Nanoparticles in the era of Personalized Medicine. Nanotheranostics 2023, 7, 424–449. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.; Sidhu, N.; Hu, C.A.; Mlady, G.; Eberhardt, S.C.; Sillerud, L.O. Iron-based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. AJR Am. J. Roentgenol. 2015, 204, W302–W313. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Micheli, M.R.; Bova, R.; Magini, A.; Polidoro, M.; Emiliani, C. Lipid-based nanocarriers for CNS-targeted drug delivery. Recent Pat. CNS Drug Discov. 2012, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int. J. Nanomed. 2019, 14, 6497–6517. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D. A Review of Patisiran (ONPATTRO(R)) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Glantz, M.J.; Jaeckle, K.A.; Chamberlain, M.C.; Phuphanich, S.; Recht, L.; Swinnen, L.J.; Maria, B.; LaFollette, S.; Schumann, G.B.; Cole, B.F.; et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999, 5, 3394–3402. [Google Scholar] [PubMed]
- Salehi, B.; Selamoglu, Z.; Mileski, K.S.; Pezzani, R.; Redaelli, M.; Cho, W.C.; Kobarfard, F.; Rajabi, S.; Martorell, M.; Kumar, P.; et al. Liposomal Cytarabine as Cancer Therapy: From Chemistry to Medicine. Biomolecules 2019, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood-brain barrier disruption: A safety study. J. Control. Release 2015, 204, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound. Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Peng, L.; Han, Y.; Wang, D.; He, X.; Wang, J.; Ou, C. Lipid nanoparticle-based mRNA vaccines in cancers: Current advances and future prospects. Front. Immunol. 2022, 13, 922301. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Dong, X.; Shi, X.; Zhang, C.; Wang, Z. Neutrophil-Based Drug Delivery Systems. Adv. Mater. 2018, 30, 1706245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hey, G.; Mehkri, I.; Mehkri, Y.; Maqbool, H.; Tahirkheli, M.; Woodford, S.; Lucke-Wold, B. Nanoparticle-Based Therapies for Management of Subarachnoid Hemorrhage, Neurotrauma, and Stroke. Biomedicines 2025, 13, 16. https://doi.org/10.3390/biomedicines13010016
Hey G, Mehkri I, Mehkri Y, Maqbool H, Tahirkheli M, Woodford S, Lucke-Wold B. Nanoparticle-Based Therapies for Management of Subarachnoid Hemorrhage, Neurotrauma, and Stroke. Biomedicines. 2025; 13(1):16. https://doi.org/10.3390/biomedicines13010016
Chicago/Turabian StyleHey, Grace, Ilyas Mehkri, Yusuf Mehkri, Hasan Maqbool, Mubariz Tahirkheli, Samuel Woodford, and Brandon Lucke-Wold. 2025. "Nanoparticle-Based Therapies for Management of Subarachnoid Hemorrhage, Neurotrauma, and Stroke" Biomedicines 13, no. 1: 16. https://doi.org/10.3390/biomedicines13010016
APA StyleHey, G., Mehkri, I., Mehkri, Y., Maqbool, H., Tahirkheli, M., Woodford, S., & Lucke-Wold, B. (2025). Nanoparticle-Based Therapies for Management of Subarachnoid Hemorrhage, Neurotrauma, and Stroke. Biomedicines, 13(1), 16. https://doi.org/10.3390/biomedicines13010016






