Novel Poly-Arginine Peptide R18D Reduces α-Synuclein Aggregation and Uptake of α-Synuclein Seeds in Cortical Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. Establishment of Rat Primary Cortical Neurons
2.1.2. Neuronal Cell Plating
2.2. Study Design
2.2.1. Establishment of Neuronal Model of Intracellular α-Syn Aggregation
2.2.2. Assessment of R18D to Inhibit α-Syn Aggregation
2.2.3. Assessment of R18D to Inhibit α-Syn Seed Uptake
2.3. Cell Assays
2.3.1. Cell Death and Cell Viability Assays
LDH Assay
MTS Assay
2.3.2. Thioflavin T (ThT) Assay
2.3.3. Homogenous Time-Resolved Fluorescence (HTRF)
2.4. Microscopy
2.4.1. Scanning Electron Microscopy
2.4.2. Confocal Microscopy
2.5. Statistical Analysis
3. Results
3.1. α-Syn Seeds Induce α-Syn Monomer Aggregation in a Cell-Free Assay
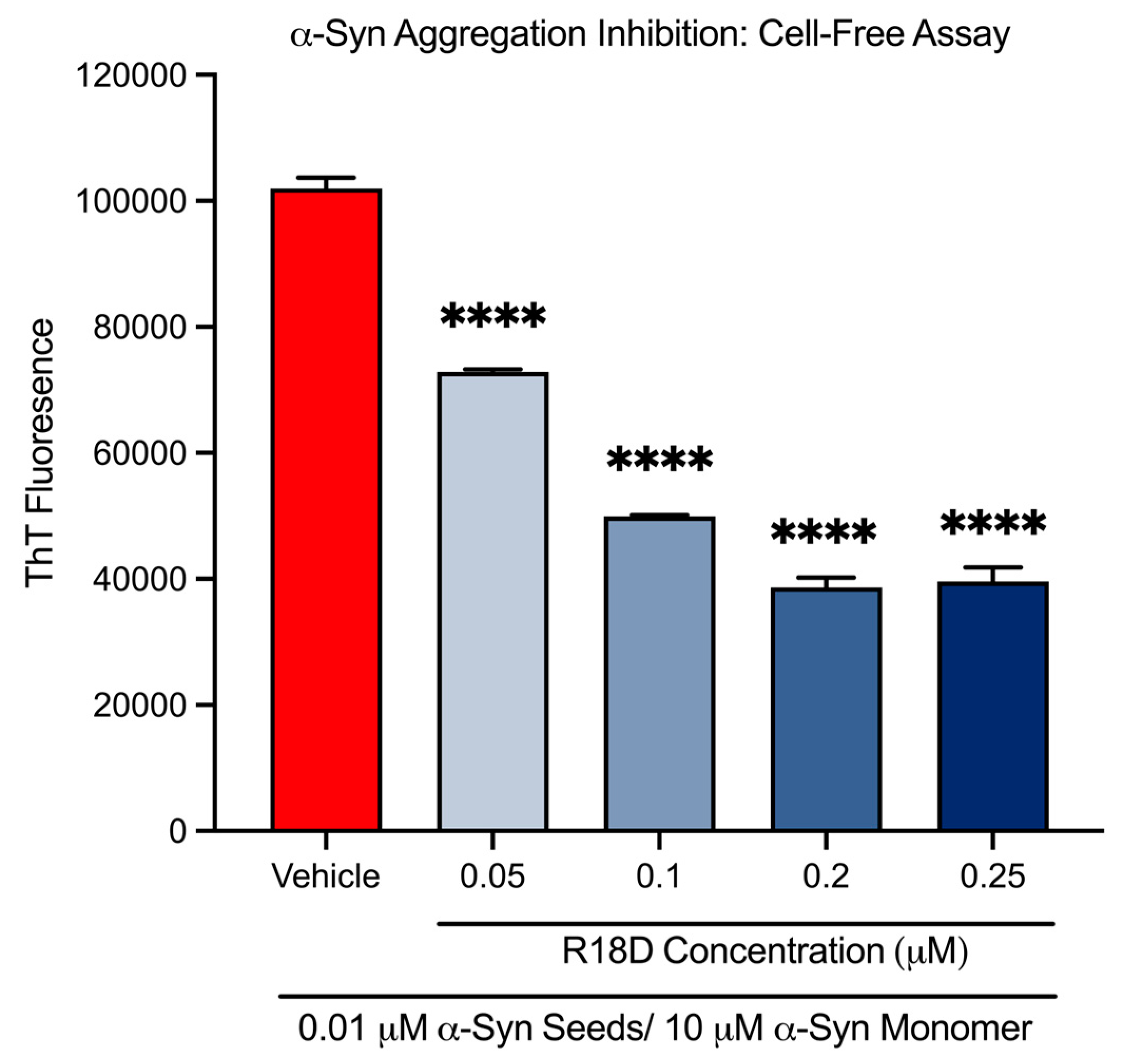
3.2. R18D Reduces α-Syn Monomer Aggregation in a Cell-Free ThT Assay
3.3. R18D Is Non-Toxic to Cortical Neurons at Concentrations Below 0.5 μM
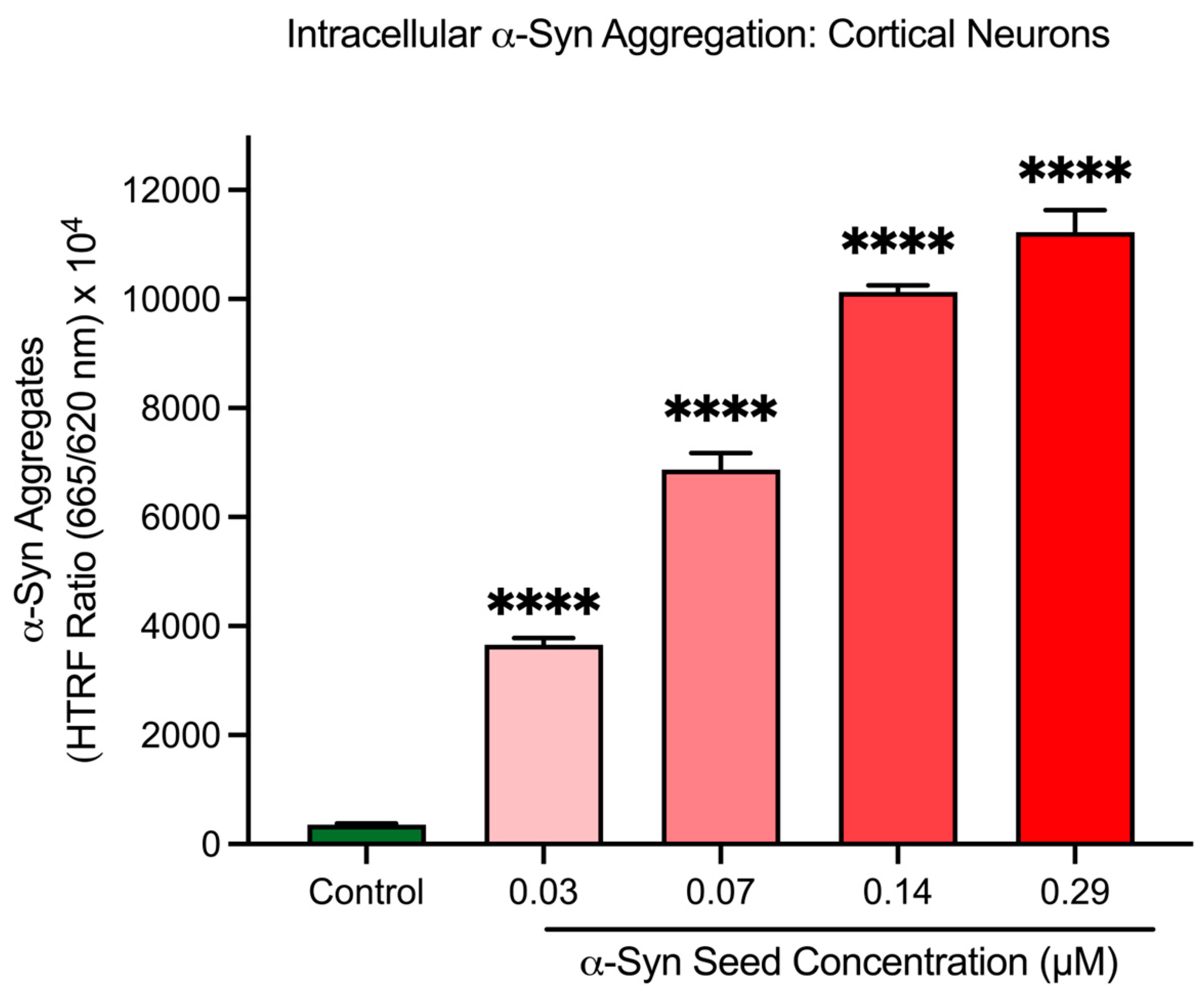
3.4. α-Syn Seeds Induce Intracellular α-Syn Aggregation in Cortical Neurons
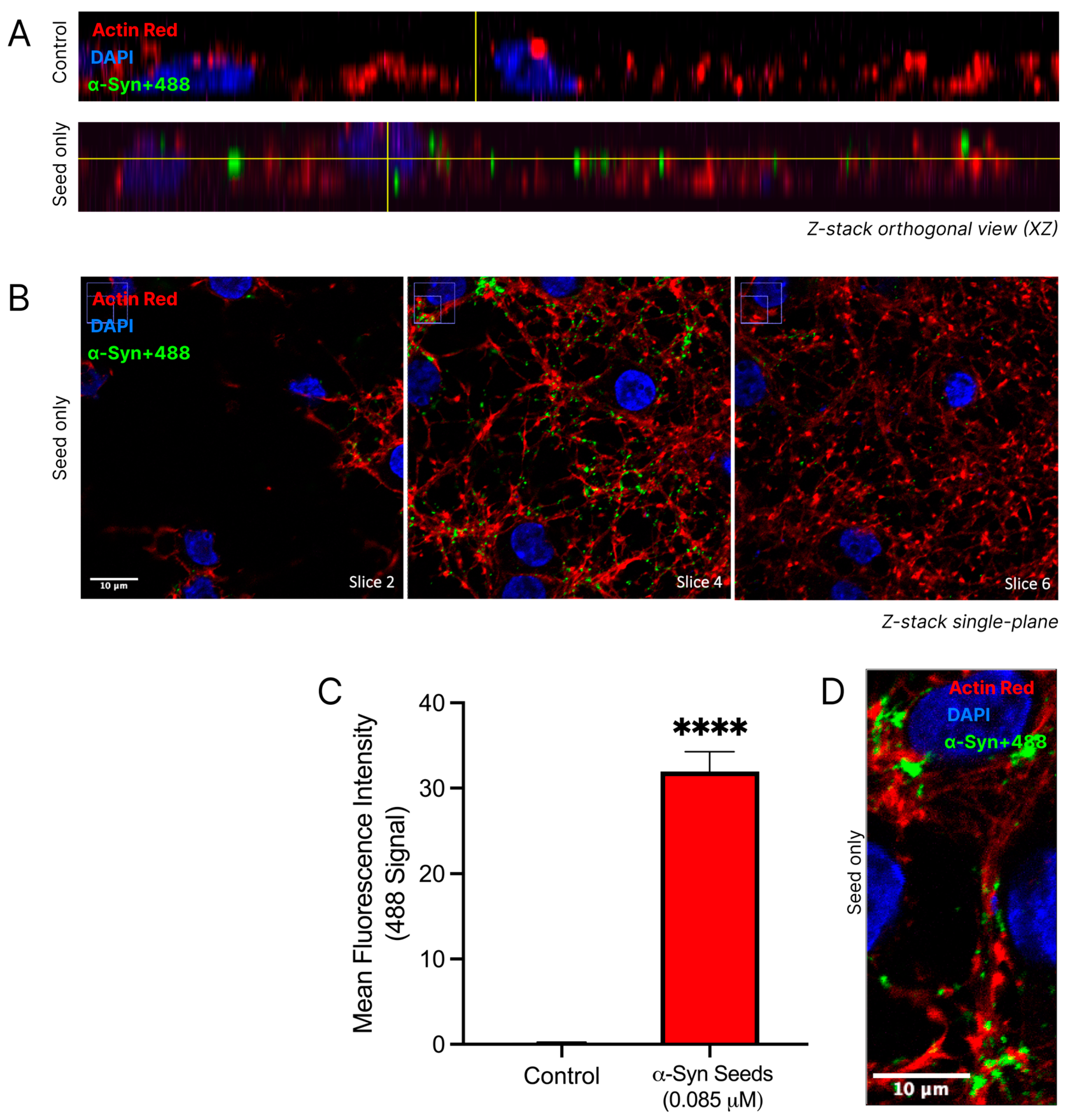
3.5. α-Syn Seeds Enter Neurons
3.6. α-Syn Seeds Induce Toxicity in Cortical Neurons
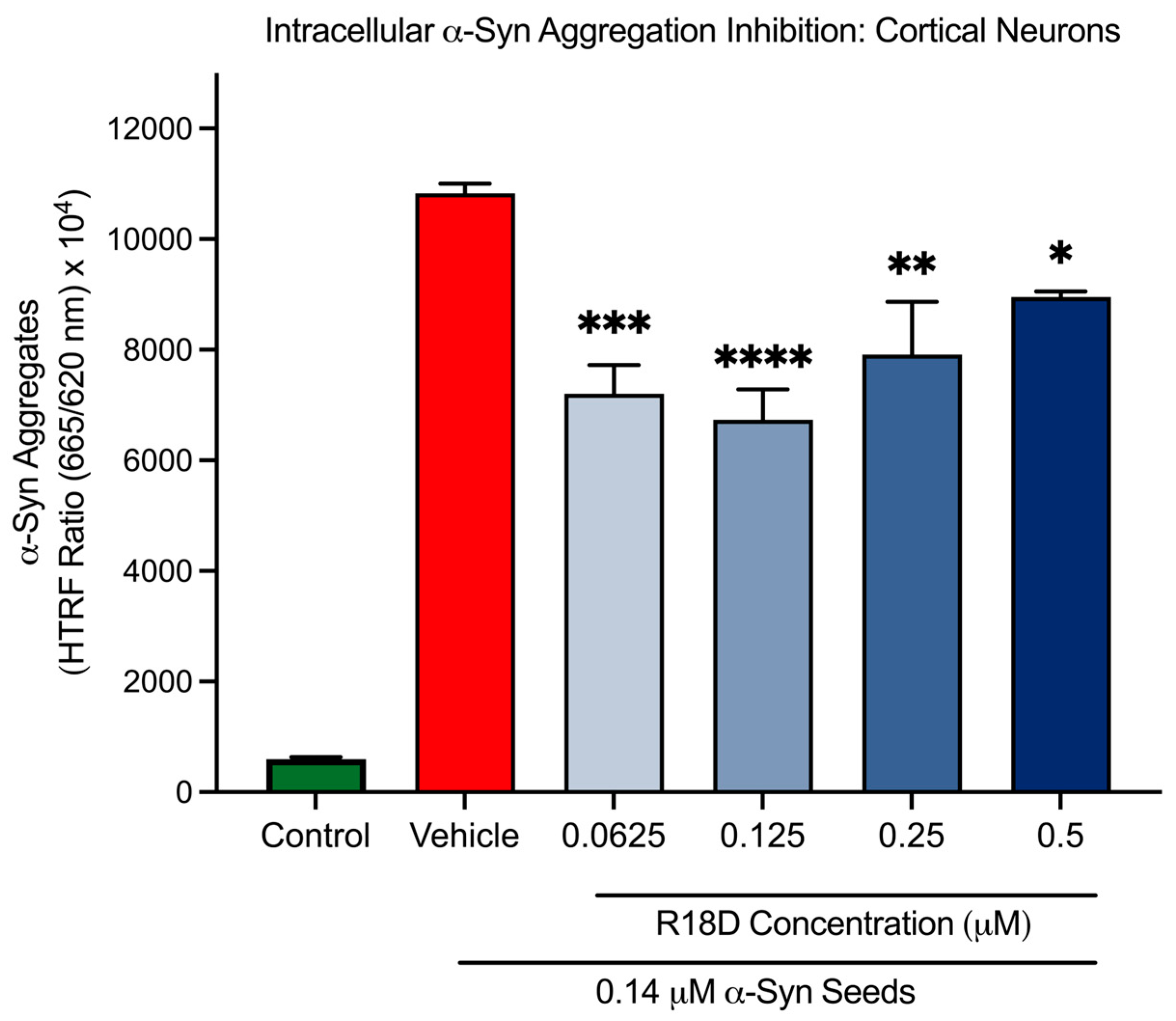
3.7. R18D Reduces Intracellular α-Syn Aggregation in Cortical Neurons
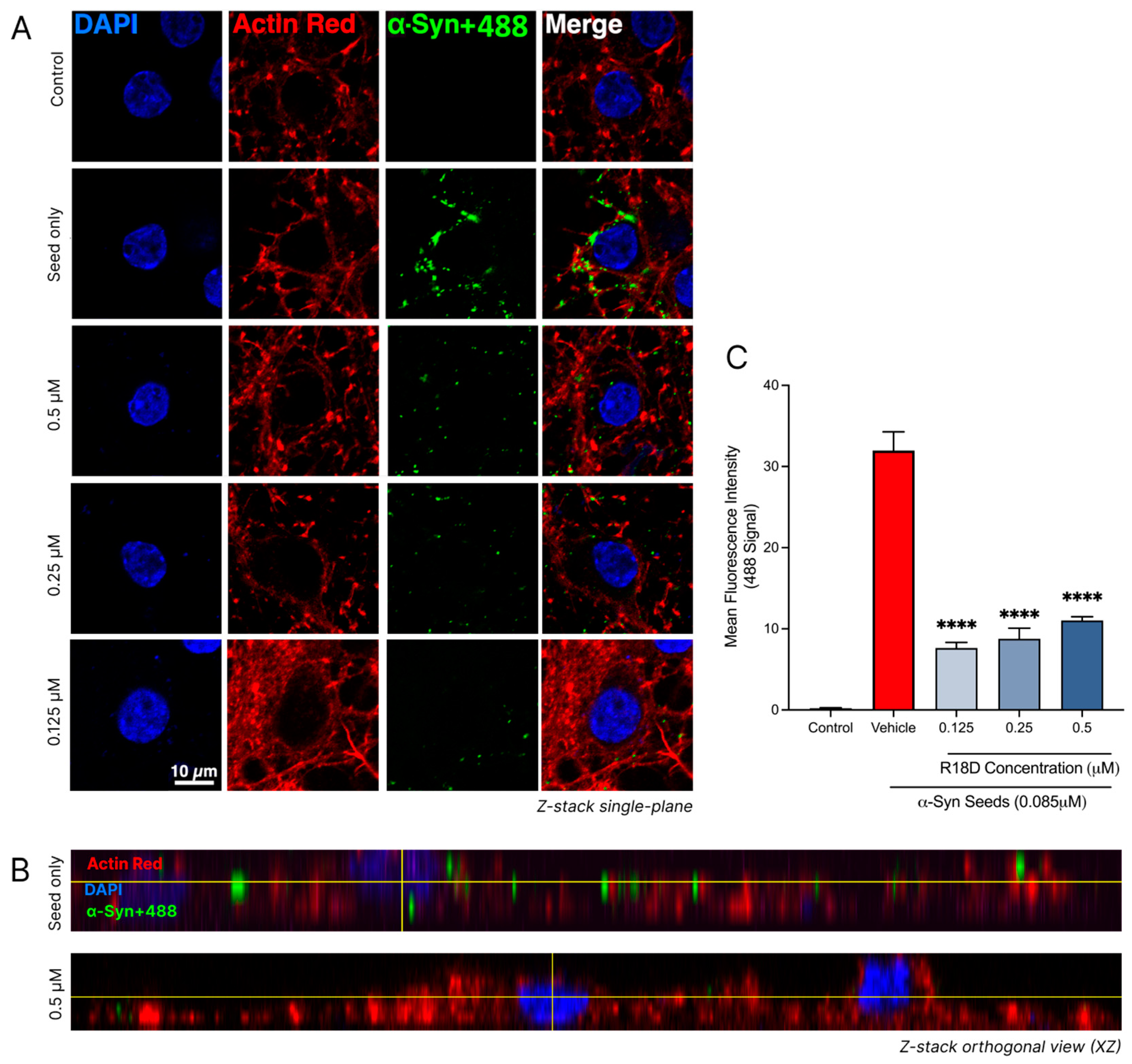
3.8. R18D Reduces α-Syn Seed Uptake in Cortical Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.J.; Campbell, C.E.; Jimenez-Corea, H.A.; Wu, G.H.; Logan, R. Neuroglial Senescence, α-Synucleinopathy, and the Therapeutic Potential of Senolytics in Parkinson’s Disease. Front. Neurosci. 2022, 16, 824191. [Google Scholar] [CrossRef] [PubMed]
- Tarutani, A.; Hasegawa, M. Prion-like propagation of α-synuclein in neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2019, 168, 323–348. [Google Scholar] [CrossRef]
- Mehra, S.; Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 890–908. [Google Scholar] [CrossRef] [PubMed]
- Mahul-Mellier, A.L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [PubMed]
- Klaips, C.L.; Jayaraj, G.G.; Hartl, F.U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 2018, 217, 51–63. [Google Scholar] [CrossRef]
- Prots, I.; Grosch, J.; Brazdis, R.M.; Simmnacher, K.; Veber, V.; Havlicek, S.; Hannappel, C.; Krach, F.; Krumbiegel, M.; Schütz, O.; et al. α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc. Natl. Acad. Sci. USA 2018, 115, 7813–7818. [Google Scholar] [CrossRef] [PubMed]
- Volles, M.J.; Lansbury, P.T., Jr. Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 2002, 41, 4595–4602. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Cedillos, R.; Choyke, S.; Lukic, Z.; McGuire, K.; Marvin, S.; Burrage, A.M.; Sudholt, S.; Rana, A.; O’Connor, C.; et al. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS ONE 2013, 8, e62143. [Google Scholar] [CrossRef] [PubMed]
- Danzer, K.M.; Haasen, D.; Karow, A.R.; Moussaud, S.; Habeck, M.; Giese, A.; Kretzschmar, H.; Hengerer, B.; Kostka, M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J. Neurosci. 2007, 27, 9220–9232. [Google Scholar] [CrossRef]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Yagi, H.; Uemura, M.T.; Hatanaka, Y.; Yamakado, H.; Takahashi, R. Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 2018, 13, 21. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Angot, E.; Bergström, A.L.; Steiner, J.A.; Pieri, L.; Paul, G.; Outeiro, T.F.; Melki, R.; Kallunki, P.; Fog, K.; et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Investig. 2011, 121, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Freundt, E.C.; Maynard, N.; Clancy, E.K.; Roy, S.; Bousset, L.; Sourigues, Y.; Covert, M.; Melki, R.; Kirkegaard, K.; Brahic, M. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol. 2012, 72, 517–524. [Google Scholar] [CrossRef]
- Reyes, J.F.; Olsson, T.T.; Lamberts, J.T.; Devine, M.J.; Kunath, T.; Brundin, P. A cell culture model for monitoring α-synuclein cell-to-cell transfer. Neurobiol. Dis. 2015, 77, 266–275. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, V.; Danzer, K.M. Release and uptake of pathologic alpha-synuclein. Cell Tissue Res. 2018, 373, 175–182. [Google Scholar] [CrossRef]
- Sapru, M.K.; Yates, J.W.; Hogan, S.; Jiang, L.; Halter, J.; Bohn, M.C. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp. Neurol. 2006, 198, 382–390. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.L.; Mak, S.K.; Henderson, J.M.; Bumcrot, D.; Farrer, M.J.; Di Monte, D.A. Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia nigra. PLoS ONE 2010, 5, e12122. [Google Scholar] [CrossRef]
- Alarcón-Arís, D.; Recasens, A.; Galofré, M.; Carballo-Carbajal, I.; Zacchi, N.; Ruiz-Bronchal, E.; Pavia-Collado, R.; Chica, R.; Ferrés-Coy, A.; Santos, M.; et al. Selective α-Synuclein Knockdown in Monoamine Neurons by Intranasal Oligonucleotide Delivery: Potential Therapy for Parkinson’s Disease. Mol. Ther. 2018, 26, 550–567. [Google Scholar] [CrossRef]
- Fountaine, T.M.; Wade-Martins, R. RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J. Neurosci. Res. 2007, 85, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. mTOR: On target for novel therapeutic strategies in the nervous system. Trends Mol. Med. 2013, 19, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, N.M.; Manfredsson, F.P. Loss of functional alpha-synuclein: A toxic event in Parkinson’s disease? J. Parkinsons Dis. 2012, 2, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Shin, Y.; Masliah, E.; Hyman, B.T.; McLean, P.J. Hsp70 Reduces alpha-Synuclein Aggregation and Toxicity. J. Biol. Chem. 2004, 279, 25497–25502. [Google Scholar] [CrossRef]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Siderowf, A.D.; Macklin, E.A.; Poewe, W.; Brooks, D.J.; Fernandez, H.H.; Rascol, O.; Giladi, N.; Stocchi, F.; Tanner, C.M.; et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Whone, A. Monoclonal Antibody Therapy in Parkinson’s Disease—The End? N. Engl. J. Med. 2022, 387, 466–467. [Google Scholar] [CrossRef]
- Kalia, L.V. First trials test targeting of α-synuclein for Parkinson disease. Nat. Rev. Neurol. 2022, 18, 703–704. [Google Scholar] [CrossRef]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374. [Google Scholar] [CrossRef]
- Liu, Y.; Sorce, S.; Nuvolone, M.; Domange, J.; Aguzzi, A. Lymphocyte activation gene 3 (Lag3) expression is increased in prion infections but does not modify disease progression. Sci. Rep. 2018, 8, 14600. [Google Scholar] [CrossRef]
- Allen, S.G.; Meade, R.M.; White Stenner, L.L.; Mason, J.M. Peptide-based approaches to directly target alpha-synuclein in Parkinson’s disease. Mol. Neurodegener. 2023, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Pandey, D.; Ashraf, G.M.; Rachana. Peptide Based Therapy for Neurological Disorders. Curr. Protein Pept. Sci. 2021, 22, 656–665. [Google Scholar] [CrossRef]
- Meloni, B.P.; Mastaglia, F.L.; Knuckey, N.W. Cationic Arginine-Rich Peptides (CARPs): A Novel Class of Neuroprotective Agents With a Multimodal Mechanism of Action. Front. Neurol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Mamsa, S.S.A.; Meloni, B.P. Arginine and Arginine-Rich Peptides as Modulators of Protein Aggregation and Cytotoxicity Associated With Alzheimer’s Disease. Front. Mol. Neurosci. 2021, 14, 759729. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.B.; Cross, J.L.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. Poly-arginine R18 and R18D (D-enantiomer) peptides reduce infarct volume and improves behavioural outcomes following perinatal hypoxic-ischaemic encephalopathy in the P7 rat. Mol. Brain 2018, 11, 8. [Google Scholar] [CrossRef]
- Gorecki, A.M.; Spencer, H.; Meloni, B.P.; Anderton, R.S. The Poly-Arginine Peptide R18D Interferes with the Internalisation of α-Synuclein Pre-Formed Fibrils in STC-1 Enteroendocrine Cells. Biomedicines 2023, 11, 2089. [Google Scholar] [CrossRef]
- MacDougall, G.; Anderton, R.S.; Mastaglia, F.L.; Knuckey, N.W.; Meloni, B.P. Proteomic analysis of cortical neuronal cultures treated with poly-arginine peptide-18 (R18) and exposed to glutamic acid excitotoxicity. Mol. Brain 2019, 12, 66. [Google Scholar] [CrossRef]
- MacDougall, G.; Anderton, R.S.; Edwards, A.B.; Knuckey, N.W.; Meloni, B.P. The Neuroprotective Peptide Poly-Arginine-12 (R12) Reduces Cell Surface Levels of NMDA NR2B Receptor Subunit in Cortical Neurons; Investigation into the Involvement of Endocytic Mechanisms. J. Mol. Neurosci. 2017, 61, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; Craig, A.J.; Milech, N.; Hopkins, R.M.; Watt, P.M.; Knuckey, N.W. The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cell. Mol. Neurobiol. 2014, 34, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M. Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095497. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. Methods Mol. Biol. 2018, 1709, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Murray, I.V.; Giasson, B.I.; Quinn, S.M.; Koppaka, V.; Axelsen, P.H.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry 2003, 42, 8530–8540. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef]
- Degorce, F.; Card, A.; Soh, S.; Trinquet, E.; Knapik, G.P.; Xie, B. HTRF: A technology tailored for drug discovery—A review of theoretical aspects and recent applications. Curr. Chem. Genom. 2009, 3, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Jethva, P.N.; Kardani, J.R.; Roy, I. Modulation of α-synuclein aggregation by dopamine in the presence of MPTP and its metabolite. FEBS J. 2011, 278, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.; Nath, S.; Engelborghs, Y.; Pountney, D.L. Raised calcium and oxidative stress cooperatively promote alpha-synuclein aggregate formation. Neurochem. Int. 2013, 62, 703–711. [Google Scholar] [CrossRef]
- Pesce, S. The Effect of Poly-Arginine Peptide R18D on α-Synuclein Aggregation and the Cellular Uptake and Toxicity of α-Synuclein Seeds. Unpublished Honours Thesis, University of Western Australia, Nedlands, Australia, 2023. [Google Scholar]
- Tarutani, A.; Arai, T.; Murayama, S.; Hisanaga, S.I.; Hasegawa, M. Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol. Commun. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Hope, J. Prions and neurodegenerative diseases. Curr. Opin. Genet. Dev. 2000, 10, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Nonaka, T.; Masuda-Suzukake, M. α-Synuclein: Experimental Pathology. Cold Spring Harb. Perspect. Med. 2016, 6, a024273. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Watanabe, S.T.; Iwatsubo, T.; Hasegawa, M. Seeded aggregation and toxicity of {alpha}-synuclein and tau: Cellular models of neurodegenerative diseases. J. Biol. Chem. 2010, 285, 34885–34898. [Google Scholar] [CrossRef] [PubMed]
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef]
- Devi, S.; Garg, D.K.; Bhat, R. Kinetic control in amyloid polymorphism: Different agitation and solution conditions promote distinct amyloid polymorphs of alpha-synuclein. Biochim. Biophys. Acta Proteins Proteom. 2023, 1871, 140917. [Google Scholar] [CrossRef]
- Choi, Y.R.; Park, S.J.; Park, S.M. Molecular events underlying the cell-to-cell transmission of α-synuclein. FEBS J. 2021, 288, 6593–6602. [Google Scholar] [CrossRef]
- Rodriguez, L.; Marano, M.M.; Tandon, A. Import and Export of Misfolded α-Synuclein. Front. Neurosci. 2018, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Yang, J.; Zhang, X.; Vikeved, E.; Nilsson, A.; Andrén, P.E.; Svenningsson, P. Caveolin-1 interacts with alpha-synuclein and mediates toxic actions of cellular alpha-synuclein overexpression. Neurochem. Int. 2011, 59, 280–289. [Google Scholar] [CrossRef]
- Holmes, B.B.; DeVos, S.L.; Kfoury, N.; Li, M.; Jacks, R.; Yanamandra, K.; Ouidja, M.O.; Brodsky, F.M.; Marasa, J.; Bagchi, D.P.; et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA 2013, 110, E3138–E3147. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Vincent, J.; Ezeanii, A.; Wakade, S.; Yerigenahally, S.; Mor, D.E. Heparan sulfate proteoglycans mediate prion-like α-synuclein toxicity in Parkinson’s in vivo models. Life Sci. Alliance 2022, 5, e202201366. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Martens, Y.A.; Meneses, A.; Ryu, D.H.; Lu, W.; Raulin, A.C.; Li, F.; Zhao, J.; Chen, Y.; Jin, Y.; et al. LRP1 is a neuronal receptor for α-synuclein uptake and spread. Mol. Neurodegener. 2022, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Ghanem, S.S.; Abdulla, S.A.; Lv, G.; Emara, M.M.; Paleologou, K.E.; Vaikath, N.N.; Lu, J.-H.; Li, M.; Vekrellis, K.; et al. Inhibition of alpha-synuclein seeded fibril formation and toxicity by herbal medicinal extracts. BMC Complement. Med. Ther. 2020, 20, 73. [Google Scholar] [CrossRef]
- Gupta, V.; Salim, S.; Hmila, I.; Vaikath, N.N.; Sudhakaran, I.P.; Ghanem, S.S.; Majbour, N.K.; Abdulla, S.A.; Emara, M.M.; Abdesselem, H.B.; et al. Fibrillar form of α-synuclein-specific scFv antibody inhibits α-synuclein seeds induced aggregation and toxicity. Sci. Rep. 2020, 10, 8137. [Google Scholar] [CrossRef]
- Devi, L.; Raghavendran, V.; Prabhu, B.M.; Avadhani, N.G.; Anandatheerthavarada, H.K. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008, 283, 9089–9100. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.Y.; Kim, J.; Paik, S.R.; Park, J.H.; Ahn, Y.S.; Chung, K.C. Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J. Biol. Chem. 2001, 276, 27441–27448. [Google Scholar] [CrossRef] [PubMed]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; González-Billault, C.; de Castro Fonseca, M. Akkermansia muciniphila induces mitochondrial calcium overload and α -synuclein aggregation in an enteroendocrine cell line. iScience 2022, 25, 103908. [Google Scholar] [CrossRef] [PubMed]
- Milani, D.; Bakeberg, M.C.; Cross, J.L.; Clark, V.W.; Anderton, R.S.; Blacker, D.J.; Knuckey, N.W.; Meloni, B.P. Comparison of neuroprotective efficacy of poly-arginine R18 and R18D (D-enantiomer) peptides following permanent middle cerebral artery occlusion in the Wistar rat and in vitro toxicity studies. PLoS ONE 2018, 13, e0193884. [Google Scholar] [CrossRef]
- Nadimidla, K.; Ismail, T.; Kanapathipillai, M. Tau peptides and tau mutant protein aggregation inhibition by cationic polyethyleneimine and polyarginine. Biopolymers 2017, 107, e23024. [Google Scholar] [CrossRef]
- Santos, J.; Gracia, P.; Navarro, S.; Peña-Díaz, S.; Pujols, J.; Cremades, N.; Pallarès, I.; Ventura, S. α-Helical peptidic scaffolds to target α-synuclein toxic species with nanomolar affinity. Nat. Commun. 2021, 12, 3752. [Google Scholar] [CrossRef]
- Spencer, H.; Gorecki, A.; Foley, H.; Phillips, L.; Abonnel, M.Y.; Meloni, B.P.; Anderton, R.S. Poly-Arginine R18 Peptide Inhibits Heat-Induced Lysozyme Protein Aggregation: Implications for a Possible Therapeutic Role in Parkinson’s Disease. Appl. Biochem. Microbiol. 2023, 59, 33–40. [Google Scholar] [CrossRef]
- Shukla, D.; Trout, B.L. Interaction of arginine with proteins and the mechanism by which it inhibits aggregation. J. Phys. Chem. B 2010, 114, 13426–13438. [Google Scholar] [CrossRef]
- Meloni, B.P.; Milani, D.; Edwards, A.B.; Anderton, R.S.; O’Hare Doig, R.L.; Fitzgerald, M.; Palmer, T.N.; Knuckey, N.W. Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol. Ther. 2015, 153, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; Blacker, D.J.; Edwards, A.B.; Knuckey, N.W. Impact of poly-arginine peptides R18D and R18 on alteplase and tenecteplase thrombolysis in vitro, and neuroprotective stability to proteolysis. J. Thromb. Thrombolysis 2022, 54, 172–182. [Google Scholar] [CrossRef]
- Milani, D.; Clark, V.W.; Feindel, K.W.; Blacker, D.J.; Bynevelt, M.; Edwards, A.B.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. Comparative Assessment of the Proteolytic Stability and Impact of Poly-Arginine Peptides R18 and R18D on Infarct Growth and Penumbral Tissue Preservation Following Middle Cerebral Artery Occlusion in the Sprague Dawley Rat. Neurochem. Res. 2021, 46, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; South, S.M.; Gill, D.A.; Marriott, A.L.; Déziel, R.A.; Jacques, A.; Blacker, D.J.; Knuckey, N.W. Poly-Arginine Peptides R18 and R18D Improve Functional Outcomes After Endothelin-1-Induced Stroke in the Sprague Dawley Rat. J. Neuropathol. Exp. Neurol. 2019, 78, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hamaguchi, T. The sulfation code for propagation of neurodegeneration. J. Biol. Chem. 2018, 293, 10841–10842. [Google Scholar] [CrossRef]
- MacDougall, G.; Anderton, R.S.; Trimble, A.; Mastaglia, F.L.; Knuckey, N.W.; Meloni, B.P. Poly-arginine-18 (R18) Confers Neuroprotection through Glutamate Receptor Modulation, Intracellular Calcium Reduction, and Preservation of Mitochondrial Function. Molecules 2020, 25, 2977. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Petsko, G.A.; Studer, L. Induced pluripotent stem cells: A tool for modeling Parkinson’s disease. Trends Neurosci. 2022, 45, 608–620. [Google Scholar] [CrossRef]
- Liu, Y.; Graetz, M.; Ho, L.; Pukala, T.L. Ion mobility-mass spectrometry-based screening for inhibition of α- synuclein aggregation. Eur. J. Mass Spectrom. 2015, 21, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin modulates α-synuclein aggregation and toxicity. ACS Chem. Neurosci. 2013, 4, 393–407. [Google Scholar] [CrossRef] [PubMed]



| Cell-Free ThT α-Syn Mononer Aggregation Assay | ||
| R18D Concentration Examined | α-Syn Monomer Aggregation—Percentage Inhibition | p Value |
| 0.05 μM | 28.6% | p < 0.001 |
| 0.1 μM | 51.0% | p < 0.001 |
| 0.2 μM | 62.1% | p < 0.001 |
| 0.25 μM | 61.2% | p < 0.001 |
| Intracellular α-Syn Aggregate HTRFAssay | ||
| R18D Concentration Examined | α-Syn Aggregation in Neurons—Percentage Inhibition | |
| 0.0625 μM | 33.5% | p = 0.003 |
| 0.125 μM | 37.8% | p < 0.0001 |
| 0.25 μM | 26.9% | p = 0.0015 |
| 0.5 μM | 17.3% | p = 0.022 |
| Confocal Microscopy of Labelled α-Syn Seeds | ||
| R18D Concentration Examined | Labelled α-Syn Seed Uptake in Neurons—Percentage Inhibition | |
| 0.125 μM | 77.74% | p < 0.001 |
| 0.25 μM | 76.0% | p < 0.001 |
| 0.5 μM | 67.0% | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, E.C.; Gorecki, A.M.; Pesce, S.R.; Bagda, V.; Anderton, R.S.; Meloni, B.P. Novel Poly-Arginine Peptide R18D Reduces α-Synuclein Aggregation and Uptake of α-Synuclein Seeds in Cortical Neurons. Biomedicines 2025, 13, 122. https://doi.org/10.3390/biomedicines13010122
Robinson EC, Gorecki AM, Pesce SR, Bagda V, Anderton RS, Meloni BP. Novel Poly-Arginine Peptide R18D Reduces α-Synuclein Aggregation and Uptake of α-Synuclein Seeds in Cortical Neurons. Biomedicines. 2025; 13(1):122. https://doi.org/10.3390/biomedicines13010122
Chicago/Turabian StyleRobinson, Emma C., Anastazja M. Gorecki, Samuel R. Pesce, Vaishali Bagda, Ryan S. Anderton, and Bruno P. Meloni. 2025. "Novel Poly-Arginine Peptide R18D Reduces α-Synuclein Aggregation and Uptake of α-Synuclein Seeds in Cortical Neurons" Biomedicines 13, no. 1: 122. https://doi.org/10.3390/biomedicines13010122
APA StyleRobinson, E. C., Gorecki, A. M., Pesce, S. R., Bagda, V., Anderton, R. S., & Meloni, B. P. (2025). Novel Poly-Arginine Peptide R18D Reduces α-Synuclein Aggregation and Uptake of α-Synuclein Seeds in Cortical Neurons. Biomedicines, 13(1), 122. https://doi.org/10.3390/biomedicines13010122






