mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Line
2.2. Cell Counting Kit 8 (CCK-8) Assay
2.3. Synergy Determination with SynergyFinder
2.4. Colony-Forming Assays
2.5. Wound Healing Test
2.6. Cell Apoptosis Experiment
2.7. Cell Cycle Detection
2.8. EdU Staining Assay
2.9. TUNEL Assay
2.10. Western Blotting Assay
2.11. In Vivo Xenograft Model
2.12. Immunohistochemical Staining (IHC) and Hematoxylin–Eosin Staining (HE)
2.13. Evaluation of Autophagy Flux
2.14. RNA Sequencing and Differentially Expressed Genes Analysis
2.15. Statistical Analysis
3. Results
3.1. Everolimus Synergistically Enhanced the Cytotoxicity of Cisplatin Against SCCOHT Cells
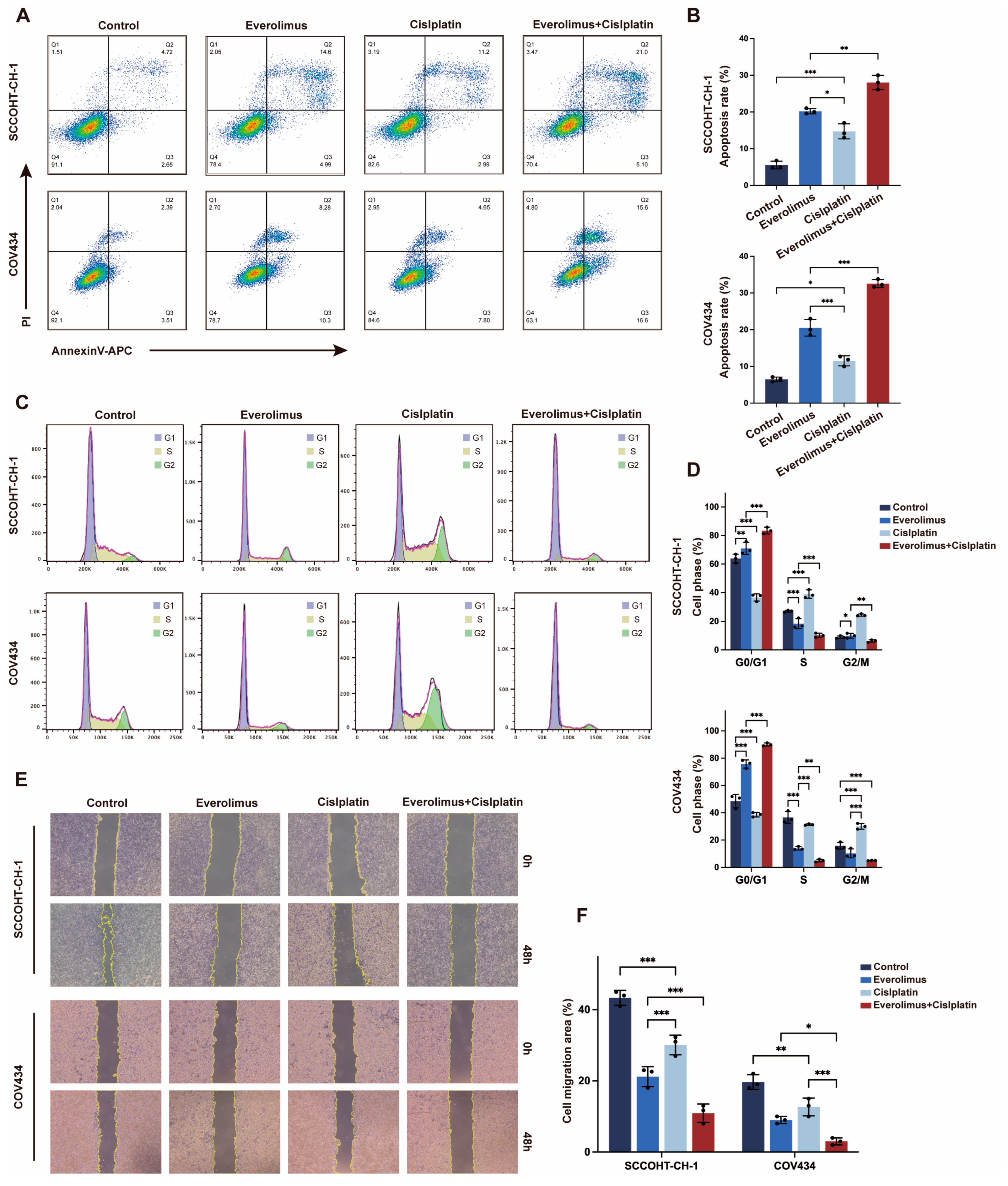
3.2. Everolimus Co-Stimulation with Cisplatin Induced Apoptosis and Cell Cycle Arrest in SCCOHT Cells
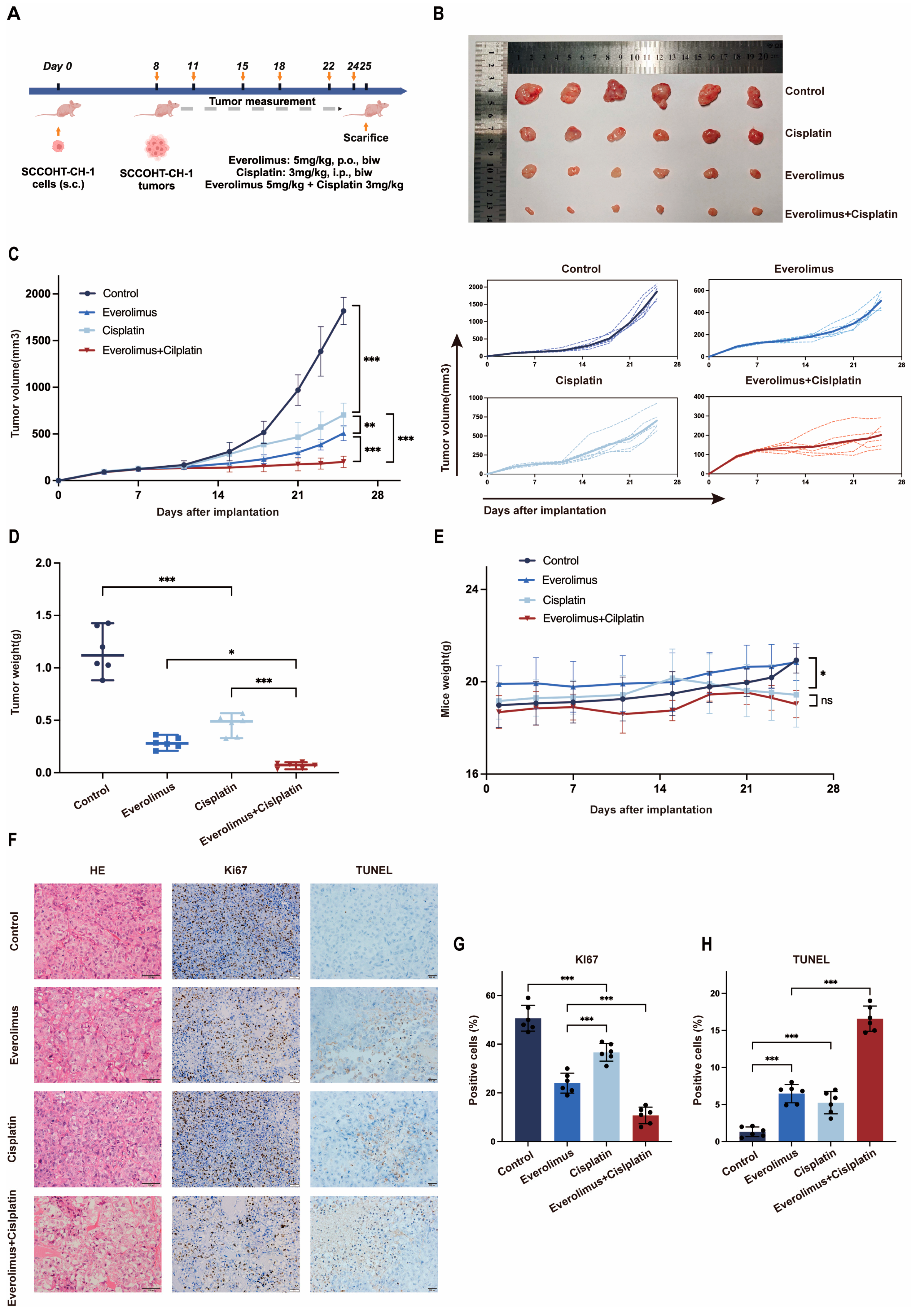
3.3. The Combination of Everolimus Plus Cisplatin Enhanced the Inhibition of Tumor Growth in SCCOHT-CH-1 Xenograft Mouse Models
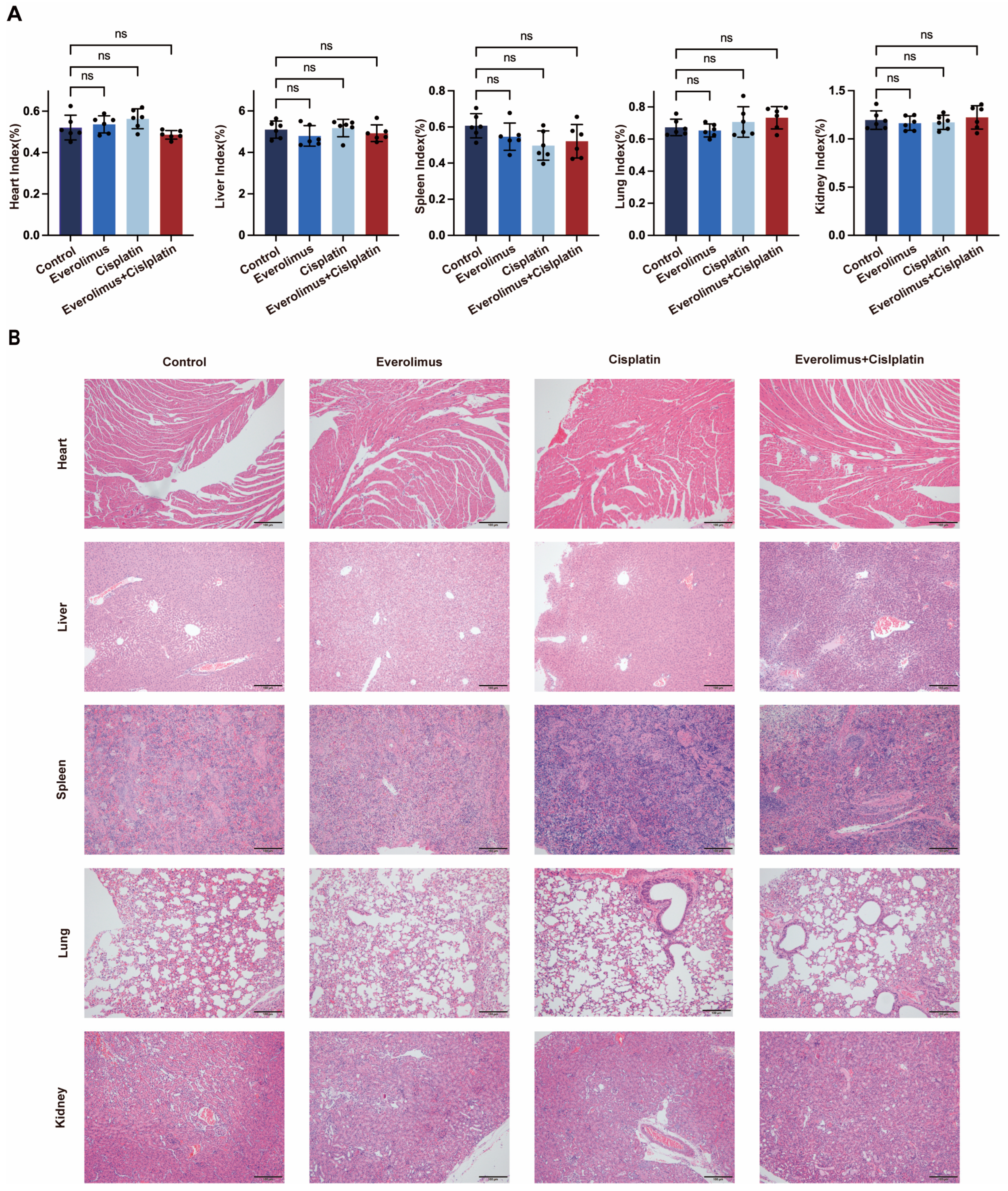
3.4. Toxicity Analysis
3.5. The Effect of Everolimus Is Mediated by the Autophagy
3.6. Regulation of Apoptosis-Related Genes by Everolimus Combined with Cisplatin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tischkowitz, M.; Huang, S.; Banerjee, S.; Hague, J.; Hendricks, W.P.D.; Huntsman, D.G.; Lang, J.D.; Orlando, K.A.; Oza, A.M.; Pautier, P.; et al. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type-Genetics, New Treatment Targets, and Current Management Guidelines. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Young, R.H.; Oliva, E.; Scully, R.E. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am. J. Surg. Pathol. 1994, 18, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Jamy, O.; Yaghmour, G.; Hare, F.; Martin, M.G. Population-based Analysis of the Clinical Features of Primary Small Cell Carcinoma of the Ovary. Anticancer Res. 2015, 35, 3091–3095. [Google Scholar]
- Harrison, M.L.; Hoskins, P.; du Bois, A.; Quinn, M.; Rustin, G.J.; Ledermann, J.A.; Baron-Hay, S.; Friedlander, M.L. Small cell of the ovary, hypercalcemic type—Analysis of combined experience and recommendation for management. A GCIG study. Gynecol. Oncol. 2006, 100, 233–238. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Gao, Y.; Xu, C.; Kang, Y. Clinical characteristics and status of treatment of small-cell carcinoma of the ovary, hypercalcemic type in the Chinese population: A meta-analysis. J. Gynecol. Oncol. 2024, 35, e96. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Tee, A.R. Mammalian target of rapamycin complex 1: Signalling inputs, substrates and feedback mechanisms. Cell. Signal. 2009, 21, 827–835. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Fakhredini, F.; Alidadi, H.; Mahdavinia, M.; Khorsandi, L. Morin promotes autophagy in human PC3 prostate cancer cells by modulating AMPK/mTOR/ULK1 signaling pathway. Tissue Cell 2024, 91, 102557. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, M.; Liu, L.; Li, H.; Liu, H.; Ren, J.; Liu, T.; Cong, M.; Zhu, Z.; Zhao, X.; et al. Targeting NPM1 inhibits proliferation and promotes apoptosis of hepatic progenitor cells via suppression of mTOR signalling pathway. Stem Cell Res. Ther. 2024, 15, 292. [Google Scholar] [CrossRef]
- Lang, J.D.; Hendricks, W.P.D.; Orlando, K.A.; Yin, H.; Kiefer, J.; Ramos, P.; Sharma, R.; Pirrotte, P.; Raupach, E.A.; Sereduk, C.; et al. Ponatinib Shows Potent Antitumor Activity in Small Cell Carcinoma of the Ovary Hypercalcemic Type (SCCOHT) through Multikinase Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1932–1943. [Google Scholar] [CrossRef]
- Huang, H.; Yan, J.; Xu, X.; Feng, Y.; Liu, H.; Liu, J.; Xie, M.; Chen, L.; Xiang, D.; Peng, W.; et al. Everolimus inhibits hepatoblastoma by inducing autophagy-dependent ferroptosis. Drug Dev. Res. 2024, 85, e22140. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Ng, W.H.; Soo, K.C. Everolimus Acts in Synergy with Vinorelbine to Suppress the Growth of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Rockhold, J.D.; Marszalkowski, H.; Sannella, M.; Gibney, K.; Murphy, L.; Zukowski, E.; Kalantar, G.H.; SantaCruz-Calvo, S.; Hart, S.N.; Kuhn, M.K.; et al. Everolimus alleviates CD4(+) T cell inflammation by regulating autophagy and cellular redox homeostasis. GeroScience 2024, 46, 5681–5699. [Google Scholar] [CrossRef] [PubMed]
- Subtil, F.S.B.; Gröbner, C.; Recknagel, N.; Parplys, A.C.; Kohl, S.; Arenz, A.; Eberle, F.; Dikomey, E.; Engenhart-Cabillic, R.; Schötz, U. Dual PI3K/mTOR Inhibitor NVP-BEZ235 Leads to a Synergistic Enhancement of Cisplatin and Radiation in Both HPV-Negative and -Positive HNSCC Cell Lines. Cancers 2022, 14, 3160. [Google Scholar] [CrossRef]
- Zhang, N.N.; Ban, Y.J.; Wang, Y.J.; He, S.Y.; Qi, P.P.; Bi, T.; Ma, Y.F.; Dong, Y.X.; Guo, B.; Weng, J.; et al. Virtual screening of novel mTOR inhibitors for the potential treatment of human colorectal cancer. Bioorg. Chem. 2023, 140, 106781. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yu, C.; Tian, Y.; Xiang, X.; Luo, Y. Inhibition of STX17-SNAP29-VAMP8 complex formation by costunolide sensitizes ovarian cancer cells to cisplatin via the AMPK/mTOR signaling pathway. Biochem. Pharmacol. 2023, 212, 115549. [Google Scholar] [CrossRef]
- Gao, Y.; Zheng, K.; Kang, M.; Xu, J.; Ning, Y.; Hu, W.; Li, K.; Kang, Y.; Xu, C. Establishment and characterization of a novel cell line (SCCOHT-CH-1) and PDX models derived from Chinese patients of small cell ovarian carcinoma of the hypercalcemic type. Hum. Cell 2023, 36, 2214–2227. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Chen, S.Y.; Chow, C.; Yang, W.; Hendricks, W.P.D.; Ramos, P.; Briones, N.; Mes-Masson, A.M.; Bosse, T.; Gilks, C.B.; et al. Re-assigning the histologic identities of COV434 and TOV-112D ovarian cancer cell lines. Gynecol. Oncol. 2021, 160, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Roberts, A.; Trapnell, C.; Donaghey, J.; Rinn, J.L.; Pachter, L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011, 12, R22. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Huang, D.; Savage, S.R.; Calinawan, A.P.; Lin, C.; Zhang, B.; Wang, P.; Starr, T.K.; Birrer, M.J.; Paulovich, A.G. A highly annotated database of genes associated with platinum resistance in cancer. Oncogene 2021, 40, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Abdel-Bakky, M.S.; Mohammed, H.A.; Mahmoud, N.I.; Amin, E.; Alsharidah, M.; Al Rugaie, O.; Ewees, M.G. Targeting the PI3K/pAKT/mTOR/NF-κB/FOXO3a signaling pathway for suppressing the development of hepatocellular carcinoma in rats: Role of the natural remedic Suaeda vermiculata forssk. Environ. Toxicol. 2024, 39, 3666–3678. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Li, L.; Zhang, Z.; Lu, M.; Xia, X. Dual PI3K/mTOR Inhibitor BEZ235 combined with BMS-1166 Promoting Apoptosis in Colorectal Cancer. Int. J. Med. Sci. 2024, 21, 1814–1823. [Google Scholar] [CrossRef]
- Kawata, T.; Tada, K.; Kobayashi, M.; Sakamoto, T.; Takiuchi, Y.; Iwai, F.; Sakurada, M.; Hishizawa, M.; Shirakawa, K.; Shindo, K.; et al. Dual inhibition of the mTORC1 and mTORC2 signaling pathways is a promising therapeutic target for adult T-cell leukemia. Cancer Sci. 2018, 109, 103–111. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, T.; McSheehy, P.M. Biomarker Development for the Clinical Activity of the mTOR Inhibitor Everolimus (RAD001): Processes, Limitations, and Further Proposals. Transl. Oncol. 2010, 3, 65–79. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Case, S. Everolimus (RAD001) in the treatment of advanced renal cell carcinoma: A review. Oncologist 2010, 15, 236–245. [Google Scholar] [CrossRef]
- Shao, Z.; Cai, L.; Wang, S.; Hu, X.; Shen, K.; Wang, H.; Li, H.; Feng, J.; Liu, Q.; Cheng, J.; et al. 238P BOLERO-5: A phase II study of everolimus and exemestane combination in Chinese post-menopausal women with ER+/HER2- advanced breast cancer. Ann. Oncol. 2021, 32, S463. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Cutsem, E.V.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; Vries, E.G.E.d.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Garcia, J.M.; Scherer, T.; Chen, J.A.; Guillory, B.; Nassif, A.; Papusha, V.; Smiechowska, J.; Asnicar, M.; Buettner, C.; Smith, R.G. Inhibition of cisplatin-induced lipid catabolism and weight loss by ghrelin in male mice. Endocrinology 2013, 154, 3118–3129. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, Y.Y.; Jeong, S.; Lee, S.W.; Lee, S.J.; Rho, M.C.; Kim, S.H.; Lee, S. Oleanolic Acid Acetate Alleviates Cisplatin-Induced Nephrotoxicity via Inhibition of Apoptosis and Necroptosis In Vitro and In Vivo. Toxics 2024, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Országhová, Z.; Kalavska, K.; Mego, M.; Chovanec, M. Overcoming Chemotherapy Resistance in Germ Cell Tumors. Biomedicines 2022, 10, 972. [Google Scholar] [CrossRef]
- Weng, H.C.; Sung, C.J.; Hsu, J.L.; Leu, W.J.; Guh, J.H.; Kung, F.L.; Hsu, L.C. The Combination of a Novel GLUT1 Inhibitor and Cisplatin Synergistically Inhibits Breast Cancer Cell Growth By Enhancing the DNA Damaging Effect and Modulating the Akt/mTOR and MAPK Signaling Pathways. Front. Pharmacol. 2022, 13, 879748. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Gao, Q.; Xiang, X.; Xu, J.; Jiao, Y.; Li, X.; Zhang, X.; Jia, X. Autophagy Inhibition Enhances the Anti-Tumor Activity of Methylseleninic Acid in Cisplatin-Resistance Human Lung Adenocarcinoma Cells. Front. Pharmacol. 2022, 13, 890974. [Google Scholar] [CrossRef]
- Kupryjańczyk, J.; Dansonka-Mieszkowska, A.; Moes-Sosnowska, J.; Plisiecka-Hałasa, J.; Szafron, L.; Podgórska, A.; Rzepecka, I.K.; Konopka, B.; Budziłowska, A.; Rembiszewska, A.; et al. Ovarian small cell carcinoma of hypercalcemic type—Evidence of germline origin and SMARCA4 gene inactivation. A pilot study. Pol. J. Pathol. Off. J. Pol. Soc. Pathol. 2013, 64, 238–246. [Google Scholar] [CrossRef]
- Jelinic, P.; Mueller, J.J.; Olvera, N.; Dao, F.; Scott, S.N.; Shah, R.; Gao, J.; Schultz, N.; Gonen, M.; Soslow, R.A.; et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat. Genet. 2014, 46, 424–426. [Google Scholar] [CrossRef]
- Ramos, P.; Karnezis, A.N.; Craig, D.W.; Sekulic, A.; Russell, M.L.; Hendricks, W.P.; Corneveaux, J.J.; Barrett, M.T.; Shumansky, K.; Yang, Y.; et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat. Genet. 2014, 46, 427–429. [Google Scholar] [CrossRef]
- Witkowski, L.; Carrot-Zhang, J.; Albrecht, S.; Fahiminiya, S.; Hamel, N.; Tomiak, E.; Grynspan, D.; Saloustros, E.; Nadaf, J.; Rivera, B.; et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat. Genet. 2014, 46, 438–443. [Google Scholar] [CrossRef]
- Morais, M.; Machado, V.; Figueiredo, P.; Dias, F.; Craveiro, R.; Lencart, J.; Palmeira, C.; Mikkonen, K.S.; Teixeira, A.L.; Medeiros, R. Silver Nanoparticles (AgNPs) as Enhancers of Everolimus and Radiotherapy Sensitivity on Clear Cell Renal Cell Carcinoma. Antioxidants 2023, 12, 2051. [Google Scholar] [CrossRef]
- Nam, D.; Park, J.; Lee, J.; Son, J.; Kim, J.E. mTOR potentiates senescent phenotypes and primary cilia formation after cisplatin-induced G2 arrest in retinal pigment epithelial cells. Cell. Signal. 2024, 124, 111402. [Google Scholar] [CrossRef]
- Elshazly, A.M.; Elzahed, A.A.; Gewirtz, D.A. The Cytoprotective and Cytotoxic Functions of Autophagy in Response to mTOR Inhibitors. Front. Biosci. (Landmark Ed.) 2024, 29, 231. [Google Scholar] [CrossRef]
- Wang, S.; Guo, S.; Guo, J.; Du, Q.; Wu, C.; Wu, Y.; Zhang, Y. Cell death pathways: Molecular mechanisms and therapeutic targets for cancer. MedComm 2024, 5, e693. [Google Scholar] [CrossRef]
- Choi, E.H.; Park, S.J. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp. Mol. Med. 2023, 55, 1348–1356. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, F.; Qu, K.; Liu, C.; Zhang, J. TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook. Oxidative Med. Cell. Longev. 2022, 2022, 7805115. [Google Scholar] [CrossRef]
- Han, S.H.; Jeon, J.H.; Ju, H.R.; Jung, U.; Kim, K.Y.; Yoo, H.S.; Lee, Y.H.; Song, K.S.; Hwang, H.M.; Na, Y.S.; et al. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene 2003, 22, 4035–4046. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Gao, Y.; Xu, J.; Kang, M.; Chai, R.; Jin, G.; Kang, Y. mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin. Biomedicines 2025, 13, 1. https://doi.org/10.3390/biomedicines13010001
Zheng K, Gao Y, Xu J, Kang M, Chai R, Jin G, Kang Y. mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin. Biomedicines. 2025; 13(1):1. https://doi.org/10.3390/biomedicines13010001
Chicago/Turabian StyleZheng, Kewei, Yi Gao, Jing Xu, Mingyi Kang, Ranran Chai, Guanqin Jin, and Yu Kang. 2025. "mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin" Biomedicines 13, no. 1: 1. https://doi.org/10.3390/biomedicines13010001
APA StyleZheng, K., Gao, Y., Xu, J., Kang, M., Chai, R., Jin, G., & Kang, Y. (2025). mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin. Biomedicines, 13(1), 1. https://doi.org/10.3390/biomedicines13010001







