Neutrophil Extracellular Trap Scores Predict 90-Day Mortality in Hepatitis B-Related Acute-on-Chronic Liver Failure
Abstract
1. Introduction
2. Materials and Methods
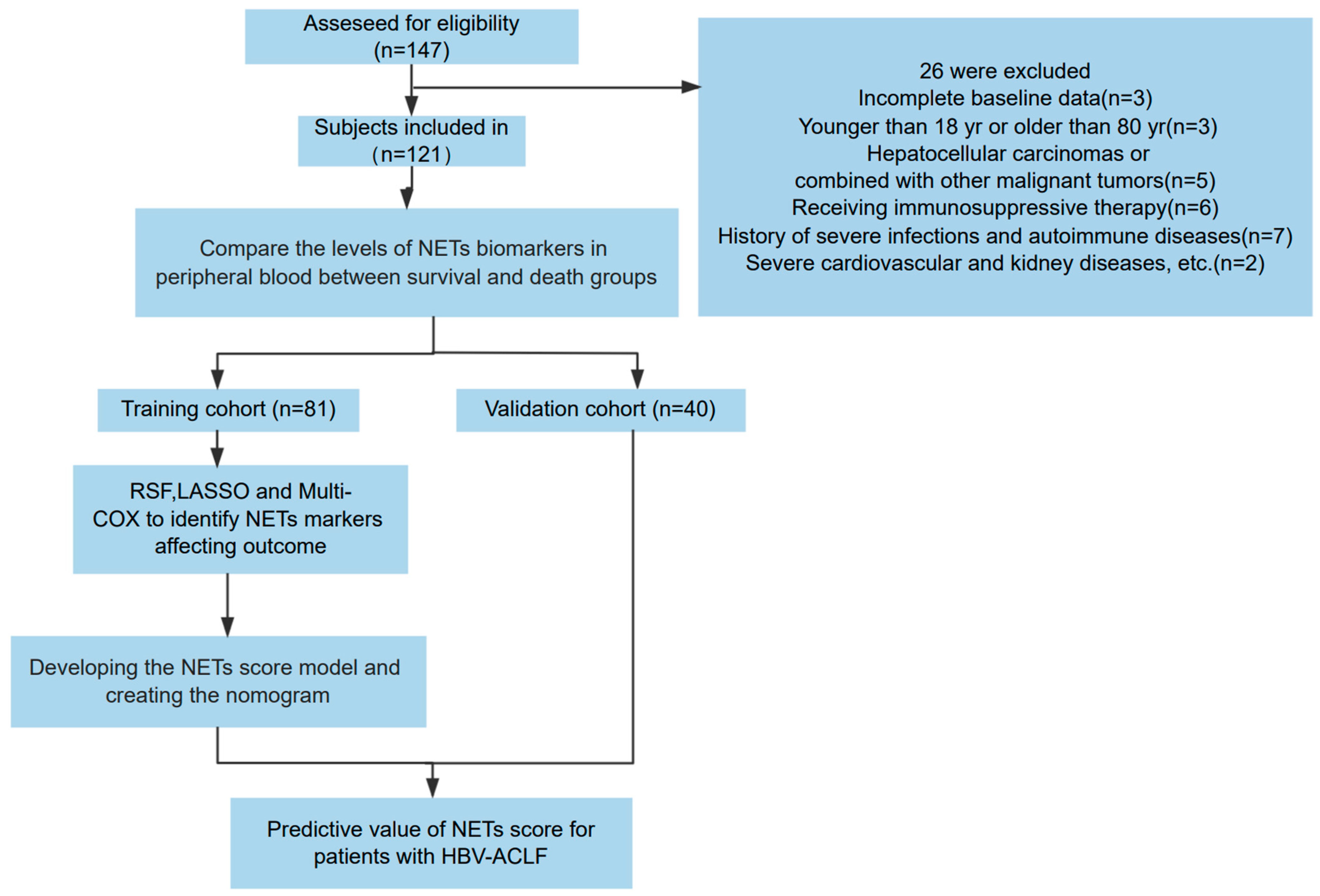
2.1. Patient Enrollment
2.2. Blood Sample Collection and Storage Process
2.3. NET Measurement
2.3.1. cfDNA Detection
2.3.2. MPO-DNA Detection
2.3.3. CitH3 Detection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with HBV-ACLF
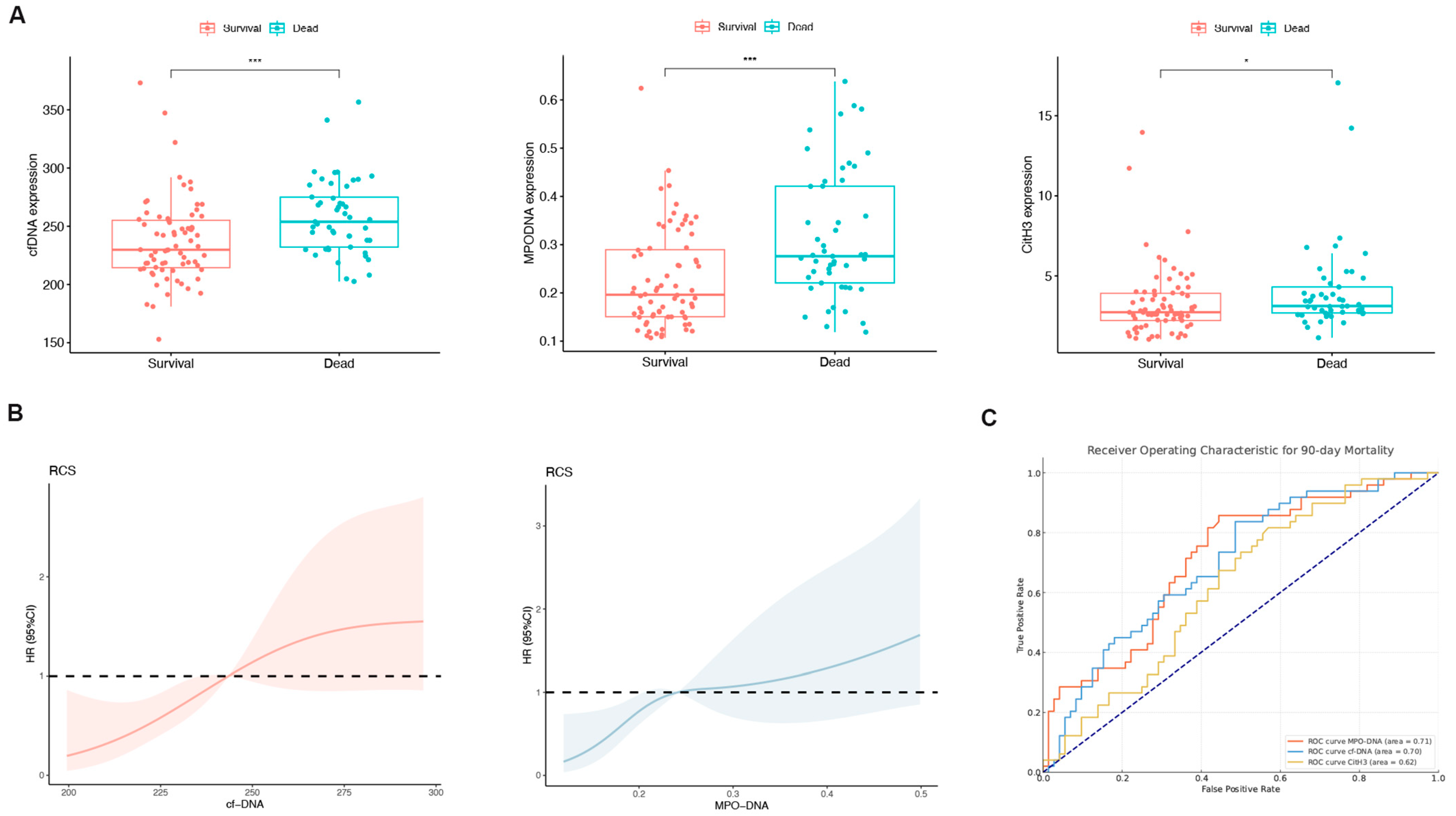
3.2. Expression Levels of NET-Related Markers in HBV-ACLF Patients
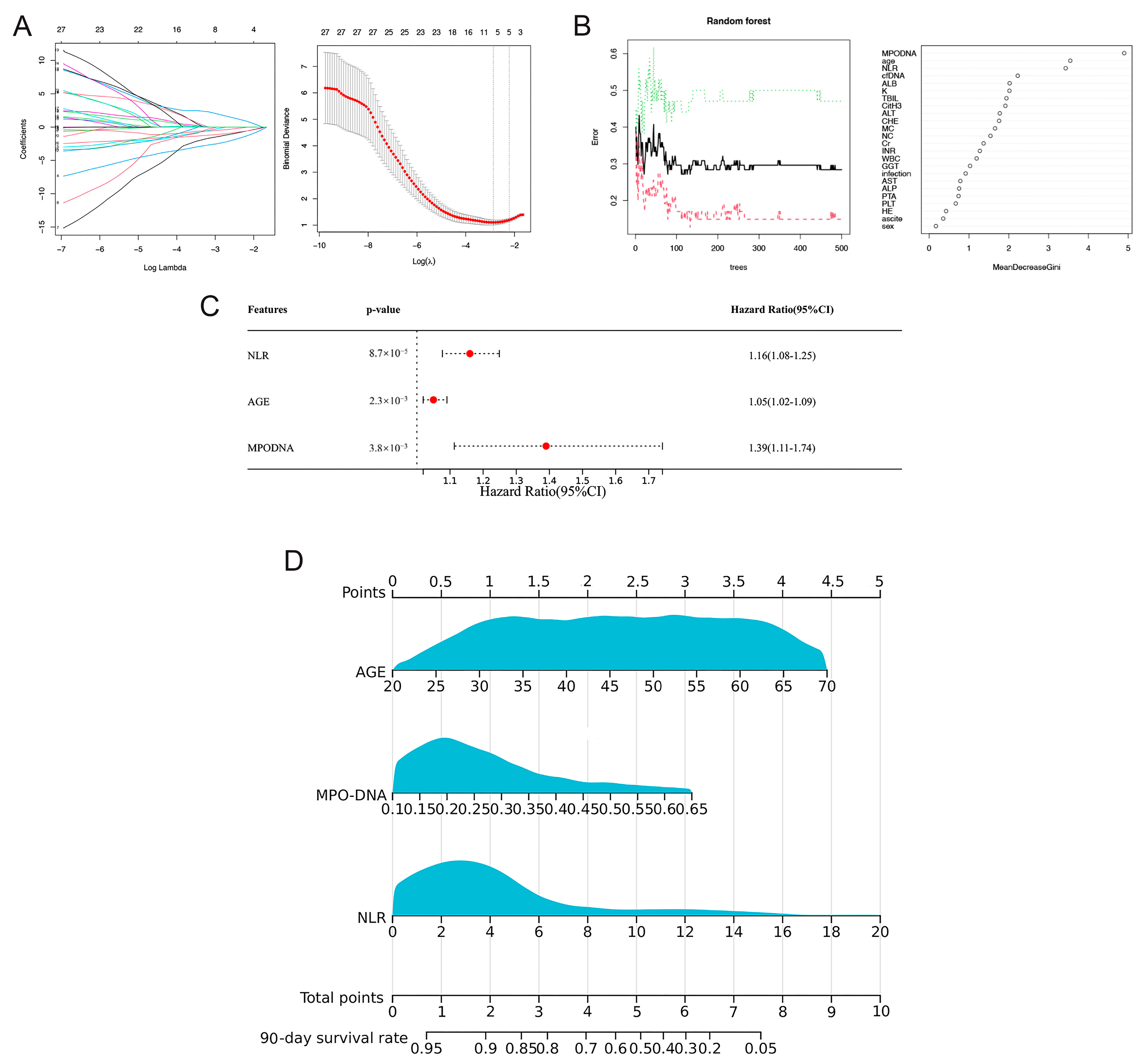
3.3. Prognostic Model Based on LASSO-Cox Regression
3.4. Prognosis Model Based on RSF-Cox Analysis
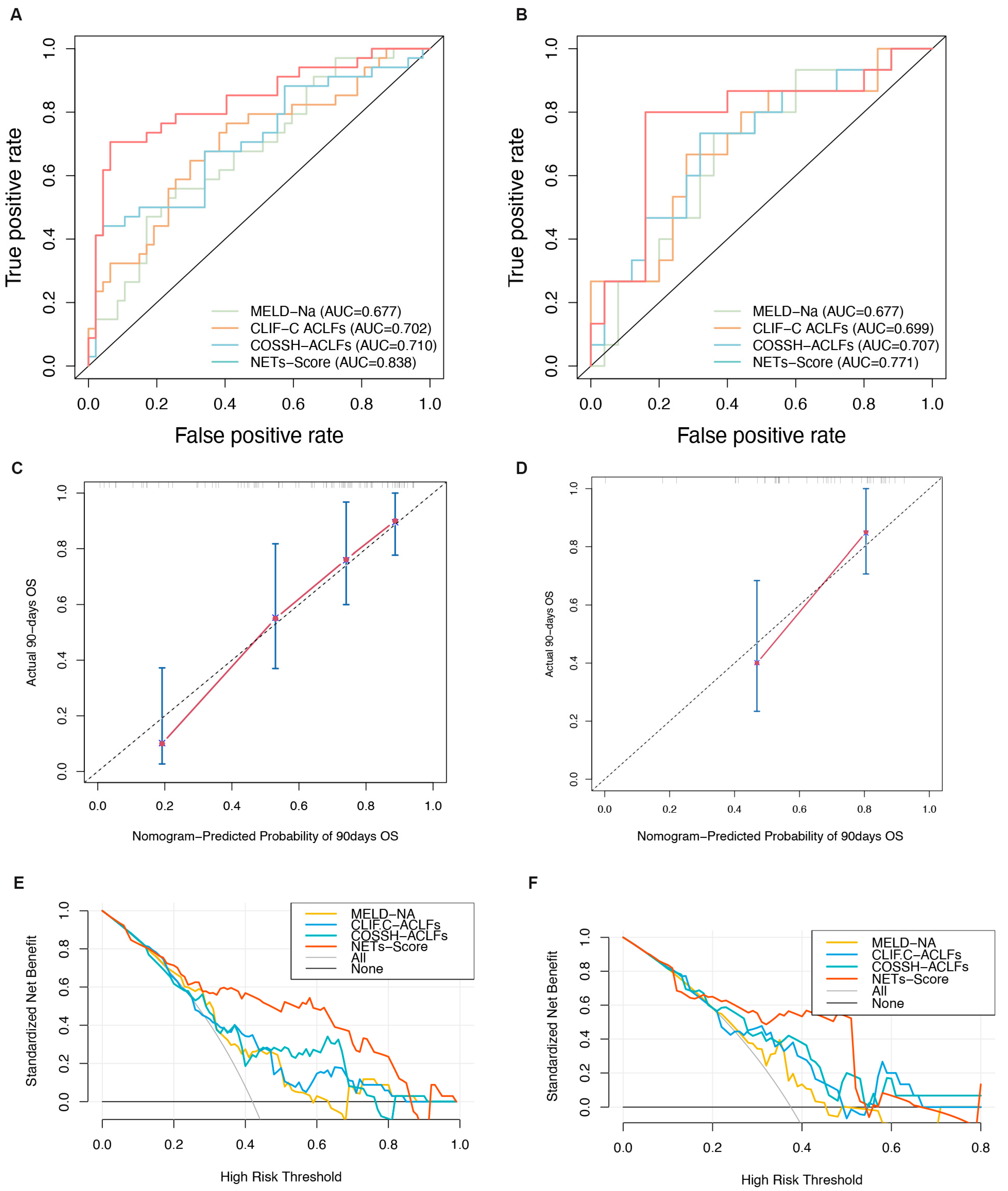
3.5. Discrimination and Calibration of the Nomogram
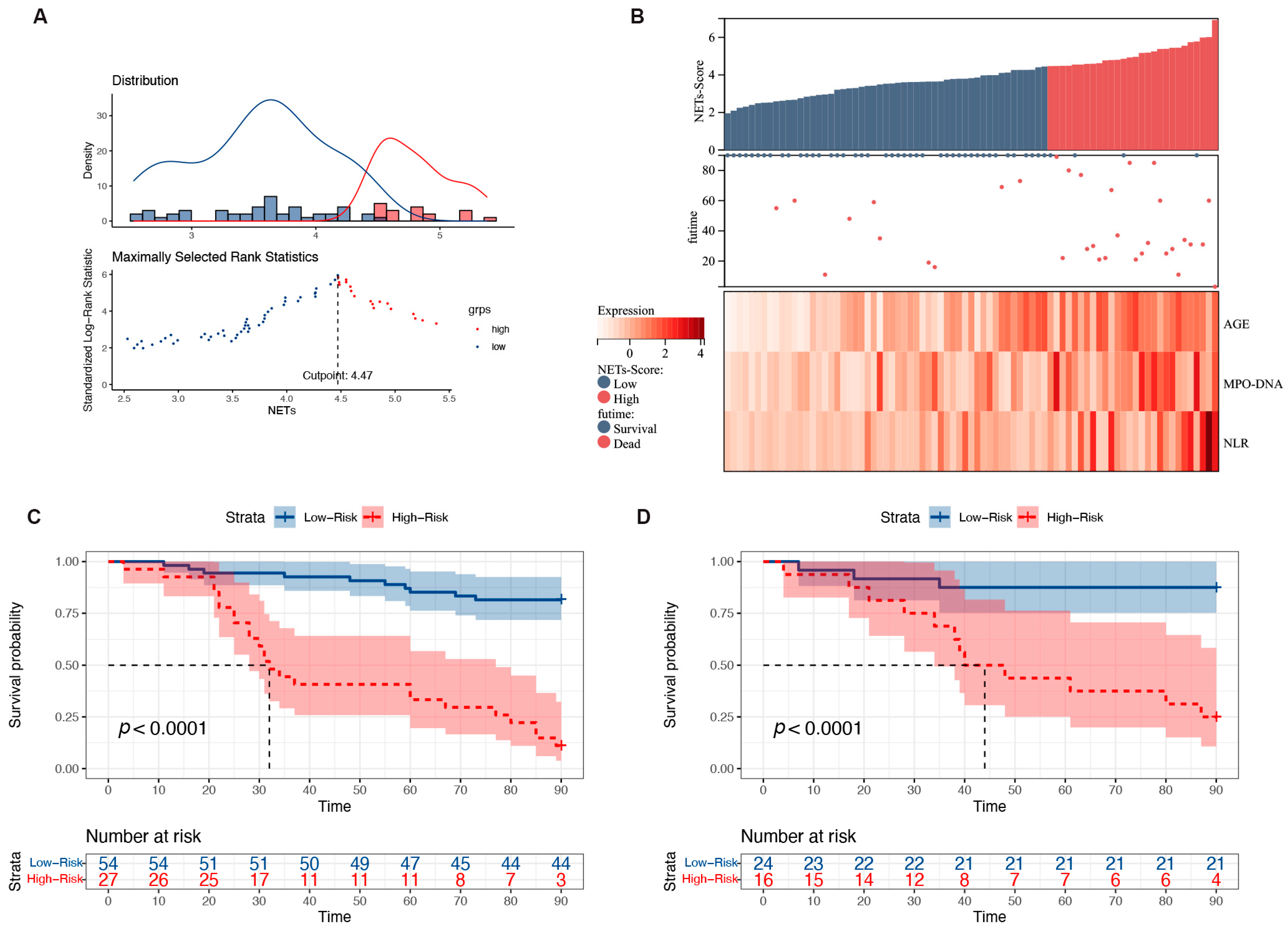
3.6. Risk Stratification for Patients with HBV-ACLF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarin, S.K.; Kedarisetty, C.K.; Abbas, Z.; Amarapurkar, D.; Bihari, C.; Chan, A.C.; Chawla, Y.K.; Dokmeci, A.K.; Garg, H.; Ghazinyan, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 2014, 8, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Kamath, P.S.; Jalan, R.; Ginès, P.; Nevens, F.; Fernández, J.; To, U.; García-Tsao, G.; Schnabl, B. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Primers 2016, 2, 16041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Han, T.; Nie, C.; Cai, J.; Liu, H.; Liu, Y. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS ONE 2015, 10, e0122158. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef]
- Hernaez, R.; Liu, Y.; Kramer, J.R.; Rana, A.; El-Serag, H.B.; Kanwal, F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J. Hepatol. 2020, 73, 1425–1433. [Google Scholar] [CrossRef]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Shao, L.; Xin, J.; Jiang, L.; Zhou, Q.; Shi, D.; Jiang, J.; Sun, S.; Jin, L.; et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018, 67, 2181–2191. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on Acute-on-Chronic Liver Failure. J. Hepatol. 2023, 79, 461–491. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Tritto, G.; Bechlis, Z.; Stadlbauer, V.; Davies, N.; Francés, R.; Shah, N.; Mookerjee, R.P.; Such, J.; Jalan, R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J. Hepatol. 2011, 55, 574–581. [Google Scholar] [CrossRef]
- Wu, L.; Gao, X.; Guo, Q.; Li, J.; Yao, J.; Yan, K.; Xu, Y.; Jiang, X.; Ye, D.; Guo, J. The role of neutrophils in innate immunity-driven nonalcoholic steatohepatitis: Lessons learned and future promise. Hepatol. Int. 2020, 14, 652–666. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- von Meijenfeldt, F.A.; Stravitz, R.T.; Zhang, J.; Adelmeijer, J.; Zen, Y.; Durkalski, V.; Lee, W.M.; Lisman, T. Generation of neutrophil extracellular traps in patients with acute liver failure is associated with poor outcome. Hepatology 2022, 75, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Biggins, S.W.; Kim, W.R.; Terrault, N.A.; Saab, S.; Balan, V.; Schiano, T.; Benson, J.; Therneau, T.; Kremers, W.; Wiesner, R.; et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006, 130, 1652–1660. [Google Scholar] [CrossRef]
- von Meijenfeldt, F.A.; Lisman, T. Unravelling the Role of Neutrophil Extracellular Traps in Acute Liver Failure. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 720–721. [Google Scholar] [CrossRef]
- He, L.; Cai, Q.; Liang, X.; Xin, J.; Shi, D.; Ren, K.; Li, Y.; Chen, J.; Sun, S.; Guo, B.; et al. ETS2 alleviates acute-on-chronic liver failure by suppressing excessive inflammation. J. Med. Virol. 2023, 95, e28710. [Google Scholar] [CrossRef]
- Yang, L.Y.; Luo, Q.; Lu, L.; Zhu, W.W.; Sun, H.T.; Wei, R.; Lin, Z.F.; Wang, X.Y.; Wang, C.Q.; Lu, M.; et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 2020, 13, 3. [Google Scholar] [CrossRef]
- Hilscher, M.B.; Shah, V.H. Neutrophil Extracellular Traps and Liver Disease. Semin. Liver Dis. 2020, 40, 171–179. [Google Scholar] [CrossRef]
- Makkar, K.; Tomer, S.; Verma, N.; Rathi, S.; Arora, S.K.; Taneja, S.; Duseja, A.; Chawla, Y.K.; Dhiman, R.K. Neutrophil dysfunction predicts 90-day survival in patients with acute on chronic liver failure: A longitudinal case-control study. JGH Open 2020, 4, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, S.; Wang, Y.; Zhao, R.; Ren, H.; Li, Z.; Zhao, H.; Zhang, Y.; Sheng, J.; Chen, Z. Circulating Neutrophil Dysfunction in HBV-Related Acute-on-Chronic Liver Failure. Front. Immunol. 2021, 12, 620365. [Google Scholar] [CrossRef] [PubMed]
- Vats, R.; Kaminski, T.W.; Brzoska, T.; Leech, J.A.; Tutuncuoglu, E.; Katoch, O.; Jonassaint, J.; Tejero, J.; Novelli, E.M.; Pradhan-Sundd, T.; et al. Liver-to-lung microembolic NETs promote gasdermin D-dependent inflammatory lung injury in sickle cell disease. Blood 2022, 140, 1020–1037. [Google Scholar] [CrossRef]
- Klimiankou, M.; Skokowa, J. Old drug revisited: Disulfiram, NETs, and sepsis. Blood 2021, 138, 2604–2605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, C.; Han, Y.; Huang, L.; Sheng, H.; Wang, J.; Zhang, Y.; Lai, J.; Yuan, J.; Chen, X. Neutrophil autophagy and NETosis in COVID-19: Perspectives. Autophagy 2023, 19, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, E.; Lemberg, C.; Küng, N.; Schraner, E.; Theocharides, A.P.A.; LeibundGut-Landmann, S. Candida albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4. Front. Immunol. 2018, 9, 1573. [Google Scholar] [CrossRef]
- Thomson, A.H. Human recombinant DNase in cystic fibrosis. J. R. Soc. Med. 1995, 88 (Suppl. 25), 24–29. [Google Scholar] [PubMed Central]
- Davis, J.C., Jr.; Manzi, S.; Yarboro, C.; Rairie, J.; Mcinnes, I.; Averthelyi, D.; Sinicropi, D.; Hale, V.G.; Balow, J.; Austin, H.; et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 1999, 8, 68–76. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lin, T.T.; Hu, L.; Xu, C.J.; Hu, F.; Wan, L.; Yang, X.; Wu, X.F.; Zhang, X.T.; Li, Y.; et al. Neutrophil extracellular traps as a unique target in the treatment of chemotherapy-induced peripheral neuropathy. EBioMedicine 2023, 90, 104499. [Google Scholar] [CrossRef]
- Ye, D.; Yao, J.; Du, W.; Chen, C.; Yang, Y.; Yan, K.; Li, J.; Xu, Y.; Zang, S.; Zhang, Y.; et al. Neutrophil Extracellular Traps Mediate Acute Liver Failure in Regulation of miR-223/Neutrophil Elastase Signaling in Mice. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 587–607. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Guo, J.; Zhang, D.; Zhang, Y.; Zhang, L.; Gong, Z. IDH1/MDH1 deacetylation promotes acute liver failure by regulating NETosis. Cell. Mol. Biol. Lett. 2024, 29, 8. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Toltl, L.J.; Swystun, L.L.; Pogue, J.; Liaw, K.L.; Weitz, J.I.; Cook, D.J.; Fox-Robichaud, A.E.; Liaw, P.C.; Canadian Critical Care Translational Biology Group. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit. Care. 2012, 16, R151. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alcázar, M.; Kim, N.; Fuchs, T.A. Circulating Extracellular DNA: Cause or Consequence of Thrombosis? Semin. Thromb. Hemost. 2017, 43, 553–561. [Google Scholar] [CrossRef]
- Hua, X.; Zhou, H.; Wu, H.C.; Furnari, J.; Kotidis, C.P.; Rabadan, R.; Genkinger, J.M.; Bruce, J.N.; Canoll, P.; Santella, R.M.; et al. Tumor detection by analysis of both symmetric- and hemi-methylation of plasma cell-free DNA. Nat. Commun. 2024, 15, 6113. [Google Scholar] [CrossRef] [PubMed]
- Okkonen, M.; Lakkisto, P.; Korhonen, A.M.; Parviai-nen, I.; Reinikainen, M.; Varpula, T.; Pettilä, V.; FINNALI Study Group. Plasma cell-free DNA in patients needing mechanical ventilation. Crit. Care. 2011, 15, R196. [Google Scholar] [CrossRef] [PubMed]
- Tillack, K.; Naegele, M.; Haueis, C.; Schippling, S.; Wandinger, K.P.; Martin, R.; Sospedra, M. Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J. Neuroimmunol. 2013, 261, 108–119. [Google Scholar] [CrossRef]
| Training Cohort (n = 81) | Validation Cohort (n = 40) | p Values | |
|---|---|---|---|
| Patients background | |||
| Age, yr | 46 (36, 58) | 47.5 (38.25, 54) | 0.732 |
| Gender (male/female) | 63 (18) | 36 (4) | 0.165 |
| Ascite, n (%) | 68 (84) | 36 (90) | 0.533 |
| Abdominal infection | 37 (46) | 16 (40) | 0.691 |
| Hepatic Encephalopathy, n (%) | 36 (44) | 15 (38) | 0.595 |
| NETs–related indicators | |||
| MPO-DNA | 0.24 (0.16, 0.33) | 0.26(0.17, 0.34) | 0.862 |
| cf-DNA | 242.67 (219, 268.86) | 246.43 (220.84, 260.84) | 0.985 |
| CitH3 | 3.03 (2.47, 3.94) | 2.84 (2.51, 3.93) | 0.217 |
| Laboratory parameters | |||
| ALT (U/L) | 334.3 (103, 695.9) | 252.1 (113.58, 541.85) | 0.932 |
| AST (U/L) | 212.4 (103, 391.6) | 268.65 (146.52, 474.12) | 0.283 |
| TBil (µmol/L) | 244.6 (177.1, 333.40) | 281.75 (219.4, 349.38) | 0.106 |
| γ-GGT (U/L) | 82.3 (46, 112.6) | 96.4 (57.08, 142.98) | 0.136 |
| ALB (g/L) | 31.1 (28.6, 33.3) | 32.05 (29.8, 35.35) | 0.069 |
| Cr (µmol/L) | 72 (60, 81.7) | 67.2(58.45, 80.55) | 0.512 |
| INR | 2.22 (1.9, 2.75) | 2.15 (2, 2.41) | 0.381 |
| PTA (%) | 35 (27, 39) | 36 (30.8, 40) | 0.178 |
| WBC (109/L) | 6.14 (4.4, 8.3) | 5.16 (4.46, 6.69) | 0.279 |
| NLR | 2.74 (2.07, 4.78) | 3.58 (2.25, 5.29) | 0.365 |
| K+ (mmol/L) | 3.74 (3.52, 4.1) | 3.99 (3.63, 4.24) | 0.026 |
| NA+ (mmol/L) | 138.5 (135.4, 142.4) | 137.5 (132.75, 139.85) | 0.081 |
| Cutoff Value | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) |
|---|---|---|---|
| Training cohort (n = 81) | |||
| NET score | 87.5% | 77.2% | 71.8% |
| MELD-Na | 61.5% | 67.3% | 47.1% |
| CLIF-C ACLF | 65.5% | 71.2% | 55.9% |
| COSSH-ACLF | 56.6% | 85.7% | 51.1% |
| Validation cohort (n = 40) | |||
| NET score | 70.6% | 86.9% | 80% |
| MELD-Na | 52.3% | 78.9% | 73.33% |
| CLIF-C ACLF | 50% | 85.7% | 48% |
| COSSH-ACLF | 55% | 80% | 64% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shi, K.; Zhu, B.; Feng, Y.; Liu, Y.; Wang, X. Neutrophil Extracellular Trap Scores Predict 90-Day Mortality in Hepatitis B-Related Acute-on-Chronic Liver Failure. Biomedicines 2024, 12, 2048. https://doi.org/10.3390/biomedicines12092048
Zhang Y, Shi K, Zhu B, Feng Y, Liu Y, Wang X. Neutrophil Extracellular Trap Scores Predict 90-Day Mortality in Hepatitis B-Related Acute-on-Chronic Liver Failure. Biomedicines. 2024; 12(9):2048. https://doi.org/10.3390/biomedicines12092048
Chicago/Turabian StyleZhang, Yi, Ke Shi, Bingbing Zhu, Ying Feng, Yao Liu, and Xianbo Wang. 2024. "Neutrophil Extracellular Trap Scores Predict 90-Day Mortality in Hepatitis B-Related Acute-on-Chronic Liver Failure" Biomedicines 12, no. 9: 2048. https://doi.org/10.3390/biomedicines12092048
APA StyleZhang, Y., Shi, K., Zhu, B., Feng, Y., Liu, Y., & Wang, X. (2024). Neutrophil Extracellular Trap Scores Predict 90-Day Mortality in Hepatitis B-Related Acute-on-Chronic Liver Failure. Biomedicines, 12(9), 2048. https://doi.org/10.3390/biomedicines12092048






