Clinical and Immunological Factors Associated with the Progression of Lupus Nephritis in a Population from the Colombian Caribbean
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Variables
2.3. Statistical Methods
3. Results
3.1. General Characteristics
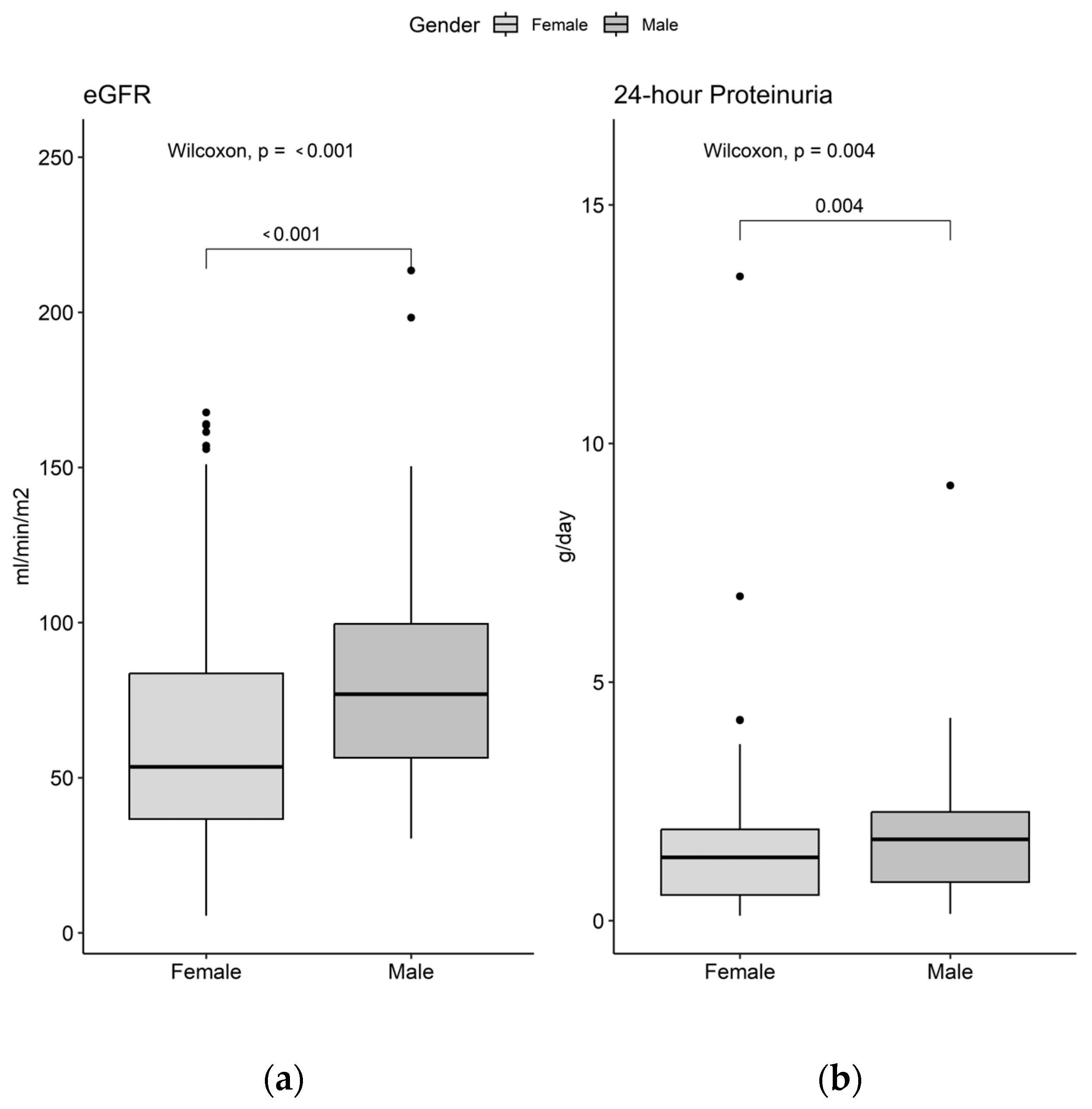
3.2. Clinical, Immunological, and Histopathological Profile by Sex
3.3. Clinical, Immunological, and Histopathological Profile by Histological Type
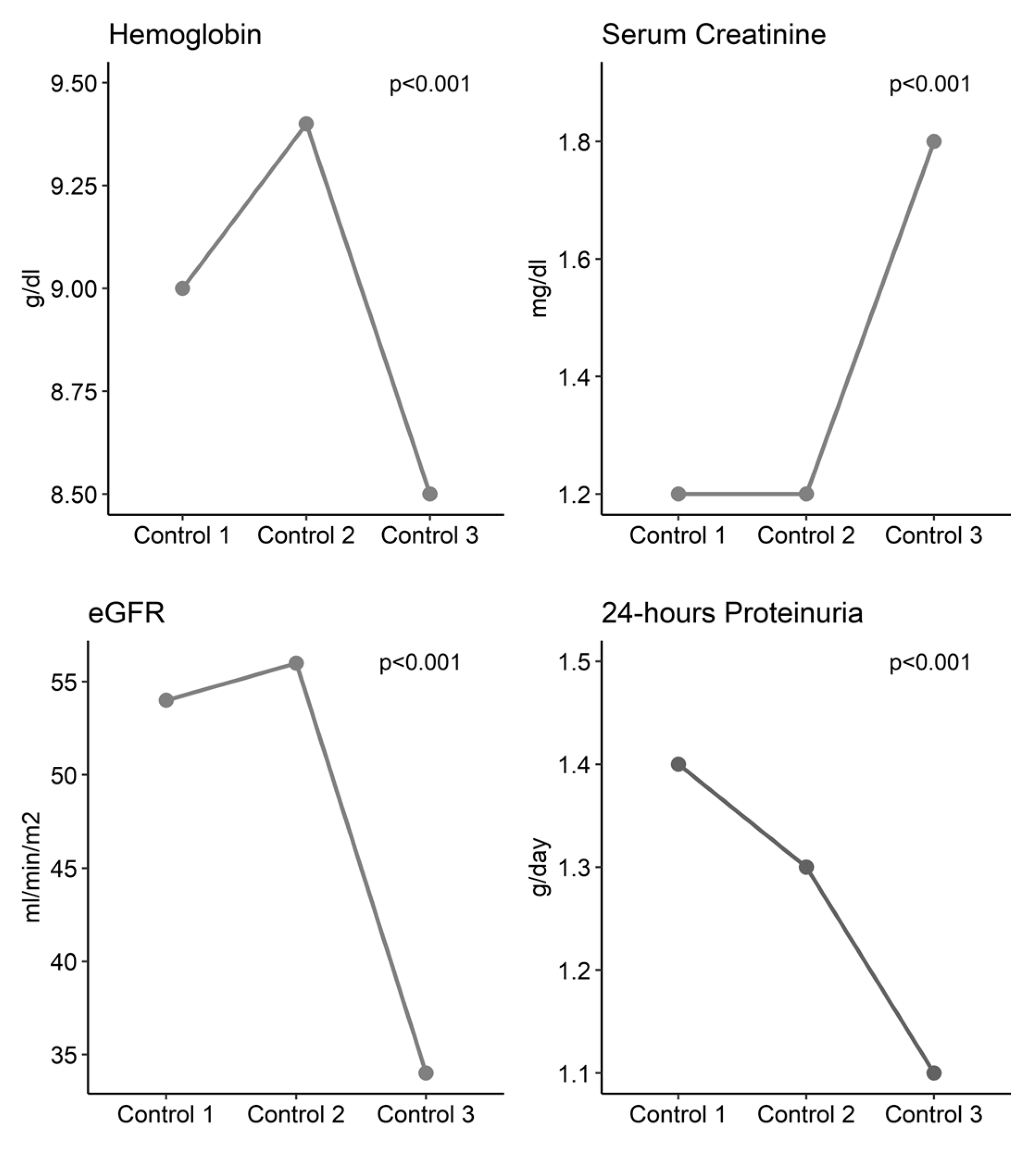
3.4. Evolution of Clinical and Immunological Parameters
3.5. Clinical, Immunological, and Histopathological Characteristics According to Survival
3.6. Predictors of Non-Response to Treatment
3.7. Predictors of Mortality in LN Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef]
- Jesus, D.; Rodrigues, M.; Matos, A.; Henriques, C.; Pereira da Silva, J.A.; Inês, L.S. Performance of SLEDAI-2K to Detect a Clinically Meaningful Change in SLE Disease Activity: A 36-Month Prospective Cohort Study of 334 Patients. Lupus 2019, 28, 607–612. [Google Scholar] [CrossRef]
- Student, M.T.; Kumar, R.R.; Omments, R.E.C.; Prajapati, A.; Blockchain, T.-A.; Ml, A.I.; Randive, P.S.N.; Chaudhari, S.; Barde, S.; Devices, E.; et al. Lupus Nephritis. En Comprehensive Clinical. Front. Neurosci. 2021, 14, 726–729. [Google Scholar]
- Saxena, R.; Mahajan, T.; Mohan, C. Lupus Nephritis: Current Update. Arthritis Res. Ther. 2011, 13, 240. [Google Scholar] [CrossRef]
- Zahab, M.; Fouda, M.A.; Elhendy, Y.; Elokely, A.; Abdul Rahim, M.; Refaie, A.F.; Alobaidi, S.; Akl, A. Treatment Outcomes of Proliferative vs. Non-Proliferative Adult Lupus Nephritis: A 10-Year Follow-Up. Cureus 2021, 13, e16955. [Google Scholar] [CrossRef]
- Duran, E.; Yıldırım, T.; Taghiyeva, A.; Bilgin, E.; Arıcı, M.; Sağlam, E.A.; Özen, S.; Üner, M.; Erdem, Y.; Kalyoncu, U.; et al. Differences and Similarities of Proliferative and Non-Proliferative Forms of Biopsy-Proven Lupus Nephritis: Single Centre, Cross-Disciplinary Experience. Lupus 2022, 31, 1147–1156. [Google Scholar] [CrossRef]
- McDonald, S.; Yiu, S.; Su, L.; Gordon, C.; Truman, M.; Lisk, L.; Solomons, N.; Bruce, I.N. MASTERPLANS Consortium Predictors of Treatment Response in a Lupus Nephritis Population: Lessons from the Aspreva Lupus Management Study (ALMS) Trial. Lupus Sci. Med. 2022, 9, e000584. [Google Scholar] [CrossRef]
- Pacheco-Lugo, L.; Sáenz-García, J.; Navarro Quiroz, E.; González Torres, H.J.; Fang, L.; Díaz-Olmos, Y.; Garavito de Egea, G.; Egea Bermejo, E.; Aroca Martínez, G. Plasma Cytokines as Potential Biomarkers of Kidney Damage in Patients with Systemic Lupus Erythematosus. Lupus 2019, 28, 34–43. [Google Scholar] [CrossRef]
- Choi, S.-E.; Park, D.-J.; Kang, J.-H.; Lee, K.-E.; Xu, H.; Lee, J.S.; Choi, Y.-D.; Lee, S.-S. Comparison of Renal Responses to Cyclophosphamide and Mycophenolate Mofetil Used as Induction Therapies in Korean Patients with Lupus Nephritis. J. Rheum. Dis. 2019, 26, 57. [Google Scholar] [CrossRef]
- Park, D.J.; Bin Joo, Y.; Bang, S.-Y.; Lee, J.; Lee, H.-S.; Bae, S.-C. Predictive Factors for Renal Response in Lupus Nephritis: A Single-Center Prospective Cohort Study. J. Rheum. Dis. 2022, 29, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, M.; Gatto, M.; Binda, V.; Doria, A.; Moroni, G. Lupus Nephritis: Clinical Presentations and Outcomes in the 21st Century. Rheumatology 2020, 59, v39–v51. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhang, X.; Feng, J.; Zhong, S.; Qi, J.; Lin, Z. Renal Response and Its Predictive Factors of Lupus Nephritis: A 2-Year Real-World Study of 56 Hospital-Based Patients. Clin. Rheumatol. 2022, 41, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Seligman, V.A.; Lum, R.F.; Olson, J.L.; Li, H.; Criswell, L.A. Demographic Differences in the Development of Lupus Nephritis: A Retrospective Analysis. Am. J. Med. 2002, 112, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Afifi, R.; Elkhayat, S.S.; Medkouri, G.; Mtioui, N.; Zamd, M.; Ramdani, B.; Benghanem, M. MO119: Lupus Nephritis in Males: About 34 Cases. Nephrol. Dial. Transplant. 2022, 37, S43–S44. [Google Scholar] [CrossRef]
- Resende, A.; Titan, S.; Barros, R.; Woronik, V. Worse Renal Outcome of Lupus Nephritis in Male Patients: A Case–Control Study. Lupus 2011, 20, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.I.; Dannoun, E.; Abu Shahin, N.; AlRyalat, S.A. Characteristics and Histological Types of Lupus Nephritis in a Jordanian Tertiary Medical Center. Biomed Res. Int. 2019, 2019, 7087461. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, K.; Kitamura, M.; Sato, S.; Eguchi, M.; Okamoto, M.; Endo, Y.; Tsuji, S.; Takatani, A.; Shimizu, T.; Umeda, M.; et al. Complete Renal Response at 12 Months after Induction Therapy Is Associated with Renal Relapse-Free Rate in Lupus Nephritis: A Single-Center, Retrospective Cohort Study. Lupus 2019, 28, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Luís, M.S.F.; Bultink, I.E.M.; da Silva, J.A.P.; Voskuyl, A.E.; Inês, L.S. Early Predictors of Renal Outcome in Patients with Proliferative Lupus Nephritis: A 36-Month Cohort Study. Rheumatology 2021, 60, 5134–5141. [Google Scholar] [CrossRef]
- Delfino, J.; dos Santos, T.A.F.G.; Skare, T.L. Comparison of Lupus Patients with Early and Late Onset Nephritis: A Study in 71 Patients from a Single Referral Center. Adv. Rheumatol. 2020, 60, 5. [Google Scholar] [CrossRef]
- Bobirca, A.; Bobira, F.; Florescui, A.; Iorgusi, C.; Tinta, R.; Musetescu, A.; Bojica, M.; Ancata, I. Evaluation of Treatment Response in Lupus Nephritis. Mod. Med. 2021, 28, 389–395. [Google Scholar] [CrossRef]
- Rossi, G.M.; Maggiore, U.; Peyronel, F.; Fenaroli, P.; Delsante, M.; Benigno, G.D.; Gianfreda, D.; Urban, M.L.; Manna, Z.; Arend, L.J.; et al. Persistent Isolated C3 Hypocomplementemia as a Strong Predictor of End-Stage Kidney Disease in Lupus Nephritis. Kidney Int. Rep. 2022, 7, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Hernández, D.; Cruz-Reyes, C.; Angeles, U.; Jara, L.J.; Saavedra, M.A. Prognostic Factors for Treatment Response in Patients with Lupus Nephritis. Reumatol. Clin. 2014, 10, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.; Stone, D.; Levesque, V.; Cisternas, M.; Wofsy, D. Identification of Biomarkers That Predict Response to Treatment of Lupus Nephritis with Mycophenolate Mofetil or Pulse Cyclophosphamide. Arthritis Care Res. 2011, 63, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.H.; Tang, C.S.O.; Ma, M.K.M.; Lam, M.F.; Chan, T.M. Survival Analysis and Causes of Mortality in Patients with Lupus Nephritis. Nephrol. Dial. Transplant. 2012, 27, 3248–3254. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Farinha, F.; Isenberg, D.A.; Rahman, A. Survival Analysis of Mortality and Development of Lupus Nephritis in Patients with Systemic Lupus Erythematosus up to 40 Years of Follow-Up. Rheumatology 2022, 62, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.L.; Phui, V.E.; Ling, G.R.; Ngu, L.-S.; Wan, S.A.; Tan, C.H.-H. Causes and Predictors of Mortality in Biopsy-Proven Lupus Nephritis: The Sarawak Experience. Clin. Kidney J. 2018, 11, 56–61. [Google Scholar] [CrossRef]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 825–835. [Google Scholar] [CrossRef]


| Characteristic | n = 401 1 |
|---|---|
| Age | 42 (19, 93) |
| Sex | |
| Female | 349 (87%) |
| Male | 52 (13%) |
| Histological Class | |
| I | 2 (0.5%) |
| II | 26 (6.5%) |
| III | 79 (20%) |
| IV | 282 (70%) |
| V | 12 (3.0%) |
| VI | 0 (0.0%) |
| Histological type | |
| Non-proliferative | 40 (10.0%) |
| Proliferative | 361 (90%) |
| Treatment Response | |
| CR | 114 (28%) |
| PR | 77 (19%) |
| NR | 210 (52%) |
| Immunosuppressant | |
| MMF + CTX | 26 (6.5%) |
| CTX | 53 (13%) |
| MMF | 281 (70%) |
| Survival | |
| Deceased | 61 (15%) |
| Survivor | 340 (85%) |
| Parameter | Female (n = 349) 1 | Male, (n = 52) 1 | p-Value | |
|---|---|---|---|---|
| Age | 41 (19, 93) | 43 (20, 75) | 0.71 2 | |
| Hemogram | Hb (g/dL) | 9.4 (5.2, 14.5) | 9.6 (5.2, 14.2) | 0.62 2 |
| Plt (103/μL) | 225 (76, 450) | 250 (76, 400) | 0.41 2 | |
| Leu (mm3 × 103) | 7.9 (2.3, 14.4) | 7.5 (2.5, 12.6) | 0.43 2 | |
| Renal Function | sCr (mg/dL) | 1.2 (0.5, 15) | 1.1 (0.5, 2.4) | 0.63 2 |
| eGFR (mL/min/m2) | 54 (6, 168) | 77 (30, 214) | <0.001 2 | |
| Urea (mg/dL) | 60 (14, 118) | 61 (16, 118) | 0.82 2 | |
| Proteinuria (g/day) | 1.3 (0.1, 13.5) | 1.7 (0.1, 9.1) | 0.004 2 | |
| Hematuria | 186 (53%) | 23 (44%) | 0.21 3 | |
| Immunological | Hypocomplementemia C3 | 257 (74%) | 35 (67%) | 0.32 3 |
| Hypocomplementemia C4 | 73 (21%) | 11 (21%) | >0.9 3 | |
| Anti ds DNA (% positive) | 122 (35%) | 16 (31%) | 0.61 3 | |
| Histopathology | Histological Type | 0.72 3 | ||
| Non-proliferative (I,II,V) | 34 (9.7%) | 6 (12%) | ||
| Proliferative (III,IV) | 315 (90%) | 46 (88%) | ||
| Activity Index | 6 (1, 15) | 5.5 (1, 13) | 0.54 2 | |
| Chronicity Index | 4 (0, 9) | 4.5 (0, 9) | 0.91 2 | |
| Outcome | Treatment Response | 0.03 3 | ||
| CR | 104 (30%) | 10 (19%) | ||
| NR | 174 (50%) | 36 (69%) | ||
| PR | 71 (20%) | 6 (12%) | ||
| Survival | 0.72 3 | |||
| Deceased | 52 (15%) | 9 (17%) | ||
| Survivor | 297 (85%) | 43 (83%) | ||
| Characteristic | Non-Proliferative (I-II-V) (n = 40) 1 | Proliferative (III-IV) (n = 361) 1 | p-Value | |
|---|---|---|---|---|
| Demographics | Age | 46 (27, 83) | 41 (19, 93) | 0.02 2 |
| Sex | 0.71 3 | |||
| Female | 34 (85%) | 315 (87%) | ||
| Male | 6 (15%) | 46 (13%) | ||
| Hemogram | Hemoglobin (g/dL) | 9.9 (5.4, 14.3) | 9.4 (5.2, 14.5) | 0.13 2 |
| Platelets (103/μL) | 241 (84, 443) | 228 (76, 450) | 0.42 2 | |
| Leu (mm3 × 103) | 7.8 (3.7, 14.2) | 8.0 (2.3, 14.4) | 0.93 2 | |
| Renal Function | Serum creatinine (mg/dL) | 1.1 (0.5, 4.5) | 1.2 (0.5, 12) | 0.41 2 |
| eGFR (mL/min/m2) | 63 (12, 198) | 55 (6, 214) | 0.62 2 | |
| Urea (mg/dL) | 58 (14, 118) | 61 (15, 118) | 0.41 2 | |
| Proteinuria (g/day) | 1.23 (0.14, 2.48) | 1.36 (0.1, 13.5) | 0.532 | |
| Hematuria | 19 (48%) | 190 (53%) | 0.52 3 | |
| Immunological | Hypocomplementemia C3 | 30 (75%) | 262 (73%) | 0.71 3 |
| Hypocomplementemia C4 | 7 (18%) | 77 (21%) | 0.62 3 | |
| Anti ds DNA (% positive) | 14 (35%) | 124 (34%) | 0.92 3 | |
| Histopathology | Activity Index | 5.0 (1.0, 13.0) | 6.0 (1.0, 15.0) | 0.31 2 |
| Chronicity Index | 5.00 (0.00, 9.00) | 4.00 (0.00, 9.00) | 0.14 2 | |
| Outcome | Treatment Response | 0.44 3 | ||
| Complete Response (CR) | 14 (35%) | 100 (28%) | ||
| No Response (NR) | 21 (53%) | 189 (52%) | ||
| Partial Response (PR) | 5 (13%) | 72 (20%) | ||
| Survival | 0.23 3 | |||
| Deceased | 3 (7.5%) | 58 (16%) | ||
| Survivor | 37 (93%) | 303 (84%) | ||
| Characteristic | First Control | Second Control | Third Control | p-Value | |
|---|---|---|---|---|---|
| Hemogram | Hb (g/dL) | 9 (4.9, 14.5) | 9.4 (5.2, 14.5) | 8.5 (5.2, 12) | <0.001 1 |
| Plt (103/μL) | 226 (328, 723) | 230 (76, 450) | 224 (76, 349) | 0.84 1 | |
| Leu (mm3 × 103) | 7.9 (5.0, 71) | 7.9 (2.3, 14.4) | 8.3 (2.5, 15.0) | 0.31 1 | |
| Renal Function | sCr (mg/dL) | 1.20 (0.40, 8.30) | 1.2 (0.5, 12) | 1.8 (0.5, 4.5) | <0.001 1 |
| eGFR (mL/min/m2) | 54 (6, 223) | 56 (6, 214) | 34 (12, 181) | <0.001 1 | |
| Urea (mg/dL) | 43 (10, 223) | 60 (14, 118) | 82 (14, 149) | <0.001 1 | |
| Proteinuria (g/day) | 1.42 (0.13, 12.10) | 1.35 (0.1, 13.5) | 1.1 (0.1, 7.5) | <0.001 1 | |
| Hematuria | 211 (53%) | 209 (52%) | 241 (61%) | <0.001 2 | |
| Immunological | Hipocomplementemia C3 | 250 (62%) | 292 (73%) | 310 (77%) | 0.19 2 |
| Hipocomplementemia C4 | 81 (20%) | 84 (21%) | 110 (27%) | 0.63 2 | |
| Anti ds DNA (% positive) | 138 (34%) | 149 (37%) | 126 (32%) | 0.23 2 | |
| Characteristic | Deceased (n= 61) 1 | Survivor (n = 340) 1 | p-Value | |
|---|---|---|---|---|
| Demographical | Age | 44 (24, 69) | 41 (19, 93) | 0.08 2 |
| Sex | 0.71 3 | |||
| Female | 52 (85%) | 297 (87%) | ||
| Male | 9 (15%) | 43 (13%) | ||
| Hemogram | Hb (g/dL) | 9.4 (5.2, 13.3) | 9.45 (5.2, 14.5) | >0.9 2 |
| Plt (103/μL) | 259 (91, 443) | 222 (76, 450) | 0.01 2 | |
| Leu (mm3 × 103) | 8.09 (3.1,14.3) | 7.9 (2.3, 14.4) | >0.9 2 | |
| Renal Function | sCrt (mg/dL) | 1.40 (0.50, 8.10) | 1.20 (0.50, 12) | 0.01 2 |
| eGFR (mL/min/m2) | 48 (6, 151) | 58 (8, 214) | 0.01 2 | |
| Urea (mg/dL) | 62 (14, 118) | 60 (15, 118) | 0.82 2 | |
| Proteinuria (g/day) | 1.06 (0.1, 9.1) | 1.36 (0.1, 13.5) | 0.23 2 | |
| Hematuria | 34 (56%) | 175 (51%) | 0.51 3 | |
| Immunological | C3 hypocomplementemia | 48 (79%) | 244 (72%) | 0.34 3 |
| C4 hypocomplementemia | 17 (28%) | 67 (20%) | 0.15 3 | |
| Anti ds DNA (% positive) | 42 (69%) | 173 (51%) | 0.01 3 | |
| Histopathological | Histological Type | 0.22 3 | ||
| Non-proliferative | 3 (4.9%) | 37 (11%) | ||
| Proliferative | 58 (95%) | 303 (89%) | ||
| Activity Index | 7 (1, 15) | 6 (1, 14) | >0.9 2 | |
| Chronicity Index | 5 (0, 9) | 4 (0, 9) | 0.61 2 | |
| Outcome | Response to Treatment | 0.12 3 | ||
| CR | 24 (39%) | 90 (26%) | ||
| NR | 27 (44%) | 183 (54%) | ||
| PR | 10 (16%) | 67 (20%) | ||
| Variable | Multivariate | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR 1 | 95% CI 2 | p-Value | OR 1 | 95% CI 2 | p-Value | |
| Age > 30 | ||||||
| Yes | 0.73 | 0.40, 1.27 | 0.31 | 0.76 | 0.43, 1.32 | 0.31 |
| Sex | ||||||
| Female | — | — | — | — | ||
| Male | 2.14 | 0.96, 5.36 | 0.07 | 1.92 | 1.62, 4.59 | 0.04 |
| Hb < 10 g/dL | ||||||
| No | — | — | — | — | ||
| Yes | 0.68 | 0.39, 1.17 | 0.22 | 0.65 | 0.38, 1.10 | 0.11 |
| Plt > 150 103/μL | ||||||
| Yes | 0.75 | 0.39, 1.42 | 0.41 | |||
| PRT > 2 g/day | ||||||
| Yes | 28.4 | 16.4, 57.9 | <0.001 | 27.3 | 15.9, 54.1 | <0.001 |
| sCRT > 1.5 mg/dL | ||||||
| No | — | — | ||||
| Yes | 0.96 | 0.46, 1.98 | >0.9 | |||
| eGFR < 60 mL/min/m2 | ||||||
| Yes | 1.45 | 0.72, 3.15 | 0.32 | |||
| Hematuria | ||||||
| Yes | 0.96 | 0.56, 1.64 | 0.91 | |||
| Hypocomplementemia C3 | ||||||
| Yes | 1.51 | 1.17, 3.91 | 0.03 | 1.8 | 1.27, 3.89 | 0.02 |
| Hypocomplementemia C4 | ||||||
| Yes | 0.68 | 0.34, 1.30 | 0.23 | |||
| Anti ds DNA Positive | ||||||
| Yes | 1.46 | 0.70, 3.78 | 0.71 | |||
| Histologic type | ||||||
| Non-proliferative | — | — | ||||
| Proliferative | 0.75 | 0.31, 1.77 | 0.52 | |||
| Activity Index > 7 | ||||||
| Yes | 1.03 | 0.60, 1.77 | >0.9 | |||
| Immunosuppressor Treatment | ||||||
| CTX | — | — | ||||
| MMF | 1.25 | 0.56, 2.97 | 0.64 | |||
| Parameter | Multivariate | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR 1 | 95% CI 2 | p-Value | OR 1 | 95% CI 2 | p-Value | |
| Age > 35 | ||||||
| Yes | 1.55 | 0.81, 3.25 | 0.2 | 1.62 | 0.87, 3.34 | 0.14 |
| Sex | ||||||
| Females | — | — | ||||
| Male | 1.31 | 0.48, 3.43 | 0.6 | |||
| Plt > 150 μL | ||||||
| Yes | 0.36 | 0.12, 0.81 | 0.024 | 0.34 | 0.12, 0.75 | 0.015 |
| PRT > 1 g/day | ||||||
| Yes | 1.41 | 0.61, 3.74 | 0.4 | |||
| sCRT > 1.5 mg/dL | ||||||
| Yes | 1.61 | 0.75, 3.75 | 0.2 | 2.08 | 1.16, 3.93 | 0.016 |
| eGFR < 60 mL/min/m2 | ||||||
| Yes | 1.46 | 0.59, 3.60 | 0.4 | |||
| Hematuria | ||||||
| Yes | 1.02 | 0.54, 1.94 | >0.9 | |||
| Hypocomplementemia C3 | ||||||
| Yes | 1.07 | 0.52, 2.29 | 0.9 | 1.17 | 0.60, 2.47 | 0.6 |
| Hypocomplementemia C4 | ||||||
| Yes | 1.69 | 0.81, 3.54 | 0.15 | |||
| Anti ds DNA Positive | ||||||
| Yes | 2.6 | 0.45, 8.75 | 0.07 | |||
| Histological Type | ||||||
| Non-proliferative | — | — | — | — | ||
| Proliferative | 1.65 | 0.55, 7.91 | 0.4 | 1.82 | 1.63, 8.68 | 0.03 |
| Activity Index > 6 | ||||||
| Yes | 1.36 | 0.73, 2.61 | 0.3 | |||
| Response to Treatment | ||||||
| No | — | — | ||||
| Yes | 1.71 | 0.72, 4.60 | 0.2 | |||
| Immunosuppressor | ||||||
| CTX | — | — | — | — | ||
| MMF | 1.99 | 0.82, 6.59 | 0.2 | 2.05 | 0.86, 6.65 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Verbel, M.; Aroca-Martínez, G.; Vélez-Verbel, D.; Domínguez-Vargas, A.; Vallejo-Patiño, M.; Sarmiento-Gutierrez, J.; Gomez-Escorcia, L.; Musso, C.G.; González-Torres, H.J. Clinical and Immunological Factors Associated with the Progression of Lupus Nephritis in a Population from the Colombian Caribbean. Biomedicines 2024, 12, 2047. https://doi.org/10.3390/biomedicines12092047
Vélez-Verbel M, Aroca-Martínez G, Vélez-Verbel D, Domínguez-Vargas A, Vallejo-Patiño M, Sarmiento-Gutierrez J, Gomez-Escorcia L, Musso CG, González-Torres HJ. Clinical and Immunological Factors Associated with the Progression of Lupus Nephritis in a Population from the Colombian Caribbean. Biomedicines. 2024; 12(9):2047. https://doi.org/10.3390/biomedicines12092047
Chicago/Turabian StyleVélez-Verbel, María, Gustavo Aroca-Martínez, David Vélez-Verbel, Alex Domínguez-Vargas, Manuela Vallejo-Patiño, Joanny Sarmiento-Gutierrez, Lorena Gomez-Escorcia, Carlos G. Musso, and Henry J. González-Torres. 2024. "Clinical and Immunological Factors Associated with the Progression of Lupus Nephritis in a Population from the Colombian Caribbean" Biomedicines 12, no. 9: 2047. https://doi.org/10.3390/biomedicines12092047
APA StyleVélez-Verbel, M., Aroca-Martínez, G., Vélez-Verbel, D., Domínguez-Vargas, A., Vallejo-Patiño, M., Sarmiento-Gutierrez, J., Gomez-Escorcia, L., Musso, C. G., & González-Torres, H. J. (2024). Clinical and Immunological Factors Associated with the Progression of Lupus Nephritis in a Population from the Colombian Caribbean. Biomedicines, 12(9), 2047. https://doi.org/10.3390/biomedicines12092047






