Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring?
Abstract
1. Introduction
2. DOHaD Concept and Effects of Developmental Exposure to Endocrine-Disrupting Chemicals on Neurobehavioral Programming
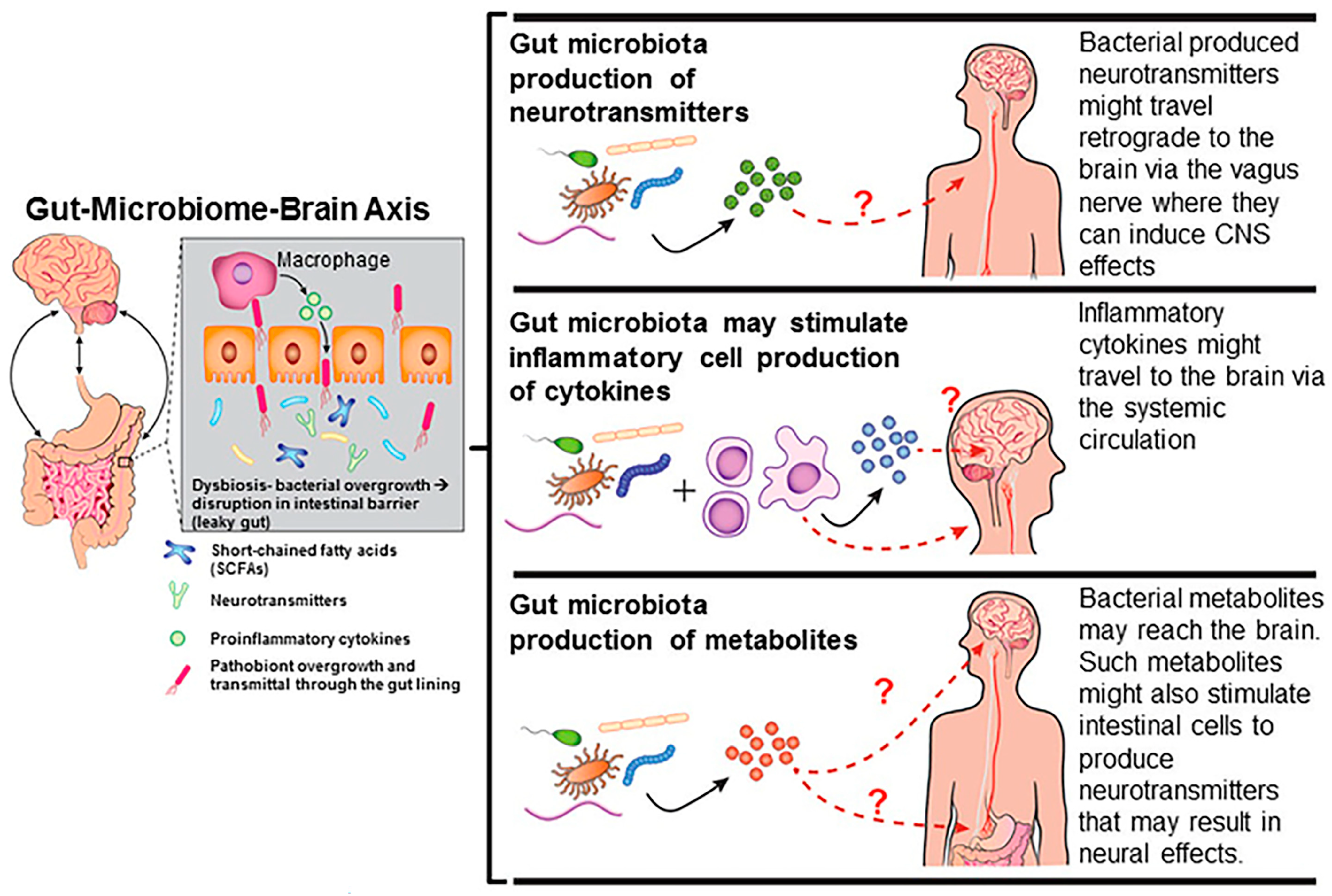
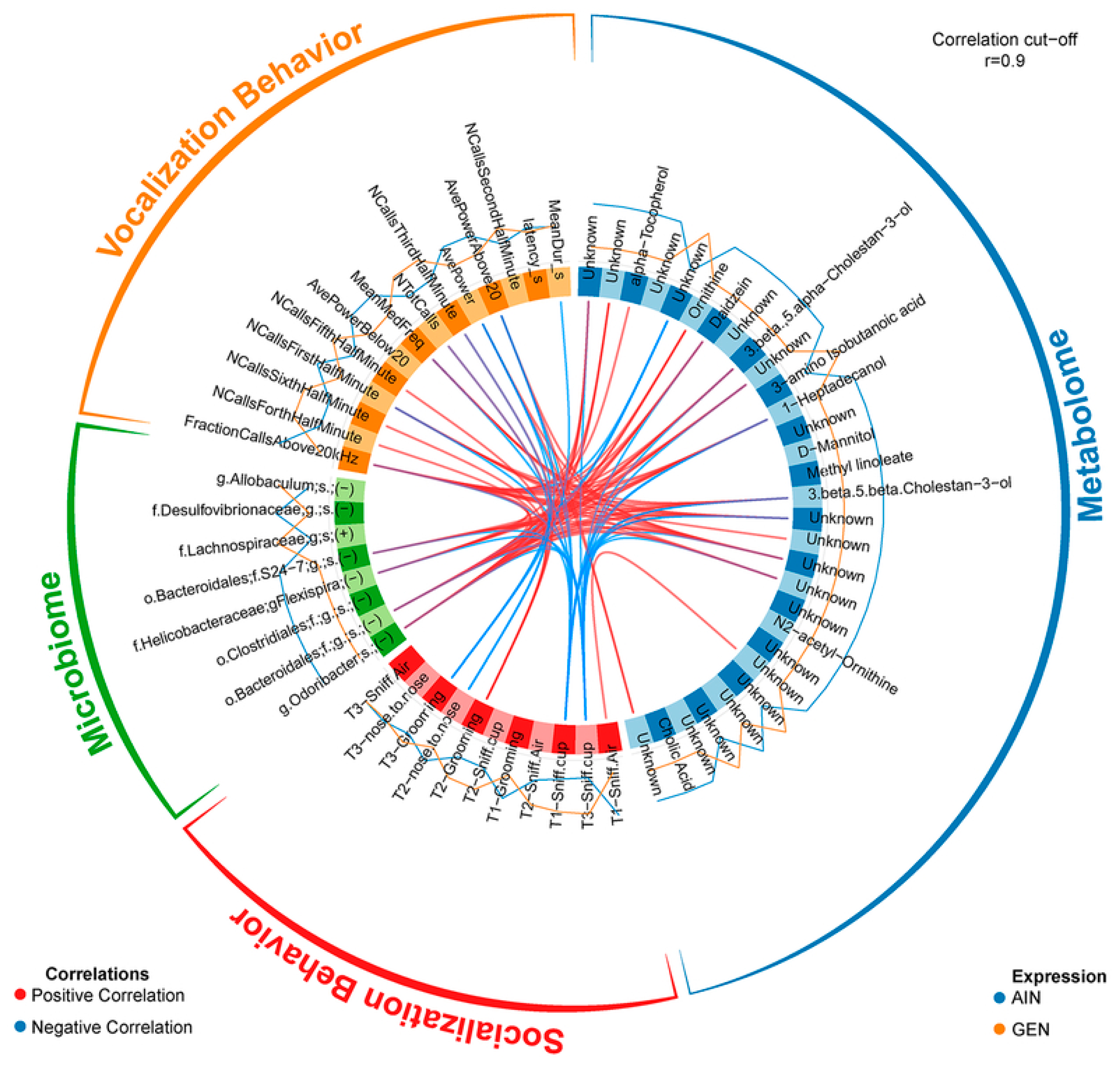
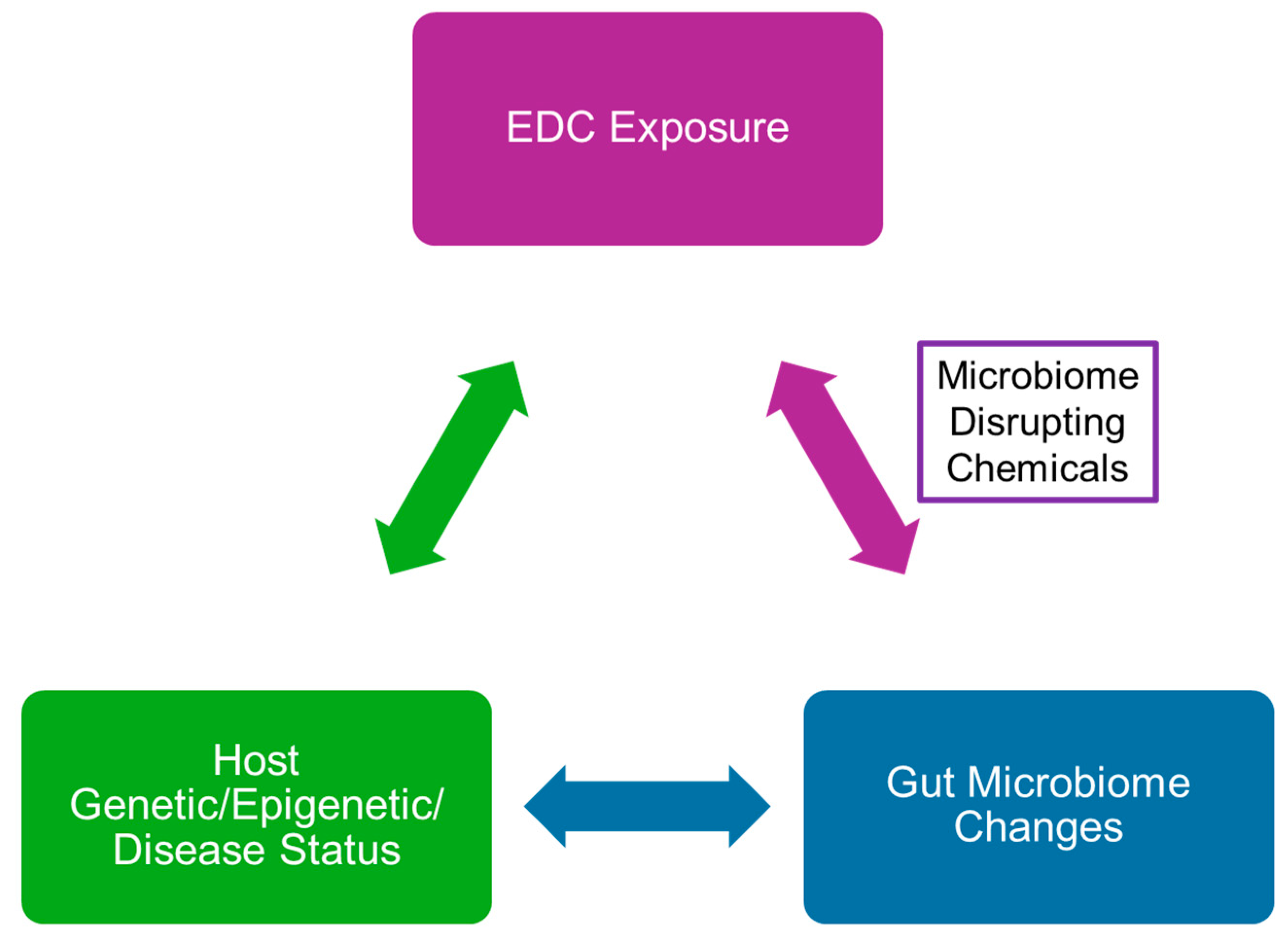
3. Effects of Endocrine-Disrupting Chemicals on the Gut-Microbiome–Brain Axis
4. Effects of Probiotics on Health of Pregnant Women
5. Effects of Maternal Probiotics on Offspring Health
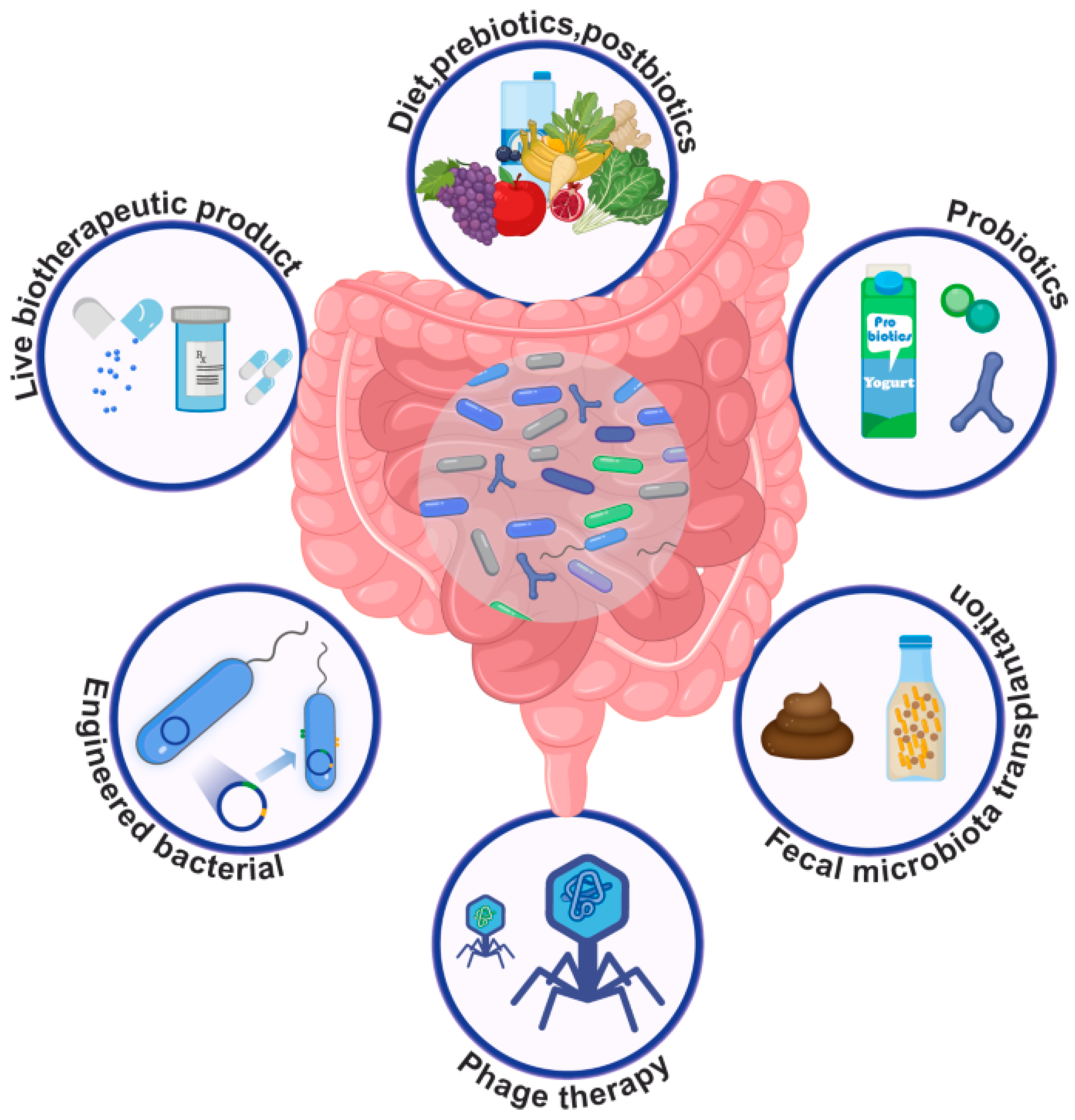
6. Usage of Probiotics to Neutralize Effects of Endocrine-Disrupting Chemicals
7. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Muckle, G.; Arbuckle, T.; Bouchard, M.F.; Fraser, W.D.; Ouellet, E.; Séguin, J.R.; Oulhote, Y.; Webster, G.M.; Lanphear, B.P. Associations of prenatal urinary bisphenol A concentrations with child behaviors and cognitive abilities. Environ. Health Perspect. 2017, 125, 067008. [Google Scholar] [CrossRef]
- Evans, S.F.; Kobrosly, R.W.; Barrett, E.S.; Thurston, S.W.; Calafat, A.M.; Weiss, B.; Stahlhut, R.; Yolton, K.; Swan, S.H. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology 2014, 45, 91–99. [Google Scholar] [CrossRef]
- Harley, K.G.; Gunier, R.B.; Kogut, K.; Johnson, C.; Bradman, A.; Calafat, A.M.; Eskenazi, B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013, 126, 43–50. [Google Scholar] [CrossRef]
- Perera, F.; Nolte, E.L.R.; Wang, Y.; Margolis, A.E.; Calafat, A.M.; Wang, S.; Garcia, W.; Hoepner, L.A.; Peterson, B.S.; Rauh, V.; et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016, 151, 195–202. [Google Scholar] [CrossRef]
- Stacy, S.L.; Papandonatos, G.D.; Calafat, A.M.; Chen, A.; Yolton, K.; Lanphear, B.P.; Braun, J.M. Early life bisphenol A exposure and neurobehavior at 8years of age: Identifying windows of heightened vulnerability. Environ. Int. 2017, 107, 258–265. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Antoniou, M.; Belcher, S.M.; Bergman, A.; Bhandari, R.K.; Birnbaum, L.S.; Cohen, A.; Collins, T.J.; Demeneix, B.; Fine, A.M.; et al. The conflict between regulatory agencies over the 20,000-fold lowering of the tolerable daily intake (TDI) for bisphenol A (BPA) by the European Food Safety Authority (EFSA). Environ. Health Perspect. 2024, 132, 45001. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Environment Canada. Screening Assessment for the Challenge Phenol, 4,4′-(1-methylethylidene)bis-(Bisphenol A) Chemical Abstracts Service Registry Number 80-05-7; Environment Canada: Quebec, CA, Canada, 2008; pp. 1–107. [Google Scholar]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M.; Birnbaum, L.S.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J., Jr.; Hauser, R.; Heindel, J.J.; et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Schonfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol A accumulation in human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef]
- Javurek, A.B.; Spollen, W.G.; Johnson, S.A.; Bivens, N.J.; Bromert, K.H.; Givan, S.A.; Rosenfeld, C.S. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 2016, 7, 471–485. [Google Scholar] [CrossRef]
- Kaur, S.; Sarma, S.J.; Marshall, B.L.; Liu, Y.; Kinkade, J.A.; Bellamy, M.M.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; et al. Developmental exposure of California mice to endocrine disrupting chemicals and potential effects on the microbiome-gut-brain axis at adulthood. Sci. Rep. 2020, 10, 10902. [Google Scholar] [CrossRef]
- Koestel, Z.L.; Backus, R.C.; Tsuruta, K.; Spollen, W.G.; Johnson, S.A.; Javurek, A.B.; Ellersieck, M.R.; Wiedmeyer, C.E.; Kannan, K.; Xue, J.; et al. Bisphenol A (BPA) in the serum of pet dogs following short-term consumption of canned dog food and potential health consequences of exposure to BPA. Sci. Total Environ. 2016, 579, 1804–1814. [Google Scholar] [CrossRef]
- Marshall, B.L.; Liu, Y.; Farrington, M.J.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; Sarma, S.J.; Lei, Z.; Sumner, L.W.; et al. Early genistein exposure of California mice and effects on the gut microbiota-brain axis. J. Endocrinol. 2019, 242, 139–157. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Xenoestrogen effects on the gut microbiome. Curr. Opin. Endocr. Metab. Res. 2021, 19, 41–45. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Gut dysbiosis in animals due to environmental chemical exposures. Front. Cell Infect. Microbiol. 2017, 7, 396. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Effects of phytoestrogens on the developing brain, gut microbiota, and risk for neurobehavioral disorders. Front. Nutr. 2019, 6, 142. [Google Scholar] [CrossRef]
- Oesterle, I.; Ayeni, K.I.; Ezekiel, C.N.; Berry, D.; Rompel, A.; Warth, B. Insights into the early-life chemical exposome of Nigerian infants and potential correlations with the developing gut microbiome. Environ. Int. 2024, 188, 108766. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Riadi, Y.; Afzal, M.; Bansal, P.; Kaur, H.; Deorari, M.; Tonk, R.K.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; et al. The hidden threat: Environmental toxins and their effects on gut microbiota. Pathol. Res. Pract. 2024, 255, 155173. [Google Scholar] [CrossRef]
- Charitos, I.A.; Topi, S.; Gagliano-Candela, R.; De Nitto, E.; Polimeno, L.; Montagnani, M.; Santacroce, L. The Toxic effects of endocrine disrupting chemicals (EDCs) on gut microbiota: Bisphenol A (BPA) a review. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 716–727. [Google Scholar] [CrossRef]
- Chi, Y.; Zhu, L.; Wang, Y.; Peng, C.; Lin, Y.; Ji, S.; Wei, J. Long-term Bisphenol S exposure induced gut microbiota dysbiosis, obesity, hepatic lipid accumulation, intestinal lesions and dyslipidemia in mice. Toxicology 2024, 504, 153798. [Google Scholar] [CrossRef]
- Chiu, K.; Bashir, S.T.; Gao, L.; Gutierrez, J.; de Godoy, M.R.C.; Drnevich, J.; Fields, C.J.; Cann, I.; Flaws, J.A.; Nowak, R.A. Subacute exposure to an environmentally relevant dose of di-(2-ethylhexyl) phthalate during gestation alters the cecal microbiome, but not pregnancy outcomes in mice. Toxics 2021, 9, 215. [Google Scholar] [CrossRef]
- Fabozzi, G.; Rebuzzini, P.; Cimadomo, D.; Allori, M.; Franzago, M.; Stuppia, L.; Garagna, S.; Ubaldi, F.M.; Zuccotti, M.; Rienzi, L. Endocrine-disrupting chemicals, gut microbiota, and human (in)fertility-it is time to consider the triad. Cells 2022, 11, 3335. [Google Scholar] [CrossRef]
- Giommi, C.; Lombó, M.; Habibi, H.R.; Rossi, G.; Basili, D.; Mangiaterra, S.; Ladisa, C.; Chemello, G.; Carnevali, O.; Maradonna, F. The probiotic SLAB51 as agent to counteract BPA toxicity on zebrafish gut microbiota -liver-brain axis. Sci. Total Environ. 2024, 912, 169303. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Ampatzoglou, A.; Aguilera-Gómez, M. Microbiota analysis for risk assessment of xenobiotics: Cumulative xenobiotic exposure and impact on human gut microbiota under One Health approach. EFSA J. 2022, 20, e200916. [Google Scholar] [CrossRef]
- Hou, X.; Zhu, L.; Zhang, X.; Zhang, L.; Bao, H.; Tang, M.; Wei, R.; Wang, R. Testosterone disruptor effect and gut microbiome perturbation in mice: Early life exposure to doxycycline. Chemosphere 2019, 222, 722–731. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, Z.; Wu, Y.; Zhang, S.; Fu, Z. Oral exposure of mice to carbendazim induces hepatic lipid metabolism disorder and gut microbiota d. Toxicol. Sci. 2015, 147, 116–126. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Gao, J.; Li, X. Effects of octylphenol exposure on the lipid metabolism and microbiome of the intestinal tract of Rana chensinensis tadpole by RNAseq and 16s amplicon sequencing. Ecotoxicol. Environ. Saf. 2020, 197, 110650. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Li, H.; Qiao, F.; Wu, J.; Du, Z.Y.; Zhang, M. Influence of endogenous and exogenous estrogenic endocrine on intestinal microbiota in zebrafish. PLoS ONE 2016, 11, e0163895. [Google Scholar] [CrossRef]
- Mousavi, S.E.; Delgado-Saborit, J.M.; Adivi, A.; Pauwels, S.; Godderis, L. Air pollution and endocrine disruptors induce human microbiome imbalances: A systematic review of recent evidence and possible biological mechanisms. Sci. Total Environ. 2022, 816, 151654. [Google Scholar] [CrossRef]
- Pu, C.; Liu, Y.; Ma, J.; Hou, L.; Cheng, Y.; Zhang, B.; Wang, B.; Wang, A.; Zhang, C. Bisphenol S exposed changes in intestinal microflora and metabolomics of freshwater crayfish, Procambarus clarkii. Aquat. Toxicol. 2024, 272, 106957. [Google Scholar] [CrossRef]
- Ramírez, V.; González-Palacios, P.; Baca, M.A.; González-Domenech, P.J.; Fernández-Cabezas, M.; Álvarez-Cubero, M.J.; Rodrigo, L.; Rivas, A. Effect of exposure to endocrine disrupting chemicals in obesity and neurodevelopment: The genetic and microbiota link. Sci. Total Environ. 2022, 852, 158219. [Google Scholar] [CrossRef]
- Hampl, R.; Stárka, L. Endocrine disruptors and gut microbiome interactions. Physiol. Res. 2020, 69, S211–s223. [Google Scholar] [CrossRef]
- Li, N.; Li, J.; Zhang, Q.; Gao, S.; Quan, X.; Liu, P.; Xu, C. Effects of endocrine disrupting chemicals in host health: Three-way interactions between environmental exposure, host phenotypic responses, and gut microbiota. Environ. Pollut. 2021, 271, 116387. [Google Scholar] [CrossRef]
- Vacca, M.; Calabrese, F.M.; Loperfido, F.; Maccarini, B.; Cerbo, R.M.; Sommella, E.; Salviati, E.; Voto, L.; De Angelis, M.; Ceccarelli, G.; et al. Maternal exposure to endocrine-disrupting chemicals: Analysis of their impact on infant gut microbiota composition. Biomedicines 2024, 12, 234. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, N.; Wang, J.; Gao, Y.; Wu, W.; Jiang, M.; Han, M. Maternal Di-(2-ethylhexyl)-Phthalate exposure during pregnancy altered energy metabolism in immature offspring and caused hyperglycemia. Ecotoxicol. Environ. Saf. 2024, 279, 116494. [Google Scholar] [CrossRef]
- Buerger, A.N.; Dillon, D.T.; Schmidt, J.; Yang, T.; Zubcevic, J.; Martyniuk, C.J.; Bisesi, J.H., Jr. Gastrointestinal dysbiosis following diethylhexyl phthalate exposure in zebrafish (Danio rerio): Altered microbial diversity, functionality, and network connectivity. Environ. Pollut. 2020, 265, 114496. [Google Scholar] [CrossRef]
- Adamovsky, O.; Buerger, A.N.; Vespalcova, H.; Sohag, S.R.; Hanlon, A.T.; Ginn, P.E.; Craft, S.L.; Smatana, S.; Budinska, E.; Persico, M.; et al. Evaluation of microbiome-host relationships in the zebrafish gastrointestinal system reveals adaptive immunity is a target of bis(2-ethylhexyl) phthalate (DEHP) exposure. Environ. Sci. Technol. 2020, 54, 5719–5728. [Google Scholar] [CrossRef]
- Castanys-Muñoz, E.; Martin, M.J.; Vazquez, E. Building a beneficial microbiome from birth. Adv. Nutr. 2016, 7, 323–330. [Google Scholar] [CrossRef]
- Hu, T.; Dong, Y.; Yang, C.; Zhao, M.; He, Q. Pathogenesis of children’s allergic diseases: Refocusing the role of the gut microbiota. Front. Physiol. 2021, 12, 749544. [Google Scholar] [CrossRef]
- Maffei, S.; Forini, F.; Canale, P.; Nicolini, G.; Guiducci, L. Gut microbiota and sex hormones: Crosstalking players in cardiometabolic and cardiovascular disease. Int. J. Mol. Sci. 2022, 23, 7154. [Google Scholar] [CrossRef]
- Moya-Alvarez, V.; Sansonetti, P.J. Understanding the pathways leading to gut dysbiosis and enteric environmental dysfunction in infants: The influence of maternal dysbiosis and other microbiota determinants during early life. FEMS Microbiol. Rev. 2022, 46, fuac004. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Microbiome disturbances and autism spectrum disorders. Drug Metabol. Dispos. 2015, 43, 1557–1571. [Google Scholar] [CrossRef]
- Babu, A.; Devi Rajeswari, V.; Ganesh, V.; Das, S.; Dhanasekaran, S.; Usha Rani, G.; Ramanathan, G. Gut microbiome and polycystic ovary syndrome: Interplay of associated microbial-metabolite pathways and therapeutic strategies. Reprod. Sci. 2024, 31, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Fu, X.; Zhou, J.; Qi, Q.; Ye, F.; Li, L.; Wang, L. The effect of the female genital tract and gut microbiome on reproductive dysfunction. Biosci. Trends 2024, 17, 458–474. [Google Scholar] [CrossRef] [PubMed]
- Cocomazzi, G.; Del Pup, L.; Contu, V.; Maggio, G.; Parmegiani, L.; Ciampaglia, W.; De Ruvo, D.; Faioli, R.; Maglione, A.; Baldini, G.M.; et al. Gynecological cancers and microbiota dynamics: Insights into pathogenesis and therapy. Int. J. Mol. Sci. 2024, 25, 2237. [Google Scholar] [CrossRef]
- Deady, C.; McCarthy, F.P.; Barron, A.; McCarthy, C.M.; O’Keeffe, G.W.; O’Mahony, S.M. An altered gut microbiome in pre-eclampsia: Cause or consequence. Front. Cell Infect. Microbiol. 2024, 14, 1352267. [Google Scholar] [CrossRef]
- Koren, O.; Konnikova, L.; Brodin, P.; Mysorekar, I.U.; Collado, M.C. The maternal gut microbiome in pregnancy: Implications for the developing immune system. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 35–45. [Google Scholar] [CrossRef]
- Mahoney, D. The Role of the Human microbiome in epithelial ovarian cancer. Adv. Exp. Med. Biol. 2024, 1452, 97–105. [Google Scholar] [CrossRef]
- Ruiz-Triviño, J.; Álvarez, D.; Cadavid, J.Á.; Alvarez, A.M. From gut to placenta: Understanding how the maternal microbiome models life-long conditions. Front. Endocrinol. 2023, 14, 1304727. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, Z.; Yang, H. Interactions between host and gut microbiota in gestational diabetes mellitus and their impacts on offspring. BMC Microbiol. 2024, 24, 161. [Google Scholar] [CrossRef] [PubMed]
- Barker, D. Commentary: Birthweight and coronary heart disease in a historical cohort. Int. J. Epidemiol. 2006, 35, 886–887. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Gillman, M.W.; Barker, D.; Bier, D.; Cagampang, F.; Challis, J.; Fall, C.; Godfrey, K.; Gluckman, P.; Hanson, M.; Kuh, D.; et al. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatr. Res. 2007, 61, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Periconceptional influences on offspring sex ratio and placental responses. Reprod. Fertil. Dev. 2011, 24, 45–58. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Effects of maternal diet and exposure to bisphenol A on sexually dimorphic responses in conceptuses and offspring. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 23–30. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Homage to the ‘H’ in developmental origins of health and disease. J. Dev. Orig. Health Dis. 2017, 8, 8–29. [Google Scholar] [CrossRef]
- Grandjean, P.; Barouki, R.; Bellinger, D.C.; Casteleyn, L.; Chadwick, L.H.; Cordier, S.; Etzel, R.A.; Gray, K.A.; Ha, E.H.; Junien, C.; et al. Life-long implications of developmental exposure to environmental stressors: New perspectives. Endocrinology 2015, 156, 3408–3415. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; Vom Saal, F.S.; Rosenfeld, C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17alpha-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2014, 214, 195–219. [Google Scholar] [CrossRef]
- Heindel, J.J.; Belcher, S.; Flaws, J.A.; Prins, G.S.; Ho, S.M.; Mao, J.; Patisaul, H.B.; Ricke, W.; Rosenfeld, C.S.; Soto, A.M.; et al. Data integration, analysis, and interpretation of eight academic CLARITY-BPA studies. Reprod. Toxicol. 2020, 98, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Jasarevic, E.; Sieli, P.T.; Twellman, E.E.; Welsh, T.H., Jr.; Schachtman, T.R.; Roberts, R.M.; Geary, D.C.; Rosenfeld, C.S. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. USA 2011, 108, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Jasarevic, E.; Williams, S.A.; Vandas, G.M.; Ellersieck, M.R.; Liao, C.; Kannan, K.; Roberts, R.M.; Geary, D.C.; Rosenfeld, C.S. Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm. Behav. 2013, 63, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Farrington, M.J.; Murphy, C.R.; Caldo, P.D.; McAllister, L.A.; Kaur, S.; Chun, C.; Ortega, M.T.; Marshall, B.L.; Hoffmann, F.; et al. Multigenerational effects of bisphenol A or ethinyl estradiol exposure on F2 California mice (Peromyscus californicus) pup vocalizations. PLoS ONE 2018, 13, e0199107. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Painter, M.S.; Javurek, A.B.; Ellersieck, M.R.; Wiedmeyer, C.E.; Thyfault, J.P.; Rosenfeld, C.S. Sex-dependent effects of developmental exposure to bisphenol A and ethinyl estradiol on metabolic parameters and voluntary physical activity. J. Dev. Orig. Health Dis. 2015, 6, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Manshack, L.K.; Conard, C.M.; Johnson, S.A.; Alex, J.M.; Bryan, S.J.; Deem, S.L.; Holliday, D.K.; Ellersieck, M.R.; Rosenfeld, C.S. Effects of developmental exposure to bisphenol A and ethinyl estradiol on spatial navigational learning and memory in painted turtles (Chrysemys picta). Horm. Behav. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 2017, 47, 123–133. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Denslow, N.D.; Orlando, E.F.; Gutierrez-Villagomez, J.M.; Trudeau, V.L. Neuroendocrine disruption of organizational and activational hormone programming in poikilothermic vertebrates. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 276–304. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Trainor, B.C. Environmental Health Factors and Sexually Dimorphic Differences in Behavioral Disruptions. Curr. Environ. Health Rep. 2014, 1, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Jasarevic, E.; Vandas, G.M.; Warzak, D.A.; Geary, D.C.; Ellersieck, M.R.; Roberts, R.M.; Rosenfeld, C.S. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): A monogamous animal model. PLoS ONE 2013, 8, e55698. [Google Scholar] [CrossRef]
- Hansen, J.B.; Bilenberg, N.; Timmermann, C.A.G.; Jensen, R.C.; Frederiksen, H.; Andersson, A.M.; Kyhl, H.B.; Jensen, T.K. Prenatal exposure to bisphenol A and autistic- and ADHD-related symptoms in children aged 2 and5 years from the Odense Child Cohort. Environ. Health 2021, 20, 24. [Google Scholar] [CrossRef]
- Kanlayaprasit, S.; Saeliw, T.; Thongkorn, S.; Panjabud, P.; Kasitipradit, K.; Lertpeerapan, P.; Songsritaya, K.; Yuwattana, W.; Jantheang, T.; Jindatip, D.; et al. Sex-specific impacts of prenatal bisphenol A exposure on genes associated with cortical development, social behaviors, and autism in the offspring’s prefrontal cortex. Biol. Sex. Differ. 2024, 15, 40. [Google Scholar] [CrossRef]
- Stein, T.P.; Schluter, M.D.; Steer, R.A.; Ming, X. Bisphenol-A and phthalate metabolism in children with neurodevelopmental disorders. PLoS ONE 2023, 18, e0289841. [Google Scholar] [CrossRef]
- Thongkorn, S.; Kanlayaprasit, S.; Panjabud, P.; Saeliw, T.; Jantheang, T.; Kasitipradit, K.; Sarobol, S.; Jindatip, D.; Hu, V.W.; Tencomnao, T.; et al. Sex differences in the effects of prenatal bisphenol A exposure on autism-related genes and their relationships with the hippocampus functions. Sci. Rep. 2021, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Geary, D.C.; Rosenfeld, C.S. Sexually selected traits: A fundamental framework for studies on behavioral epigenetics. ILAR J. 2012, 53, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.S.; Chang, E.B. The microbiome: Composition and locations. Prog. Mol. Biol. Transl. Sci. 2020, 176, 1–42. [Google Scholar] [CrossRef]
- Martino, C.; Dilmore, A.H.; Burcham, Z.M.; Metcalf, J.L.; Jeste, D.; Knight, R. Microbiota succession throughout life from the cradle to the grave. Nat. Rev. Microbiol. 2022, 20, 707–720. [Google Scholar] [CrossRef]
- Nuriel-Ohayon, M.; Neuman, H.; Koren, O. Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 2016, 7, 1031. [Google Scholar] [CrossRef]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral. Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Manos, J. The human microbiome in disease and pathology. Apmis 2022, 130, 690–705. [Google Scholar] [CrossRef]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef]
- Weinstock, G.M. Genomic approaches to studying the human microbiota. Nature 2012, 489, 250–256. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, K.A.; Kang, N.; Bienenstock, J.; Foster, J.A. Effects of intestinal microbiota on anxiety-like behavior. Commun. Integr. Biol. 2011, 4, 492–494. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; Macqueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19, pyw020. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Napolioni, V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol Teratol. 2013, 36, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Claud, E.C. Gut microbiome-brain axis as an explanation for the risk of poor neurodevelopment outcome in preterm infants with necrotizing enterocolitis. Microorganisms 2023, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Schmidt, R.R.; Martin, R.E.; Green, M.T.; Kinkade, J.A.; Mao, J.; Bivens, N.J.; Joshi, T.; Rosenfeld, C.S. Long-term effects of developmental exposure to oxycodone on gut microbiota and relationship to adult behaviors and metabolism. mSystems 2022, 7, e0033622. [Google Scholar] [CrossRef] [PubMed]
- Monday, L.; Tillotson, G.; Chopra, T. Microbiota-based live biotherapeutic products for Clostridioides difficile infection- the devil is in the details. Infect. Drug Resist. 2024, 17, 623–639. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Kuang, L.; Jiang, Y. Effect of probiotic supplementation in pregnant women: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2020, 123, 870–880. [Google Scholar] [CrossRef]
- Łagowska, K.; Malinowska, A.M.; Zawieja, B.; Zawieja, E. Improvement of glucose metabolism in pregnant women through probiotic supplementation depends on gestational diabetes status: Meta-analysis. Sci. Rep. 2020, 10, 17796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Xiao, C.L.; Ren, H.; Li, W.R.; Guo, Z.; Luo, J.Q. Comparison of the effectiveness of probiotic supplementation in glucose metabolism, lipid profile, inflammation and oxidative stress in pregnant women. Food Funct. 2024, 15, 3479–3495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, X.; Wang, Y.; He, C.; Yu, J.; Fang, X.; Zhang, Y.; Xu, X.; Yang, J. Effects of probiotic supplementation on glucose metabolism in pregnant women without diabetes: A systematic review and meta-analysis. Food Funct. 2022, 13, 8388–8398. [Google Scholar] [CrossRef] [PubMed]
- Al-Dughaishi, T.; Nikolic, D.; Zadjali, F.; Al-Hashmi, K.; Al-Waili, K.; Rizzo, M.; Al-Rasadi, K. Nutraceuticals as lipid-lowering treatment in pregnancy and their effects on the metabolic syndrome. Curr. Pharm. Biotechnol. 2016, 17, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Wong, M.M.H.; Pang, S.S.H.; Lo, K.K.H. Dietary supplementation for gestational diabetes prevention and management: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2021, 303, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Machairiotis, N.; Vasilakaki, S.; Minns, L.; Malakasis, A. Nutrients that modulate gestational diabetes mellitus: A systematic review of cohort studies Jan 2019–Jan 2020. Int. J. Clin. Pract. 2021, 75, e14033. [Google Scholar] [CrossRef] [PubMed]
- Mahdizade Ari, M.; Teymouri, S.; Fazlalian, T.; Asadollahi, P.; Afifirad, R.; Sabaghan, M.; Valizadeh, F.; Ghanavati, R.; Darbandi, A. The effect of probiotics on gestational diabetes and its complications in pregnant mother and newborn: A systematic review and meta-analysis during 2010–2020. J. Clin. Lab. Anal. 2022, 36, e24326. [Google Scholar] [CrossRef] [PubMed]
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of probiotic supplementation during pregnancy on the future maternal risk of metabolic syndrome. Int. J. Mol. Sci. 2022, 23, 8253. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of probiotics on metabolic outcomes in pregnant women with gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Walsh, C.A.; Brennan, L.; McAuliffe, F.M. Probiotics in pregnancy and maternal outcomes: A systematic review. J. Matern. Fetal Neonatal Med. 2013, 26, 772–778. [Google Scholar] [CrossRef]
- Halemani, K.; Shetty, A.P.; Thimmappa, L.; Issac, A.; Dhiraaj, S.; Radha, K.; Mishra, P.; Mathias, E.G. Impact of probiotic on anxiety and depression symptoms in pregnant and lactating women and microbiota of infants: A systematic review and meta-analysis. J. Glob. Health 2023, 13, 04038. [Google Scholar] [CrossRef] [PubMed]
- Movaghar, R.; Abbasalizadeh, S.; Vazifekhah, S.; Farshbaf-Khalili, A.; Shahnazi, M. The effects of synbiotic supplementation on blood pressure and other maternal outcomes in pregnant mothers with mild preeclampsia: A triple-blinded randomized controlled trial. BMC Womens Health 2024, 24, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, T.; Wang, J.; Zhao, G.; Hou, Y. Protective effect of Akkermansia muciniphila on the preeclampsia-like mouse model. Reprod. Sci. 2023, 30, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: A prospective observational cohort study in Norway. BMJ Open 2018, 8, e018021. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of probiotic food and risk of preeclampsia in primiparous women: The Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.M.; Meng, L.; Liu, H.; Bao, D. Changes in intestinal flora in preeclampsia rats and effects of probiotics on their inflammation and blood pressure. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10155–10161. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Tain, Y.L.; Hsu, C.N. Maternal supplementation of probiotics, prebiotics or postbiotics to prevent offspring metabolic syndrome: The gap between preclinical results and clinical translation. Int. J. Mol. Sci. 2022, 23, 10173. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Agostoni, C.; Davanzo, R.; Hyppönen, E.; Isolauri, E.; Meltzer, H.M.; Steegers-Theunissen, R.P.; Cetin, I. Early-life nutritional exposures and lifelong health: Immediate and long-lasting impacts of probiotics, vitamin D, and breastfeeding. Nutr. Rev. 2017, 75, 83–97. [Google Scholar] [CrossRef]
- Colquitt, A.S.; Miles, E.A.; Calder, P.C. Do probiotics in pregnancy reduce allergies and asthma in infancy and childhood? A systematic review. Nutrients 2022, 14, 1852. [Google Scholar] [CrossRef]
- Savilahti, E. Probiotics in the treatment and prevention of allergies in children. Biosci. Microflora 2011, 30, 119–128. [Google Scholar] [CrossRef]
- Alsharairi, N.A.; Li, L. Gut microbiota, inflammation, and probiotic supplementation in fetal growth restriction-a comprehensive review of human and animal studies. Life 2023, 13, 2239. [Google Scholar] [CrossRef] [PubMed]
- Cuinat, C.; Stinson, S.E.; Ward, W.E.; Comelli, E.M. Maternal intake of probiotics to program offspring health. Curr. Nutr. Rep. 2022, 11, 537–562. [Google Scholar] [CrossRef] [PubMed]
- Radford-Smith, D.E.; Probert, F.; Burnet, P.W.J.; Anthony, D.C. Modifying the maternal microbiota alters the gut-brain metabolome and prevents emotional dysfunction in the adult offspring of obese dams. Proc. Natl. Acad. Sci. USA 2022, 119, e2108581119. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, T.; Shen, H.; Jiang, Y.; Yang, Q.; Su, S.; Wu, L.; Fan, X.; Gao, M.; Wu, Y.; et al. Mixed probiotics modulated gut microbiota to improve spermatogenesis in bisphenol A-exposed male mice. Ecotoxicol. Environ. Saf. 2024, 270, 115922. [Google Scholar] [CrossRef] [PubMed]
- Baralić, K.; Živančević, K.; Jorgovanović, D.; Javorac, D.; Radovanović, J.; Gojković, T.; Buha Djordjevic, A.; Ćurčić, M.; Mandinić, Z.; Bulat, Z.; et al. Probiotic reduced the impact of phthalates and bisphenol A mixture on type 2 diabetes mellitus development: Merging bioinformatics with in vivo analysis. Food Chem. Toxicol. 2021, 154, 112325. [Google Scholar] [CrossRef]
- Giommi, C.; Habibi, H.R.; Candelma, M.; Carnevali, O.; Maradonna, F. Probiotic administration mitigates bisphenol A reproductive toxicity in zebrafish. Int. J. Mol. Sci. 2021, 22, 9314. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenfeld, C.S. Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring? Biomedicines 2024, 12, 1628. https://doi.org/10.3390/biomedicines12081628
Rosenfeld CS. Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring? Biomedicines. 2024; 12(8):1628. https://doi.org/10.3390/biomedicines12081628
Chicago/Turabian StyleRosenfeld, Cheryl S. 2024. "Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring?" Biomedicines 12, no. 8: 1628. https://doi.org/10.3390/biomedicines12081628
APA StyleRosenfeld, C. S. (2024). Should Pregnant Women Consume Probiotics to Combat Endocrine-Disrupting Chemical-Induced Health Risks to Their Unborn Offspring? Biomedicines, 12(8), 1628. https://doi.org/10.3390/biomedicines12081628







