Instant and Multifunctional Nanofibers Loaded with Proanthocyanidins and Hyaluronic Acid for Skincare Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospinning Solution
2.3. Preparation of Nanofiber Mats
2.4. Characterization of Nanofibrous Mats
2.5. Moisturizing and Hygroscopic Properties of Nanofibrous Mats
2.6. Measurement of Mechanical Properties
2.7. Hydrophilicity Measurement Test
2.8. In Vitro Antioxidant Activity
2.8.1. ABTS Free Radical Scavenging
2.8.2. DPPH Free Radical Scavenging
2.9. Antioxidant Assay of Cells
2.10. Toxicological Safety Test
2.11. Cytocompatibility Assessment
2.11.1. Cell Culture
2.11.2. Cell Proliferation
2.11.3. Dead Cell Staining Assay
2.12. Statistical Analysis
3. Results and Discussion
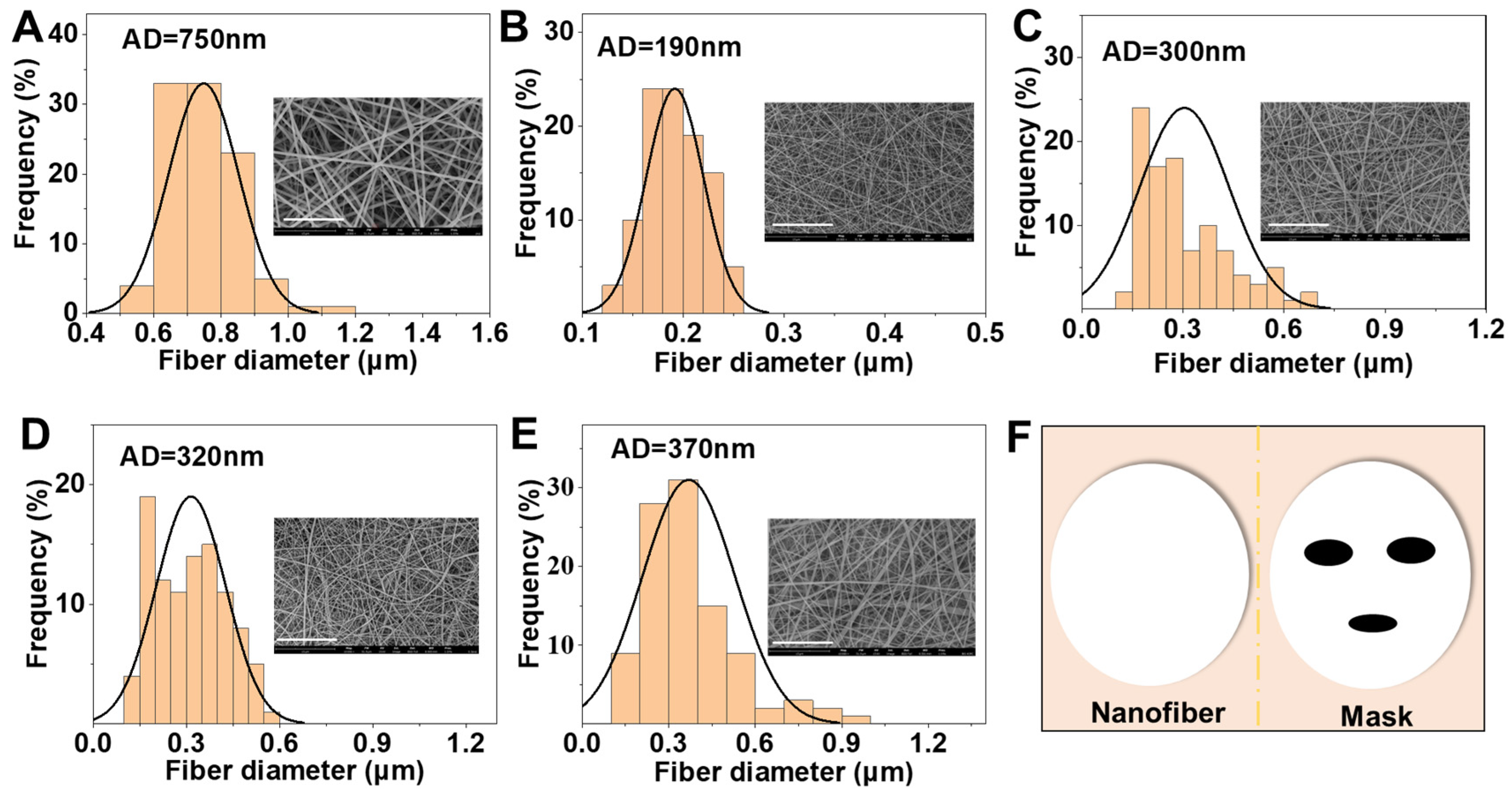
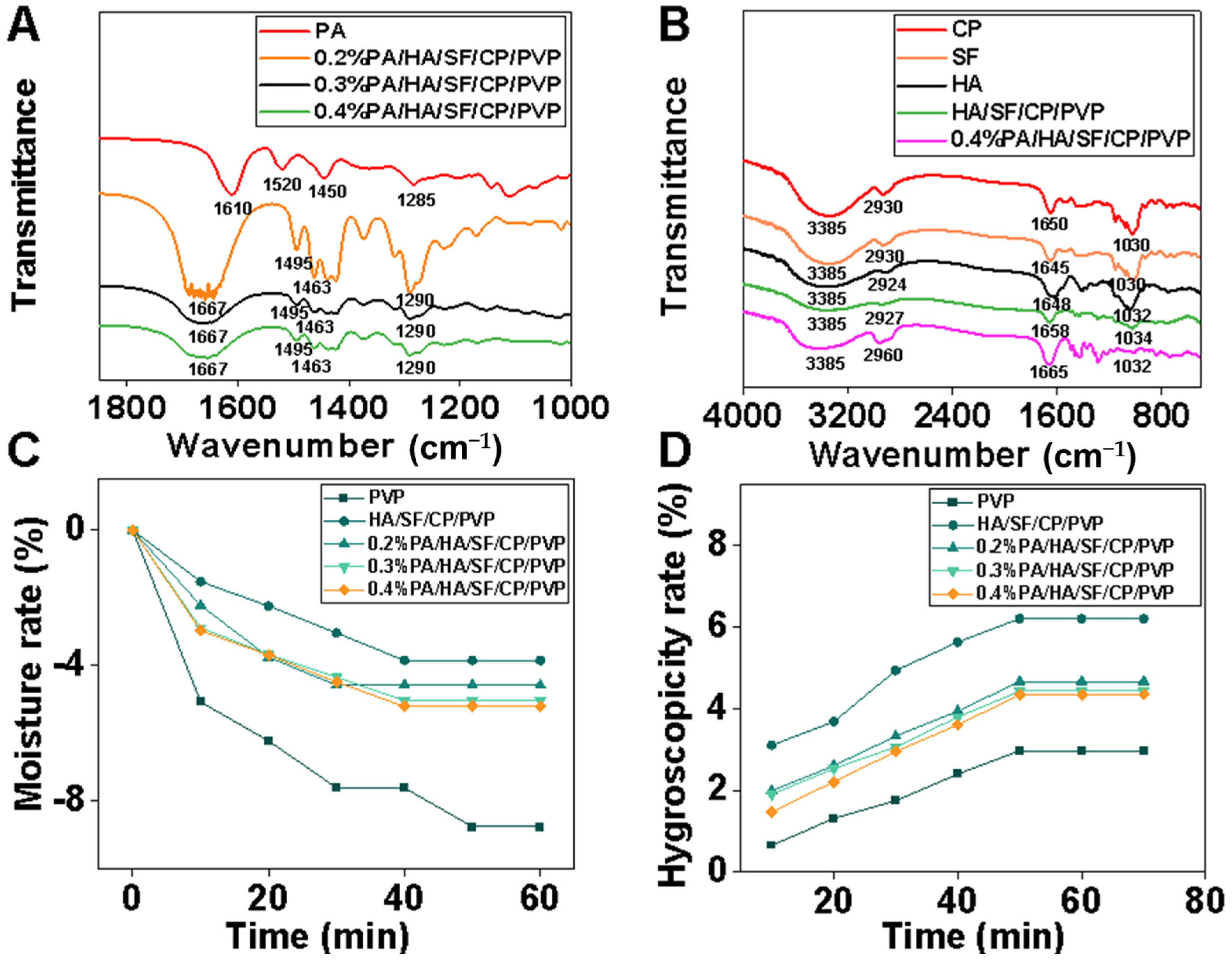
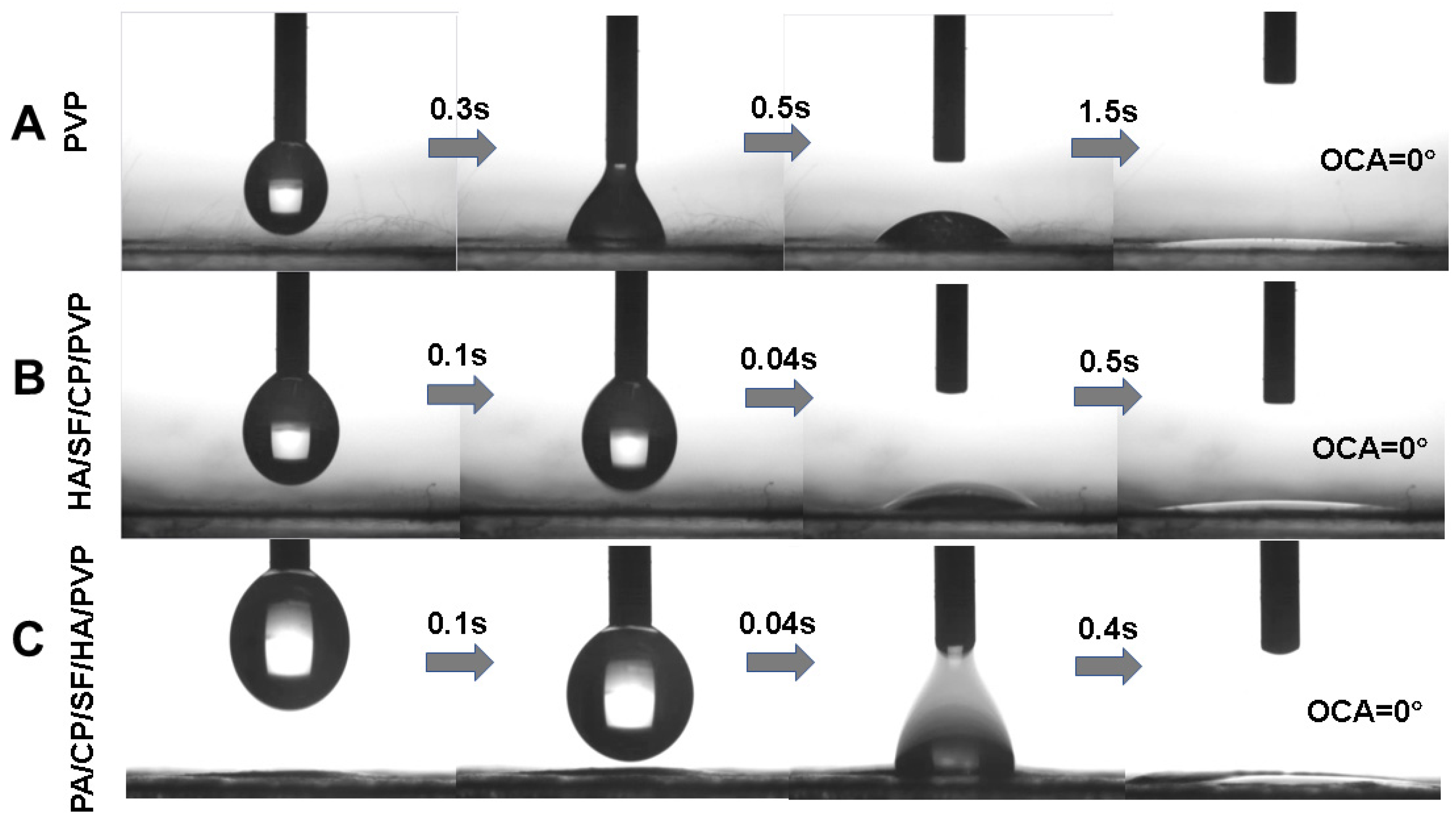
3.1. Characterization of Nanofibrous Mats
3.2. Antioxidant Capacity
3.2.1. DPPH and ABTS Free Radical Scavenging Capacity
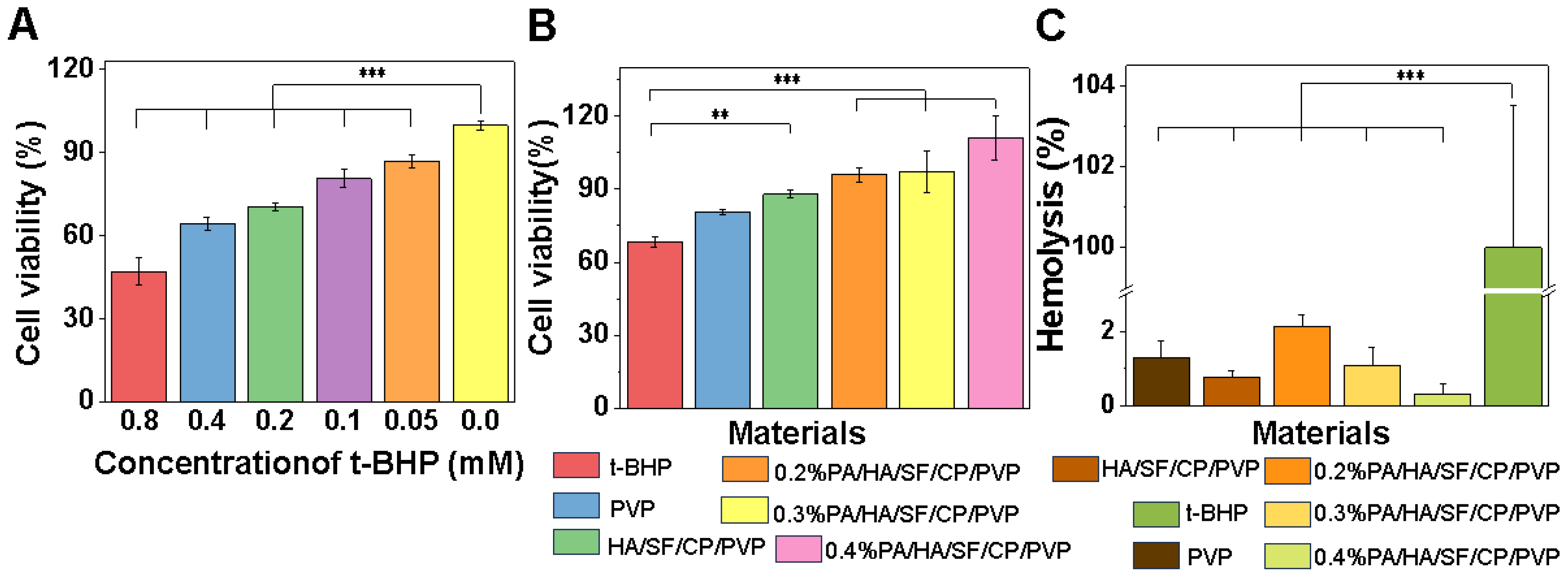
3.2.2. Cellular Antioxidant Capacity
3.3. Toxicological Safety Evaluation
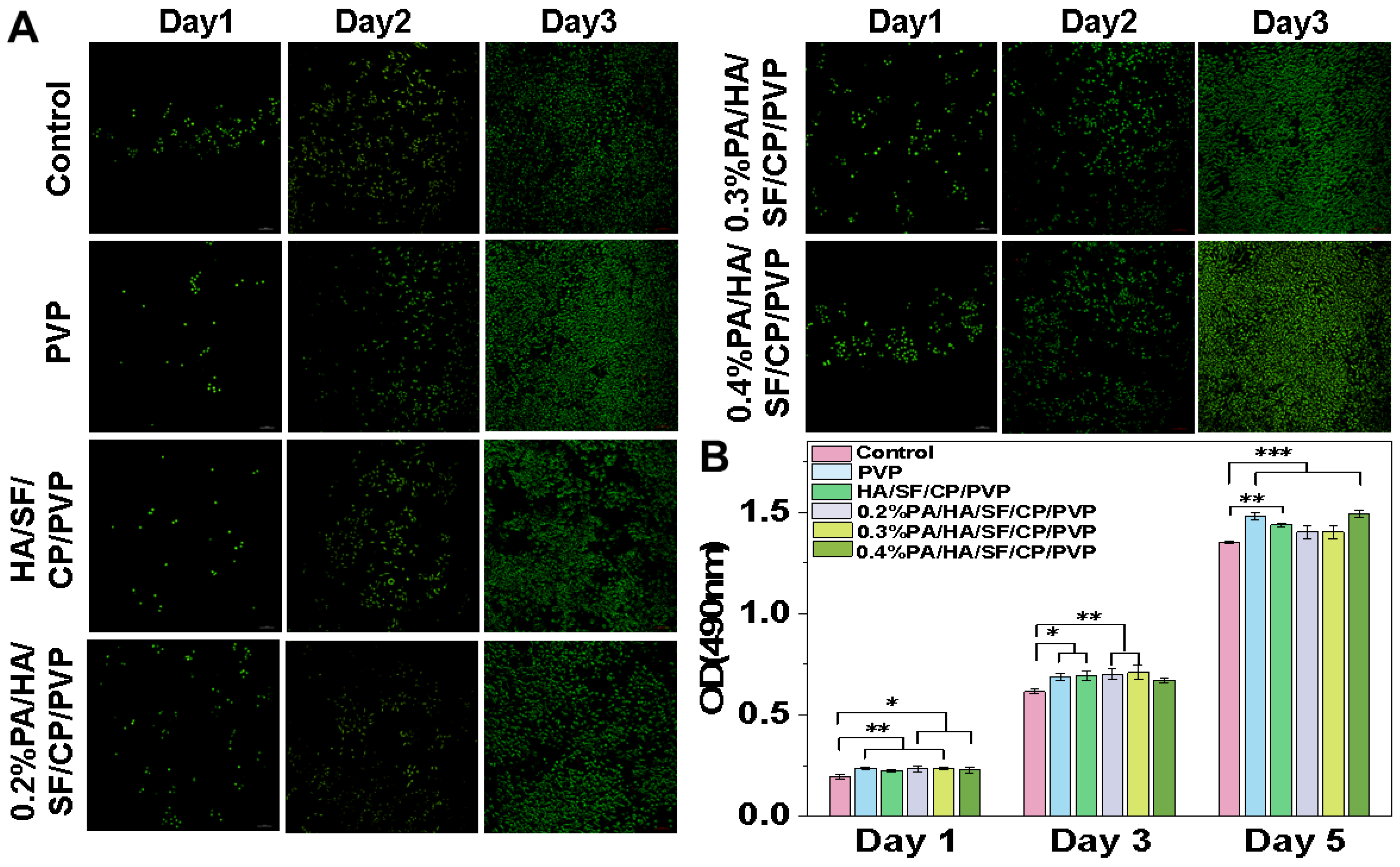
3.4. Biocompatibility Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, S.; Wang, Y.; Ni, J.; Xiao, G. The efficacy of a novel tomato extracts formulation on skin aging and pigmentation: A randomized, double-blind, parallel-controlled trial. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100005. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, S.; Wang, Y.; Ni, J.; Zhao, T.; Xiao, G. Anti-skin aging effects and bioavailability of collagen tripeptide and elastin peptide formulations in young and middle-aged women. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100019. [Google Scholar] [CrossRef]
- Jia, L.; Wang, L.; Zhang, X.; Zhang, Q.; Lei, P.; Chang, Y.; Han, L.; Chai, X.; Yang, W.; Wang, Y.; et al. Investigation of oligomeric proanthocyanidins extracted from Rhodiolae Crenulatae Radix et Rhizomes using deep eutectic solvents and identified via data-dependent-acquisition mass-spectroscopy. J. Pharm. Anal. 2024; in press. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2022, 39, 4581–4609. [Google Scholar] [CrossRef]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef]
- Yin, M.; Yang, Z.; Min, K.; Yang, Q.; Ma, M.; Chen, B. Rapid microwave-assisted Porter method for determination of proanthocyanidins. Phytochem. Anal. 2020, 31, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kang, H.; Chen, L.; Shen, X.; Zheng, B. Exploring the relationship between nutritional properties and structure of chestnut resistant starch constructed by extrusion with starch-proanthocyanidins interactions. Carbohydr. Polym. 2024, 324, 121535. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Jiang, H.; Fan, L.; Sun, B.; Yu, F.; Yang, X.; Lou, X.; He, C.; Wang, H. Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids Surf. B Biointerfaces 2016, 139, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xing, Y.; Yuen, M.; Yuen, T.; Yuen, H.; Peng, Q. Anti-Aging Effect and Mechanism of Proanthocyanidins Extracted from Sea buckthorn on Hydrogen Peroxide-Induced Aging Human Skin Fibroblasts. Antioxidants 2022, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuen, M.; Yuen, T.; Yuen, H.; Wang, M.; Peng, Q. Anti-skin aging effect of sea buckthorn proanthocyanidins in D-galactose-induced aging mice. Food Sci. Nutr. 2024, 12, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Chaala, M.; Sebba, F.Z.; Fuster, M.G.; Moulefera, I.; Montalbán, M.G.; Carissimi, G.; Víllora, G. Accelerated Simple Preparation of Curcumin-Loaded Silk Fibroin/Hyaluronic Acid Hydrogels for Biomedical Applications. Polymers 2023, 15, 504. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef]
- Rahman, M.; Dip, T.M.; Nur, M.G.; Padhye, R.; Houshyar, S. Fabrication of Silk Fibroin-Derived Fibrous Scaffold for Biomedical Frontiers. Macromol. Mater. Eng. 2024, 309, 2300422. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Sapru, S.; Samadikuchaksaraei, A.; Reis, R.L.; Kaplan, D.L.; Kundu, S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2020, 153, 28–53. [Google Scholar] [CrossRef]
- Selvaraj, S.; Inbasekar, C.; Pandurangan, S.; Nishter, N.F. Collagen-coated silk fibroin nanofibers with antioxidants for enhanced wound healing. J. Biomater. Sci. Polym. Ed. 2023, 34, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.; Yu, S.; Zheng, Y. Biomimetic AgNPs@antimicrobial peptide/silk fibroin coating for infection-trigger antibacterial capability and enhanced osseointegration. Bioact. Mater. 2023, 20, 64–80. [Google Scholar] [CrossRef]
- Lupu, M.A.; Gradisteanu Pircalabioru, G.; Chifiriuc, M.C.; Albulescu, R.; Tanase, C. Beneficial effects of food supplements based on hydrolyzed collagen for skin care (Review). Exp. Ther. Med. 2020, 20, 12–17. [Google Scholar] [CrossRef]
- Hou, Y.; Chitrakar, B.; Mao, K.; Wang, K.; Gu, X.; Gao, J.; Zhang, Q.; Bekhit, A.E.-D.A.; Sang, Y. Bioactivity of collagen peptides derived from commercial animals: In silico investigation. LWT-Food Sci. Technol. 2023, 187, 115381. [Google Scholar] [CrossRef]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Nasrine, A.; Gulzar Ahmed, M.; Sultana, R.; Jaswanth Gowda, B.H.; Surya, S.; Almuqbil, M.; Asdaq, S.M.B.; Alshehri, S.; Arif Hussain, S. Potential benefits of using chitosan and silk fibroin topical hydrogel for managing wound healing and coagulation. Saudi Pharm. J. 2023, 31, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yin, H.; Guo, Y.; Gao, Y.; Zhao, Y. Fabrication of multifunctional facial masks from phenolic acid grafted chitosan/collagen peptides via aqueous electrospinning. Int. J. Biol. Macromol. 2024, 267, 131443. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral Intake of Collagen Peptide Attenuates Ultraviolet B Irradiation-Induced Skin Dehydration In Vivo by Regulating Hyaluronic Acid Synthesis. Int. J. Mol. Sci. 2018, 19, 3551. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2021, 20, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Himeno, A.; Tsujikami, M.; Koizumi, S.; Watanabe, T.; Igase, M. Effect of Reducing Pigmentation by Collagen Peptide Intake: A Randomized, Double-Blind, Placebo-Controlled Study. Dermatol. Ther. 2022, 12, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Lv, L.; Guo, J.; Yang, X.; Liao, M.; Zhao, T.; Sun, H.; Zhang, S.; Li, W. Preparation of Cod Skin Collagen Peptides/Chitosan-Based Temperature-Sensitive Gel and Its Anti-Photoaging Effect in Skin. Drug Des. Dev. Ther. 2023, 17, 419–437. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Zhang, T.; Zhao, J.; Cui, S.; Chen, W. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: A review. Carbohydr. Polym. 2023, 299, 120153. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery—A review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Shiu, B.-C.; Hsu, P.-W.; Lou, C.-W.; Lin, J.-H. PVP/CS/Phyllanthus emblica Nanofiber Membranes for Dry Facial Masks: Manufacturing Process and Evaluations. Polymers 2022, 14, 4470. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Pamornpathomkul, B. HPMC/PVP Dissolving Microneedles: A Promising Delivery Platform to Promote Trans-Epidermal Delivery of Alpha-Arbutin for Skin Lightening. AAPS PharmSciTech 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, C.; Cao, X.; Wang, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, J.; Li, A. Mechanically robust conjugated microporous polymer membranes prepared using polyvinylpyrrolidone (PVP) electrospun nanofibers as a template for efficient PM capture. J. Colloid Interface Sci. 2023, 637, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Tipduangta, P.; Watcharathirawongs, W.; Waritdecha, P.; Sirithunyalug, B.; Leelapornpisid, P.; Chaiyana, W.; Goh, C.F. Electrospun cellulose acetate/polyvinylpyrrolidone fiber mats as potential cosmetic under-eye masks for caffeine delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104732. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by ABTS and DPPH Assays-A Practical Approach. Molecules 2021, 27, 50. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Antonijević, M.R.; Avdović, E.H.; Simijonović, D.M.; Milanović, Ž.B.; Amić, A.D.; Marković, Z.S. Radical Scavenging Activity and Pharmacokinetic Properties of Coumarin-Hydroxybenzohydrazide Hybrids. Int. J. Mol. Sci. 2022, 23, 490. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Puiggròs, F.; Salvadó, M.; Bladé, C.; Arola, L. Differential Modulation of Apoptotic Processes by Proanthocyanidins as a Dietary Strategy for Delaying Chronic Pathologies. Crit. Rev. Food Sci. Nutr. 2014, 54, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, C.; Cao, S.; Sun, Z.; Zhang, Y.; Li, M.; He, W.; Wang, Y.; Chen, Q.; Zhang, Y.; et al. Proanthocyanidins Delay Fruit Coloring and Softening by Repressing Related Gene Expression during Strawberry (Fragaria × ananassa Duch.) Ripening. Int. J. Mol. Sci. 2023, 24, 3139. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.N.; Presgrave Rde, F.; Presgrave, O.A.; Sabagh, F.P.; de Freitas, J.C.; Corrado, A.P. A reassessment of the in vitro RBC haemolysis assay with defibrinated sheep blood for the determination of the ocular irritation potential of cosmetic products: Comparison with the in vivo Draize rabbit test. Altern. Lab. Anim. 2008, 36, 275–284. [Google Scholar] [CrossRef]
- Abdelkader, H.; Fathalla, Z.; Moharram, H.; Ali, T.F.S.; Pierscionek, B. Cyclodextrin Enhances Corneal Tolerability and Reduces Ocular Toxicity Caused by Diclofenac. Oxidative Med. Cell. Longev. 2018, 13, 5260976. [Google Scholar] [CrossRef]



| Number | PA | HA | SF | CP | PVP | Name |
|---|---|---|---|---|---|---|
| 1 | — | — | — | — | √ | PVP |
| 2 | — | √ | √ | √ | √ | HA/SF/CP/PVP |
| 3 | 0.2% | √ | √ | √ | √ | 0.2% PA/HA/SF/CP/PVP |
| 4 | 0.3% | √ | √ | √ | √ | 0.3% PA/HA/SF/CP/PVP |
| 5 | 0.4% | √ | √ | √ | √ | 0.4% PA/HA/SF/CP/PVP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Gu, P.; Jiang, Q.; Cheng, X.; Fan, J.; Bai, Y. Instant and Multifunctional Nanofibers Loaded with Proanthocyanidins and Hyaluronic Acid for Skincare Applications. Biomedicines 2024, 12, 1584. https://doi.org/10.3390/biomedicines12071584
Yang X, Gu P, Jiang Q, Cheng X, Fan J, Bai Y. Instant and Multifunctional Nanofibers Loaded with Proanthocyanidins and Hyaluronic Acid for Skincare Applications. Biomedicines. 2024; 12(7):1584. https://doi.org/10.3390/biomedicines12071584
Chicago/Turabian StyleYang, Xuan, Pengcheng Gu, Qiang Jiang, Xiting Cheng, Jia Fan, and Yan Bai. 2024. "Instant and Multifunctional Nanofibers Loaded with Proanthocyanidins and Hyaluronic Acid for Skincare Applications" Biomedicines 12, no. 7: 1584. https://doi.org/10.3390/biomedicines12071584
APA StyleYang, X., Gu, P., Jiang, Q., Cheng, X., Fan, J., & Bai, Y. (2024). Instant and Multifunctional Nanofibers Loaded with Proanthocyanidins and Hyaluronic Acid for Skincare Applications. Biomedicines, 12(7), 1584. https://doi.org/10.3390/biomedicines12071584






