Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review
Abstract
1. Introduction
1.1. Pharmacogenomics: A Core Component of Personalized Medicine
1.2. Cytochrome P450 Enzymes: Their Function, Characteristics, and Role in Disease
2. CYP450 Enzyme Families and Their Substrates
2.1. CYP 450 Enzymes Functions, Mechanism, and Regulation
2.2. Effect of CYP450 Genes Polymorphisms on Enzyme Activity and Pharmacological Response
2.2.1. Single Nucleotide Polymorphisms
2.2.2. Premature Stop Codons
2.2.3. Variable Number Tandem Repeat
2.2.4. Gene Deletions
2.2.5. Copy Number Variants
2.3. The Role of Molecular Techniques in Detecting CYP450 Polymorphisms for Personalized Medicine
2.3.1. DNA Microarray Technology
2.3.2. DNA Sequencing
2.3.3. Polymerase Chain Reaction
2.3.4. Next-Generation Sequencing
3. Endogenous Metabolism
3.1. Hormone Metabolism
3.1.1. Mechanisms of CYP450 Enzymes in Steroid Hormone Regulation
CYP11A1
CYP17A1
CYP21A2
CYP11B2
CYP19A1
3.2. Fatty Acids and Lipid Metabolism
3.2.1. Overview of CYP450 Mechanism of Oxidation
3.2.2. Role of Microbial CYP450 in Fatty Acid Oxidation and the Potential Therapeutic Opportunities
3.2.3. Role of CYP450 in Polyunsaturated Fatty Acid Metabolism
3.2.4. Role of CYP450 Enzymes in Cholesterol, Endogenous Toxins (Bile Acids), and Their Associated Diseases
3.2.5. CYP450 Enzymes and Bile Acids-Binding Nuclear Receptors
Nuclear Receptor Regulating Bile Acid Synthesis: FXR
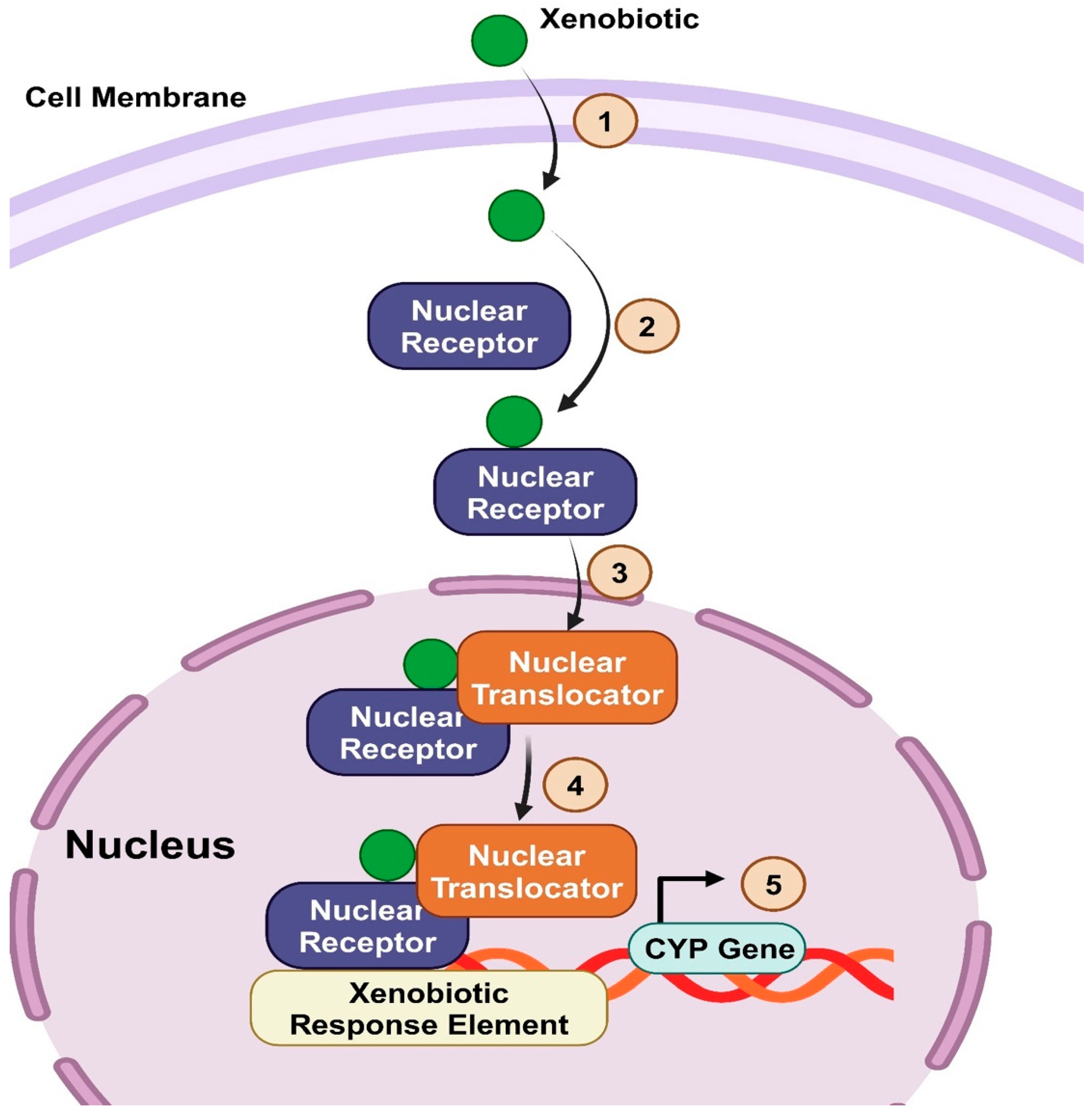
Nuclear Receptor Regulating Bile Acid Metabolism (Detoxification): PXR and CAR
4. CYP450 Enzymes and Vitamin D
4.1. Role of CYP450 Enzymes in Vitamin D Metabolism
4.1.1. CYP27B1 and Vitamin D Activation
4.1.2. CYP24A1 and the Inactivation of Vitamin D
4.1.3. Role of Additional CYP450 Enzymes in Vitamin D Metabolism
5. CYP450 Enzymes as Central Players in Disease
5.1. CYP450 and Endocrine Disorders
5.1.1. PCOS
5.1.2. Adrenal Insufficiency
5.2. CYP450 and Liver Disease
5.2.1. Nonalcoholic Fatty Liver Disease
5.2.2. Cirrhosis
6. CYP450 and Cancer
6.1. Mechanisms of CYP450-Inducing Cancers
6.2. CYP450 and Chemotherapy
6.3. BC
6.4. Ovarian and EC
6.5. Hepatocellular Carcinoma
7. Future Perspectives
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ACTH | Adrenocorticotropic hormone |
| AhR | Aryl hydrocarbon receptor |
| AI | Artificial intelligence |
| ALS | Amyotrophic lateral sclerosis |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| AS-PCR | Allele-specific PCR |
| BC | Breast cancer |
| BMI | Body mass index |
| CA | Cholic acid |
| CAH | Congenital adrenal hyperplasia |
| CAR | Constitutive androstane receptor |
| CDCA | Chenodeoxycholic acid |
| CETSA | Cellular thermal shift assay |
| CNVs | Copy number variations |
| CYP 450 | Cytochrome P450 |
| DHA | Docosahexaenoic acid |
| EC | Endometrial cancer |
| EETs | Epoxyeicosatrienoic acids |
| EGCG | Epigallocatechin gallate |
| EM | Extensive metabolizers |
| EP | Epoxy |
| EPA | Eicosapentaenoic acid |
| EpOMEs | Epoxy Octadecanoic Acids |
| FDA | Food and Drug Administration |
| FXR | Farnesoid X receptor |
| HCC | Hepatocellular carcinoma |
| HETE | Hydroxyeicosatetraenoic acid |
| HGF | Hepatocyte growth factor |
| HNF4 | Hepatocyte Nuclear Factor 4 alpha |
| HSD | Hydroxysteroid dehydrogenase |
| IMs | Intermediate metabolizers |
| LA | Linoleic acid |
| LCA | Lithocholic Acid |
| LRH-1 | Liver Receptor Homolog 1 |
| MET | Mesenchymal-epithelial transition factor |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NMD | Nonsense-mediated mRNA decay |
| NRs | Nuclear receptors |
| PBREM | Phenobarbital Responsive Enhancer Module |
| PCOS | Polycystic ovary syndrome |
| PCR | Polymerase chain reaction |
| PMs | Poor metabolizers |
| PTX | Paclitaxel |
| PUFAs | Polyunsaturated fatty acids |
| PXR | Pregnane X Receptor |
| PXREs | PXR Response Elements |
| RFLP | Restriction fragment length polymorphism |
| RT-PCR | Real-time PCR |
| RXR | Retinoid X Receptor |
| sER | Smooth endoplasmic reticulum |
| SHP | Small Heterodimer Partner |
| SLE | Systemic lupus erythematosus |
| SNPs | Single nucleotide polymorphisms |
| StAR | Steroidogenic acute regulatory |
| STARD3 | StAR-related lipid transfer domain-3 |
| UDP | Uridine diphosphate |
| UM | Ultra-rapid metabolizers |
| UTR | Untranslated region |
| VNTRs | Variable number tandem repeats |
| XREs | Xenobiotic response elements |
References
- Judge, A.; Dodd, M.S. Metabolism. Essays Biochem. 2020, 64, 607–647. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Barker, J.; ElShaer, A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 8224. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Huang, J.; Moorthy, B.; Wang, C.; Hu, M.; Gao, S.; Ghose, R. Potential role of drug metabolizing enzymes in chemotherapy-induced gastrointestinal toxicity and hepatotoxicity. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Pedretti, A.; Vistoli, G. Reactions and enzymes in the metabolism of drugs and other xenobiotics. Drug Discov. Today 2012, 17, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Squassina, A.; Manchia, M.; Manolopoulos, V.G.; Artac, M.; Lappa-Manakou, C.; Karkabouna, S.; Mitropoulos, K.; Del Zompo, M.; Patrinos, G.P. Realities and expectations of pharmacogenomics and personalized medicine: Impact of translating genetic knowledge into clinical practice. Pharmacogenomics 2010, 11, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, E.; Stocco, G. Pharmacogenomics and Personalized Medicine. Genes 2020, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef]
- Dere, W.H.; Suto, T.S. The role of pharmacogenetics and pharmacogenomics in improving translational medicine. Clin. Cases Miner. Bone Metab. 2009, 6, 13–16. [Google Scholar]
- Singh, D.B. The Impact of Pharmacogenomics in Personalized Medicine. Adv. Biochem. Eng. Biotechnol. 2020, 171, 369–394. [Google Scholar] [CrossRef]
- Song, Y.; Li, C.; Liu, G.; Liu, R.; Chen, Y.; Li, W.; Cao, Z.; Zhao, B.; Lu, C.; Liu, Y. Drug-Metabolizing Cytochrome P450 Enzymes Have Multifarious Influences on Treatment Outcomes. Clin. Pharmacokinet. 2021, 60, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H. Cytochrome P450: Genotype to phenotype. Xenobiotica 2020, 50, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Preissner, S.C.; Hoffmann, M.F.; Preissner, R.; Dunkel, M.; Gewiess, A.; Preissner, S. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS ONE 2013, 8, e82562. [Google Scholar] [CrossRef] [PubMed]
- Preissner, S.; Kroll, K.; Dunkel, M.; Senger, C.; Goldsobel, G.; Kuzman, D.; Guenther, S.; Winnenburg, R.; Schroeder, M.; Preissner, R. SuperCYP: A comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP-drug interactions. Nucleic Acids Res. 2010, 38, D237–D243. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, A.M.; Dang, C.H. Basic review of the cytochrome p450 system. J. Adv. Pract. Oncol. 2013, 4, 263–268. [Google Scholar] [CrossRef]
- McKinnon, R.A.; Sorich, M.J.; Ward, M.B. Cytochrome P450 Part 1: Multiplicity and Function. J. Pharm. Pract. Res. 2008, 38, 55–57. [Google Scholar] [CrossRef]
- Wrighton, S.A.; VandenBranden, M.; Ring, B.J. The human drug metabolizing cytochromes P450. J. Pharmacokinet. Biopharm. 1996, 24, 461–473. [Google Scholar] [CrossRef]
- Stark, K.; Guengerich, F.P. Characterization of orphan human cytochromes P450. Drug Metab. Rev. 2007, 39, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B.; Tulsyan, S.; Kumar, S.; Mittal, R.D.; Agarwal, G. Cytochrome P450 in Cancer Susceptibility and Treatment. Adv. Clin. Chem. 2015, 71, 77–139. [Google Scholar] [CrossRef] [PubMed]
- Stading, R.; Couroucli, X.; Lingappan, K.; Moorthy, B. The role of cytochrome P450 (CYP) enzymes in hyperoxic lung injury. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 171–178. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.; Beisson, F.; Bishop, G.; Hamberger, B.; Hofer, R.; Paquette, S.; Werck-Reichhart, D. Cytochromes p450. Arabidopsis Book 2011, 9, e0144. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xiao, B.; Deng, J.; Gong, L.; Li, Y.; Li, J.; Zhong, Y. The Role of Cytochrome P450 Enzymes in COVID-19 Pathogenesis and Therapy. Front. Pharmacol. 2022, 13, 791922. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.C.; Ingelman-Sundberg, M. The Human Cytochrome P450 (CYP) Allele Nomenclature website: A peer-reviewed database of CYP variants and their associated effects. Hum. Genom. 2010, 4, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.F. 57 varieties: The human cytochromes P450. Pharmacogenomics 2004, 5, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Sinha, K.; Sil, P.C. Cytochrome P450s: Mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr. Drug Metab. 2014, 15, 719–742. [Google Scholar] [CrossRef] [PubMed]
- Strolin Benedetti, M.; Whomsley, R.; Baltes, E. Involvement of enzymes other than CYPs in the oxidative metabolism of xenobiotics. Expert. Opin. Drug Metab. Toxicol. 2006, 2, 895–921. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.W.; McLean, K.J.; Grant, J.L.; Makris, T.M. Structure and function of the cytochrome P450 peroxygenase enzymes. Biochem. Soc. Trans. 2018, 46, 183–196. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Q.; Neuschwander-Tetri, B.A.; Portincasa, P.; Pandak, W.M. Interactions between Bile Acids and Nuclear Receptors and Their Effects on Lipid Metabolism and Liver Diseases. J. Lipids 2012, 2012, 560715. [Google Scholar] [CrossRef] [PubMed]
- Fang, S. Bile Acid Receptor Farnesoid X Receptor: A Novel Therapeutic Target for Metabolic Diseases. JLA 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, Y.Y.; Ren, H.W.; Li, C.J.; Xiang, Z.X.; Luan, Z.L. The role of pregnane X receptor (PXR) in substance metabolism. Front Endocrinol. 2022, 13, 959902. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Redinbo, M.R.; Kliewer, S.A. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 1–23. [Google Scholar] [CrossRef]
- Jin, J.; Zhong, X.B. Epigenetic Mechanisms Contribute to Intraindividual Variations of Drug Metabolism Mediated by Cytochrome P450 Enzymes. Drug Metab. Dispos. 2023, 51, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, S. Epigenetic Regulation of Cytochrome P450 Enzymes and Clinical Implication. Curr. Drug Metab. 2015, 16, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Michaud, V.; Frappier, M.; Dumas, M.C.; Turgeon, J. Metabolic activity and mRNA levels of human cardiac CYP450s involved in drug metabolism. PLoS ONE 2010, 5, e15666. [Google Scholar] [CrossRef]
- Lang, D.; Radtke, M.; Bairlein, M. Highly Variable Expression of CYP1A1 in Human Liver and Impact on Pharmacokinetics of Riociguat and Granisetron in Humans. Chem. Res. Toxicol. 2019, 32, 1115–1122. [Google Scholar] [CrossRef]
- Sachidanandam, R.; Weissman, D.; Schmidt, S.C.; Kakol, J.M.; Stein, L.D.; Marth, G.; Sherry, S.; Mullikin, J.C.; Mortimore, B.J.; Willey, D.L.; et al. A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 2001, 409, 928–933. [Google Scholar] [CrossRef]
- Guttman, Y.; Nudel, A.; Kerem, Z. Polymorphism in Cytochrome P450 3A4 Is Ethnicity Related. Front. Genet. 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Miladi, M.; Dukare, S.; Boulay, K.; Caudron-Herger, M.; Gross, M.; Backofen, R.; Diederichs, S. A pan-cancer analysis of synonymous mutations. Nat. Commun. 2019, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (synonymous) SNPs: Should we care about them? Methods Mol. Biol. 2009, 578, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castejon, M.; Marin, F.; Soler-Rivas, C.; Reglero, G.; Visioli, F.; Rodriguez-Casado, A. Functional non-synonymous polymorphisms prediction methods: Current approaches and future developments. Curr. Med. Chem. 2011, 18, 5095–5103. [Google Scholar] [CrossRef] [PubMed]
- Arendse, L.B.; Blackburn, J.M. Effects of polymorphic variation on the thermostability of heterogenous populations of CYP3A4 and CYP2C9 enzymes in solution. Sci. Rep. 2018, 8, 11876. [Google Scholar] [CrossRef] [PubMed]
- Nahid, N.A.; Johnson, J.A. CYP2D6 pharmacogenetics and phenoconversion in personalized medicine. Expert. Opin. Drug Metab. Toxicol. 2022, 18, 769–785. [Google Scholar] [CrossRef]
- Savas, S.; Tuzmen, S.; Ozcelik, H. Human SNPs resulting in premature stop codons and protein truncation. Hum. Genom. 2006, 2, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Schilff, M.; Sargsyan, Y.; Hofhuis, J.; Thoms, S. Stop Codon Context-Specific Induction of Translational Readthrough. Biomolecules 2021, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Klauer, A.A.; van Hoof, A. Degradation of mRNAs that lack a stop codon: A decade of nonstop progress. Wiley Interdiscip. Rev. RNA 2012, 3, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, M.; Park, J.; Ding, Y.C.; Shleizer-Burko, S.; Neuhausen, S.L.; Halldorsson, B.V.; Stefansson, K.; Gymrek, M.; Bafna, V. Variable number tandem repeats mediate the expression of proximal genes. Nat. Commun. 2021, 12, 2075. [Google Scholar] [CrossRef]
- Jafari, P.; Baghernia, S.; Moghanibashi, M.; Mohamadynejad, P. Significant Association of Variable Number Tandem Repeat Polymorphism rs58335419 in the MIR137 Gene with the Risk of Gastric and Colon Cancers. Br. J. Biomed. Sci. 2022, 79, 10095. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, I.; Naselli, F.; Saverini, M.; Giacalone, A.; Montalto, G.; Caradonna, F. Cytochrome P450 2E1 variable number tandem repeat polymorphisms and health risks: A genotype-phenotype study in cancers associated with drinking and/or smoking. Mol. Med. Rep. 2012, 6, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, N.A.; AlJahdali, I.A.; Abumansour, I.S.; Elhawary, E.N.; Gaboon, N.; Dandini, M.; Madkhali, A.; Alosaimi, W.; Alzahrani, A.; Aljohani, F.; et al. Genetic etiology and clinical challenges of phenylketonuria. Hum. Genom. 2022, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, L.; Dahl, M.L.; Dalen, P.; Al-Shurbaji, A. Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs. Br. J. Clin. Pharmacol. 2002, 53, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Pos, O.; Radvanszky, J.; Buglyo, G.; Pos, Z.; Rusnakova, D.; Nagy, B.; Szemes, T. DNA copy number variation: Main characteristics, evolutionary significance, and pathological aspects. Biomed. J. 2021, 44, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Li-Wan-Po, A.; Girard, T.; Farndon, P.; Cooley, C.; Lithgow, J. Pharmacogenetics of CYP2C19: Functional and clinical implications of a new variant CYP2C19*17. Br. J. Clin. Pharmacol. 2010, 69, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Crosby, I.; Yip, V.; Maguire, P.; Pirmohamed, M.; Turner, R.M. A Review of the Important Role of CYP2D6 in Pharmacogenomics. Genes 2020, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.L.; Lim, C.W.; Sim, W.C.; Toh, L.X.; Leong, K.P. Analysis of Genetic Variation in CYP450 Genes for Clinical Implementation. PLoS ONE 2017, 12, e0169233. [Google Scholar] [CrossRef] [PubMed]
- Bumgarner, R. Overview of DNA microarrays: Types, applications, and their future. Curr. Protoc. Mol. Biol. 2013, 101, 22.1.1–22.1.11. [Google Scholar] [CrossRef]
- de Leon, J.; Susce, M.T.; Murray-Carmichael, E. The AmpliChip CYP450 genotyping test: Integrating a new clinical tool. Mol. Diagn. Ther. 2006, 10, 135–151. [Google Scholar] [CrossRef]
- Biswas, M. Global distribution of CYP2C19 risk phenotypes affecting safety and effectiveness of medications. Pharmacogenom. J. 2021, 21, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.; Cox, G.F.; Ibrahim, J.; Peterschmitt, M.J.; Ross, L.; Thibault, N.; Turpault, S. Effects of paroxetine, ketoconazole, and rifampin on the metabolism of eliglustat, an oral substrate reduction therapy for Gaucher disease type 1. Mol. Genet. Metab. Rep. 2020, 22, 100552. [Google Scholar] [CrossRef]
- Zhou, S.F.; Liu, J.P.; Chowbay, B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef] [PubMed]
- Britto, C.; Cardoso, A.; Silveira, C.; Macedo, V.; Fernandes, O. Polymerase chain reaction (PCR) as a laboratory tool for evaluating the parasitological cure in Chagas disease after specific treatment. Medicina 1999, 59 (Suppl. S2), 176–178. [Google Scholar] [PubMed]
- Sohda, T. Allele-specific polymerase chain reaction for genotyping human cytochrome P450 2E1. J. Clin. Lab. Anal. 1999, 13, 205–208. [Google Scholar] [CrossRef]
- Parker, K.; Aasebo, W.; Haslemo, T.; Stavem, K. Relationship between cytochrome P450 polymorphisms and prescribed medication in elderly hemodialysis patients. SpringerPlus 2016, 5, 350. [Google Scholar] [CrossRef] [PubMed]
- Kane, M. Siponimod Therapy and CYP2C9 Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Abubakar, M.B.; Tan, H.L.; Gan, S.H. A Novel Multiplex PCR-RFLP Method for Simultaneous Genotyping of CYP3A4*4 A>G, CYP3A4*18B G>A and CYP3A4*22 C>T. Malays. J. Med. Sci. 2018, 25, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Koutsilieri, S.; Eliasson, E.; Lauschke, V.M. A paradigm shift in pharmacogenomics: From candidate polymorphisms to comprehensive sequencing. Basic. Clin. Pharmacol. Toxicol. 2022, 131, 452–464. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Gulilat, M.; Lamb, T.; Teft, W.A.; Wang, J.; Dron, J.S.; Robinson, J.F.; Tirona, R.G.; Hegele, R.A.; Kim, R.B.; Schwarz, U.I. Targeted next generation sequencing as a tool for precision medicine. BMC Med. Genom. 2019, 12, 81. [Google Scholar] [CrossRef]
- Zhou, Y.; Lauschke, V.M. The genetic landscape of significant drug metabolizing cytochrome P450 genes-an updated analysis of population-scale sequencing data. Pharmacogenom. J. 2022, 22, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Shen, W.J.; Azhar, S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Pramanik, J.; Mahata, B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes Immun. 2021, 22, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, K.; Hino, T.; Nagano, S. Diverse reactions catalyzed by cytochrome P450 and biosynthesis of steroid hormone. Biophys. Physicobiol. 2022, 19, e190021. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ramirez, J.; Han, J.; Jia, Y.; Domenico, J.; Seibold, M.A.; Hagman, J.R.; Gelfand, E.W. The steroidogenic enzyme Cyp11a1 is essential for development of peanut-induced intestinal anaphylaxis. J. Allergy Clin. Immunol. 2013, 132, 1174–1183 e1178. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Burris-Hiday, S.D.; Scott, E.E. Steroidogenic cytochrome P450 17A1 structure and function. Mol. Cell Endocrinol. 2021, 528, 111261. [Google Scholar] [CrossRef] [PubMed]
- Pallan, P.S.; Wang, C.; Lei, L.; Yoshimoto, F.K.; Auchus, R.J.; Waterman, M.R.; Guengerich, F.P.; Egli, M. Human Cytochrome P450 21A2, the Major Steroid 21-Hydroxylase: Structure of The Enzyme·Progesterone Substrate Complex and Rate-Limiting C-H Bond Cleavage. J. Biol. Chem. 2015, 290, 13128–13143. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Anderko, S.; Hannemann, F.; Eiden-Plach, A.; Bernhardt, R. The CYP11B subfamily. J. Steroid Biochem. Mol. Biol. 2015, 151, 38–51. [Google Scholar] [CrossRef]
- Chan, H.J.; Petrossian, K.; Chen, S. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and -resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2016, 161, 73–83. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Waterman, M.R. Unusual properties of the cytochrome P450 superfamily. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120434. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef] [PubMed]
- Burris-Hiday, S.D.; Scott, E.E. Allosteric modulation of cytochrome P450 enzymes by the NADPH cytochrome P450 reductase FMN-containing domain. J. Biol. Chem. 2023, 299, 105112. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torrado, R.; Querol, A. Opportunistic Strains of Saccharomyces cerevisiae: A Potential Risk Sold in Food Products. Front. Microbiol. 2015, 6, 1522. [Google Scholar] [CrossRef] [PubMed]
- Jorda, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Warrilow, A.G.; Parker, J.E.; Kelly, D.E.; Kelly, S.L. Azole affinity of sterol 14alpha-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens. Antimicrob. Agents Chemother. 2013, 57, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Warrilow, A.G.; Price, C.L.; Mullins, J.G.; Kelly, D.E.; Kelly, S.L. Resistance to antifungals that target CYP51. J. Chem. Biol. 2014, 7, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, H.; Johnston, J.B.; Ortiz de Montellano, P.R. The Mycobacterium tuberculosis cytochrome P450 system. Arch. Biochem. Biophys. 2010, 493, 82–95. [Google Scholar] [CrossRef]
- Johnston, J.B.; Kells, P.M.; Podust, L.M.; Ortiz de Montellano, P.R. Biochemical and structural characterization of CYP124: A methyl-branched lipid omega-hydroxylase from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2009, 106, 20687–20692. [Google Scholar] [CrossRef] [PubMed]
- Holsclaw, C.M.; Sogi, K.M.; Gilmore, S.A.; Schelle, M.W.; Leavell, M.D.; Bertozzi, C.R.; Leary, J.A. Structural characterization of a novel sulfated menaquinone produced by stf3 from Mycobacterium tuberculosis. ACS Chem. Biol. 2008, 3, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Senaratne, R.H.; Petzold, C.J.; Jain, M.; Lee, D.H.; Schelle, M.W.; Leavell, M.D.; Cox, J.S.; Leary, J.A.; Riley, L.W.; et al. A sulfated metabolite produced by stf3 negatively regulates the virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4258–4263. [Google Scholar] [CrossRef]
- Campomizzi, C.S.; Kumar, A.; Uttamrao, P.P.; Stallone, J.J.; Ghanatios, G.E.; Rathinavelan, T.; Estrada, D.F. Active Site Aromatic Residues Play a Dual Role in the Substrate Interaction and Protein Structure in Functional Dimers of CYP121A1 of Mycobacterium tuberculosis. ACS Infect. Dis. 2023, 9, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R. Potential drug targets in the Mycobacterium tuberculosis cytochrome P450 system. J. Inorg. Biochem. 2018, 180, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Muggeo, A.; Coraux, C.; Guillard, T. Current concepts on Pseudomonas aeruginosa interaction with human airway epithelium. PLoS Pathog. 2023, 19, e1011221. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.; Groeneboer, S.; Saerens, K.; Soetaert, W. The role of cytochrome P450 monooxygenases in microbial fatty acid metabolism. FEBS J. 2011, 278, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Price, C.L.; Warrilow, A.G.S.; Rolley, N.J.; Parker, J.E.; Thoss, V.; Kelly, D.E.; Corcionivoschi, N.; Kelly, S.L. Cytochrome P450 168A1 from Pseudomonas aeruginosa is involved in the hydroxylation of biologically relevant fatty acids. PLoS ONE 2022, 17, e0265227. [Google Scholar] [CrossRef]
- Sarparast, M.; Dattmore, D.; Alan, J.; Lee, K.S.S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients 2020, 12, 3523. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef]
- Xu, L.H.; Du, Y.L. Rational and semi-rational engineering of cytochrome P450s for biotechnological applications. Synth. Syst. Biotechnol. 2018, 3, 283–290. [Google Scholar] [CrossRef]
- Shoieb, S.M.; El-Ghiaty, M.A.; Alqahtani, M.A.; El-Kadi, A.O.S. Cytochrome P450-derived eicosanoids and inflammation in liver diseases. Prostaglandins Other Lipid Mediat. 2020, 147, 106400. [Google Scholar] [CrossRef] [PubMed]
- Al-Saraireh, Y.M.; Alshammari, F.; Abu-Azzam, O.H.; Al-Dalain, S.M.; Al-Sarayra, Y.M.; Haddad, M.; Makeen, H.; Al-Qtaitat, A.; Almermesh, M.; Al-Sarayreh, S.A. Targeting Cytochrome P450 Enzymes in Ovarian Cancers: New Approaches to Tumor-Selective Intervention. Biomedicines 2023, 11, 2898. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Itagaki, M.; Ito, Y.; Naoe, S.; Kojima, K.; Ikeguchi, M.; Arita, M. Comprehensive analysis of the mouse cytochrome P450 family responsible for omega-3 epoxidation of eicosapentaenoic acid. Sci. Rep. 2018, 8, 7954. [Google Scholar] [CrossRef] [PubMed]
- Konkel, A.; Schunck, W.H. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta 2011, 1814, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Fer, M.; Dreano, Y.; Lucas, D.; Corcos, L.; Salaun, J.P.; Berthou, F.; Amet, Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch. Biochem. Biophys. 2008, 471, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Westphal, C.; Konkel, A.; Schunck, W.H. Cytochrome p450 enzymes in the bioactivation of polyunsaturated Fatty acids and their role in cardiovascular disease. Adv. Exp. Med. Biol. 2015, 851, 151–187. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; von Schacky, C.; Luft, F.C.; Muller, D.N.; et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Sicard, E.; Markovic, M.; Honeck, H.; Christ, B.; Muller, D.N.; Schunck, W.H. Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem. Biophys. Res. Commun. 2005, 329, 1275–1281. [Google Scholar] [CrossRef]
- Nguyen, X.; Wang, M.H.; Reddy, K.M.; Falck, J.R.; Schwartzman, M.L. Kinetic profile of the rat CYP4A isoforms: Arachidonic acid metabolism and isoform-specific inhibitors. Am. J. Physiol. 1999, 276, R1691–R1700. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Sarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front. Pharmacol. 2018, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A. Cholesterol-metabolizing cytochromes P450: Implications for cholesterol lowering. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Klaassen, C.D. Molecular Regulation of Bile Acid Homeostasis. Drug Metab. Dispos. 2022, 50, 425–455. [Google Scholar] [CrossRef] [PubMed]
- Dubrac, S.; Lear, S.R.; Ananthanarayanan, M.; Balasubramaniyan, N.; Bollineni, J.; Shefer, S.; Hyogo, H.; Cohen, D.E.; Blanche, P.J.; Krauss, R.M.; et al. Role of CYP27A in cholesterol and bile acid metabolism. J. Lipid Res. 2005, 46, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Kastrinou Lampou, V.; Poller, B.; Huth, F.; Fischer, A.; Kullak-Ublick, G.A.; Arand, M.; Schadt, H.S.; Camenisch, G. Novel insights into bile acid detoxification via CYP, UGT and SULT enzymes. Toxicol. In Vitro 2023, 87, 105533. [Google Scholar] [CrossRef]
- Jurica, J.; Dovrtelova, G.; Noskova, K.; Zendulka, O. Bile acids, nuclear receptors and cytochrome P450. Physiol. Res. 2016, 65, S427–S440. [Google Scholar] [CrossRef]
- Chun, M.Y.; Heo, N.J.; Seo, S.W.; Jang, H.; Suh, Y.L.; Jang, J.H.; Kim, Y.E.; Kim, E.J.; Moon, S.Y.; Jung, N.Y.; et al. Case report: Cerebrotendinous xanthomatosis with a novel mutation in the CYP27A1 gene mimicking behavioral variant frontotemporal dementia. Front. Neurol. 2023, 14, 1131888. [Google Scholar] [CrossRef]
- Lambert, G.; Amar, M.J.; Guo, G.; Brewer, H.B., Jr.; Gonzalez, F.J.; Sinal, C.J. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 2003, 278, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.P.H.; Kolyvas, P.; Dang, N.; Hyon, A.; Bailey, K.; Anakk, S. Loss of Hepatic Small Heterodimer Partner Elevates Ileal Bile Acids and Alters Cell Cycle-related Genes in Male Mice. Endocrinology 2022, 163, bqac052. [Google Scholar] [CrossRef]
- Li, S.; Ni, A.; Feng, G.S. Bridging cell surface receptor with nuclear receptors in control of bile acid homeostasis. Acta Pharmacol. Sin. 2015, 36, 113–118. [Google Scholar] [CrossRef]
- Xun, Z.; Yao, X.; Ou, Q. Emerging roles of bile acids in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Cell Mol. Immunol. 2023, 20, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Escudero, R.; Herraez, E.; Sanchez-Martin, A.; Sanchon-Sanchez, P.; Marin, J.J.G.; Monte, M.J. Cholestasis associated to inborn errors in bile acid synthesis. Explor. Dig. Dis. 2022, 1, 137–153. [Google Scholar] [CrossRef]
- Li, T.; Apte, U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv. Pharmacol. 2015, 74, 263–302. [Google Scholar] [CrossRef]
- Ding, L.; Yang, L.; Wang, Z.; Huang, W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 2015, 5, 135–144. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Willson, T.M. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J. Lipid Res. 2002, 43, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Halilbasic, E.; Marschall, H.U.; Zollner, G.; Fickert, P.; Langner, C.; Zatloukal, K.; Denk, H.; Trauner, M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 2005, 42, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Lickteig, A.J.; Csanaky, I.L.; Pratt-Hyatt, M.; Klaassen, C.D. Activation of Constitutive Androstane Receptor (CAR) in Mice Results in Maintained Biliary Excretion of Bile Acids Despite a Marked Decrease of Bile Acids in Liver. Toxicol. Sci. 2016, 151, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Modica, S.; Gadaleta, R.M.; Moschetta, A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl. Recept. Signal 2010, 8, e005. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Thirouard, L.; Sedes, L.; Monrose, M.; Holota, H.; Caira, F.; Volle, D.H.; Beaudoin, C. Nuclear Receptor Metabolism of Bile Acids and Xenobiotics: A Coordinated Detoxification System with Impact on Health and Diseases. Int. J. Mol. Sci. 2018, 19, 3630. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, A.D.; DeMorrow, S. Farnesoid X Receptor as Target for Therapies to Treat Cholestasis-Induced Liver Injury. Cells 2021, 10, 1846. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W.; Liddle, C.; Downes, M. FXR and PXR: Potential therapeutic targets in cholestasis. J. Steroid Biochem. Mol. Biol. 2012, 130, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, K.N.; Chen, C. The role of CYP3A4 in the biotransformation of bile acids and therapeutic implication for cholestasis. Ann. Transl. Med. 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Marschall, H.U.; Wagner, M.; Trauner, M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: Pathogenetic and therapeutic considerations. Mol. Pharm. 2006, 3, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, E.G.; Strom, S.; Yasuda, K.; Lecureur, V.; Assem, M.; Brimer, C.; Lamba, J.; Kim, R.B.; Ramachandran, V.; Komoroski, B.J.; et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J. Biol. Chem. 2001, 276, 39411–39418. [Google Scholar] [CrossRef] [PubMed]
- Lorbek, G.; Lewinska, M.; Rozman, D. Cytochrome P450s in the synthesis of cholesterol and bile acids--from mouse models to human diseases. FEBS J. 2012, 279, 1516–1533. [Google Scholar] [CrossRef]
- Hassan, H.M.; Onabote, O.; Isovic, M.; Passos, D.T.; Dick, F.A.; Torchia, J. Regulation of Chromatin Accessibility by the Farnesoid X Receptor Is Essential for Circadian and Bile Acid Homeostasis In Vivo. Cancers 2022, 14, 6191. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Wikvall, K. Cytochrome P450 enzymes in the bioactivation of vitamin D to its hormonal form (review). Int. J. Mol. Med. 2001, 7, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Kazamel, M.; Kwon, Y.; Lee, I.; Anderson, T.; Zhou, S.; Bamman, M.; Wiggins, D.; Kwan, T.; King, P.H. The vitamin D activator CYP27B1 is upregulated in muscle fibers in denervating disease and can track progression in amyotrophic lateral sclerosis. J. Steroid Biochem. Mol. Biol. 2020, 200, 105650. [Google Scholar] [CrossRef] [PubMed]
- Lauter, K.; Arnold, A. Analysis of CYP27B1, encoding 25-hydroxyvitamin D-1alpha-hydroxylase, as a candidate tumor suppressor gene in primary and severe secondary/tertiary hyperparathyroidism. J. Bone Miner. Res. 2009, 24, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18. [Google Scholar] [CrossRef]
- Dusso, A.S.; Gomez-Alonso, C.; Cannata-Andia, J.B. The hypercalcaemia of CYP24A1 inactivation: New ways to improve diagnosis and treatment. Clin. Kidney J. 2015, 8, 456–458. [Google Scholar] [CrossRef][Green Version]
- Young, K.A.; Munroe, M.E.; Guthridge, J.M.; Kamen, D.L.; Niewold, T.B.; Gilkeson, G.S.; Weisman, M.H.; Ishimori, M.L.; Kelly, J.; Gaffney, P.M.; et al. Combined role of vitamin D status and CYP24A1 in the transition to systemic lupus erythematosus. Ann. Rheum. Dis. 2017, 76, 153–158. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Fronczek, M.; Strzelczyk, J.K.; Biernacki, K.; Salatino, S.; Osadnik, T.; Ostrowska, Z. New Variants of the Cytochrome P450 2R1 (CYP2R1) Gene in Individuals with Severe Vitamin D-Activating Enzyme 25(OH)D Deficiency. Biomolecules 2021, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A.; Waterman, M.R. Cytochromes p450: Roles in diseases. J. Biol. Chem. 2013, 288, 17091–17098. [Google Scholar] [CrossRef] [PubMed]
- Norlin, M.; Wikvall, K. Enzymatic activation in vitamin D signaling—Past, present and future. Arch. Biochem. Biophys. 2023, 742, 109639. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Binti Osman, M.; Sakinah, M.; Nordin, N.; Abdul Hamid, H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes 2022, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Rasool, S.U.A.; Nabi, M.; Ganie, M.A.; Masoodi, S.R.; Amin, S. Impact of rs2414096 polymorphism of CYP19 gene on susceptibility of polycystic ovary syndrome and hyperandrogenism in Kashmiri women. Sci. Rep. 2021, 11, 12942. [Google Scholar] [CrossRef] [PubMed]
- Munawar Lone, N.; Babar, S.; Sultan, S.; Malik, S.; Nazeer, K.; Riaz, S. Association of the CYP17 and CYP19 gene polymorphisms in women with polycystic ovary syndrome from Punjab, Pakistan. Gynecol. Endocrinol. 2021, 37, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R.A.; Al-Sherbeeny, M.M.; Abdelazim, I.A.; Fahmy, A.A.; Farghali, M.M.; Abdel-Fatah, M.A.; Mahran, M.Z. Relation between aromatase gene CYP19 variation and hyperandrogenism in Polycystic Ovary Syndrome Egyptian women. J. Infertil. Reprod. Biol. 2016, 4, 1–5. [Google Scholar]
- MacKenzie, S.M.; Davies, E.; Alvarez-Madrazo, S. Analysis of the Aldosterone Synthase (CYP11B2) and 11beta-Hydroxylase (CYP11B1) Genes. Methods Mol. Biol. 2017, 1527, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Sahakitrungruang, T.; Tee, M.K.; Blackett, P.R.; Miller, W.L. Partial defect in the cholesterol side-chain cleavage enzyme P450scc (CYP11A1) resembling nonclassic congenital lipoid adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2011, 96, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H. CYP21 mutations and congenital adrenal hyperplasia. Clin. Genet. 2001, 59, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Fatunde, O.A.; Brown, S.A. The Role of CYP450 Drug Metabolism in Precision Cardio-Oncology. Int. J. Mol. Sci. 2020, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Wong, R.; Fraysse, J.; Shreay, S.; Li, S.; Harrison, S.; Gordon, S.C. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: A real world analysis of Medicare data. Aliment. Pharmacol. Ther. 2020, 51, 1149–1159. [Google Scholar] [CrossRef]
- Jamwal, R.; de la Monte, S.M.; Ogasawara, K.; Adusumalli, S.; Barlock, B.B.; Akhlaghi, F. Nonalcoholic Fatty Liver Disease and Diabetes Are Associated with Decreased CYP3A4 Protein Expression and Activity in Human Liver. Mol. Pharm. 2018, 15, 2621–2632. [Google Scholar] [CrossRef]
- Woolsey, S.J.; Mansell, S.E.; Kim, R.B.; Tirona, R.G.; Beaton, M.D. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab. Dispos. 2015, 43, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.D.; Novak, P.; Fisher, C.D.; Jackson, J.P.; Hardwick, R.N.; Billheimer, D.D.; Klimecki, W.T.; Cherrington, N.J. Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab. Dispos. 2011, 39, 1954–1960. [Google Scholar] [CrossRef]
- Powell, N.R.; Liang, T.; Ipe, J.; Cao, S.; Skaar, T.C.; Desta, Z.; Qian, H.R.; Ebert, P.J.; Chen, Y.; Thomas, M.K.; et al. Clinically important alterations in pharmacogenetic expression in histologically severe nonalcoholic fatty liver disease. Nat. Commun. 2023, 14, 1474. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Jian, T.; Wu, Y.; Ding, X.; Lv, H.; Ma, L.; Zuo, Y.; Ren, B.; Zhao, L.; Tong, B.; Chen, J.; et al. A novel sesquiterpene glycoside from Loquat leaf alleviates oleic acid-induced steatosis and oxidative stress in HepG2 cells. Biomed. Pharmacother. 2018, 97, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Tian, X.; Zhu, L.; Wang, H.; Sun, C. USP14 governs CYP2E1 to promote nonalcoholic fatty liver disease through deubiquitination and stabilization of HSP90AA1. Cell Death Dis. 2023, 14, 566. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tan, W.; Liu, X.; Deng, L.; Huang, L.; Wang, X.; Gao, X. New insight and potential therapy for NAFLD: CYP2E1 and flavonoids. Biomed. Pharmacother. 2021, 137, 111326. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; John, A. In vitro protection of reactive oxygen species-induced degradation of lipids, proteins and 2-deoxyribose by tea catechins. Food Chem. Toxicol. 2007, 45, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Q.; Wu, T.; Liu, K.; Xiao, Y.; Liao, Q.; Qi, X.; Li, Y.; Zhou, L. 5-Methoxyflavone ameliorates non-alcoholic fatty liver disease through targeting the cytochrome P450 1A1. Free Radic. Biol. Med. 2023, 195, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Baumgartner, K.; Bositis, C. Cirrhosis: Diagnosis and Management. Am. Fam. Physician 2019, 100, 759–770. [Google Scholar] [PubMed]
- Ye, F.; Zhai, M.; Long, J.; Gong, Y.; Ren, C.; Zhang, D.; Lin, X.; Liu, S. The burden of liver cirrhosis in mortality: Results from the global burden of disease study. Front. Public. Health 2022, 10, 909455. [Google Scholar] [CrossRef]
- Lourdes, R.-F.; Anahí, R.-L.; Janet, S.-Q. Models of Hepatotoxicity for the Study of Chronic Liver Disease. In Animal Models and Experimental Research in Medicine; Mahmut, K., Volkan, G., Abdulsamed, K., Eds.; IntechOpen: Rijeka, Croatia, 2022; p. 162. [Google Scholar]
- Akhtar, T.; Sheikh, N. An overview of thioacetamide-induced hepatotoxicity. Toxin Rev. 2013, 32, 43–46. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, G.; Wang, H.; Yao, X.; Jiang, S.; Kang, A.; Zhou, F.; Xie, T.; Hao, H. Cytochrome P450 dysregulations in thioacetamide-induced liver cirrhosis in rats and the counteracting effects of hepatoprotective agents. Drug Metab. Dispos. 2012, 40, 796–802. [Google Scholar] [CrossRef]
- Bastien, M.C.; Leblond, F.; Pichette, V.; Villeneuve, J.P. Differential alteration of cytochrome P450 isoenzymes in two experimental models of cirrhosis. Can. J. Physiol. Pharmacol. 2000, 78, 912–919. [Google Scholar] [CrossRef]
- Chandrashekar, D.V.; DuBois, B.N.; Rashid, M.; Mehvar, R. Effects of chronic cirrhosis induced by intraperitoneal thioacetamide injection on the protein content and Michaelis-Menten kinetics of cytochrome P450 enzymes in the rat liver microsomes. Basic. Clin. Pharmacol. Toxicol. 2023, 132, 197–210. [Google Scholar] [CrossRef]
- Frye, R.F.; Zgheib, N.K.; Matzke, G.R.; Chaves-Gnecco, D.; Rabinovitz, M.; Shaikh, O.S.; Branch, R.A. Liver disease selectively modulates cytochrome P450-mediated metabolism. Clin. Pharmacol. Ther. 2006, 80, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Duthaler, U.; Bachmann, F.; Suenderhauf, C.; Grandinetti, T.; Pfefferkorn, F.; Haschke, M.; Hruz, P.; Bouitbir, J.; Krahenbuhl, S. Liver Cirrhosis Affects the Pharmacokinetics of the Six Substrates of the Basel Phenotyping Cocktail Differently. Clin. Pharmacokinet. 2022, 61, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Burim, R.V.; Canalle, R.; Martinelli Ade, L.; Takahashi, C.S. Polymorphisms in glutathione S-transferases GSTM1, GSTT1 and GSTP1 and cytochromes P450 CYP2E1 and CYP1A1 and susceptibility to cirrhosis or pancreatitis in alcoholics. Mutagenesis 2004, 19, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hashem, E.M.; El-Emshaty, W.M.; Farag Rel, S.; Zakaria, S.; Abd El-Aziz, M.; Ghonaim, A. Genetic Polymorphisms of Cytochrome P4501A1 (CYP1A1) and Glutathione S-Transferase P1 (GSTP1) and Risk of Hepatocellular Carcinoma Among Chronic Hepatitis C Patients in Egypt. Biochem. Genet. 2016, 54, 696–713. [Google Scholar] [CrossRef] [PubMed]
- Stipp, M.C.; Acco, A. Involvement of cytochrome P450 enzymes in inflammation and cancer: A review. Cancer Chemother. Pharmacol. 2021, 87, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Bozina, N.; Bradamante, V.; Lovric, M. Genetic polymorphism of metabolic enzymes P450 (CYP) as a susceptibility factor for drug response, toxicity, and cancer risk. Arh. Hig. Rada Toksikol. 2009, 60, 217–242. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P. The Multifarious Link between Cytochrome P450s and Cancer. Oxid. Med. Cell Longev. 2020, 2020, 3028387. [Google Scholar] [CrossRef]
- Oyama, T.; Sugio, K.; Uramoto, H.; Kawamoto, T.; Kagawa, N.; Nadaf, S.; Carbone, D.; Yasumoto, K. Cytochrome P450 expression (CYP) in non-small cell lung cancer. Front. Biosci. 2007, 12, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Moriya, N.; Kataoka, H.; Fujino, H.; Nishikawa, J.; Kugawa, F. Effect of lipopolysaccharide on the xenobiotic-induced expression and activity of hepatic cytochrome P450 in mice. Biol. Pharm. Bull. 2012, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Feng, S. Role of Metabolic Enzymes P450 (CYP) on Activating Procarcinogen and their Polymorphisms on the Risk of Cancers. Curr. Drug Metab. 2015, 16, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K. Polymorphic Variants of Cytochrome P450: Relevance to Cancer and Other Diseases. Adv. Pharmacol. 2015, 74, 85–111. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and Roles in Cancer, Diabetes and Atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Seredina, T.A.; Goreva, O.B.; Talaban, V.O.; Grishanova, A.Y.; Lyakhovich, V.V. Association of cytochrome P450 genetic polymorphisms with neoadjuvant chemotherapy efficacy in breast cancer patients. BMC Med. Genet. 2012, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.T.; Lee, J.M.; Wu, D.C.; Ho, C.K.; Wang, Y.T.; Lee, Y.C.; Hsu, H.K.; Kao, E.L. Genetic polymorphisms of cytochrome P4501A1 and oesophageal squamous-cell carcinoma in Taiwan. Br. J. Cancer 2002, 87, 529–532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, F.F.; Lu, S.S.; Hu, C.Y.; Qian, Z.Z.; Feng, F.; Wu, Y.L.; Yang, H.Y.; Sun, Y.H. Cytochrome P450 1A1 (CYP1A1) polymorphism and susceptibility to esophageal cancer: An updated meta-analysis of 27 studies. Tumour Biol. 2014, 35, 10351–10361. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, Q.; Qian, Q.; Wei, S.Z.; Yu, L.K. CYP1A1 MspI and exon7 gene polymorphisms and lung cancer risk: An updated meta-analysis and review. J. Exp. Clin. Cancer Res. 2011, 30, 99. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, Z.; Xiao, Z.; Li, S.; Ma, Q. The cytochrome P4501A1 gene polymorphisms and endometriosis: A meta-analysis. J. Assist. Reprod. Genet. 2016, 33, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Z.; He, J.; Wang, W.; Xue, W.; Wang, Y.; Zheng, L.; Zhu, M.L. Associations between CYP1A1 rs1048943 A > G and rs4646903 T > C genetic variations and colorectal cancer risk: Proof from 26 case-control studies. Oncotarget 2016, 7, 51365–51374. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.F.; Mai, C.W.; Ng, P.Y.; Leong, C.O. Cytochrome P450 2W1 (CYP2W1) in Colorectal Cancers. Curr. Cancer Drug Targets 2016, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Go, R.E.; Hwang, K.A.; Choi, K.C. Cytochrome P450 1 family and cancers. J. Steroid Biochem. Mol. Biol. 2015, 147, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, K.C.; Zeka, K.; Ijaz, T.; Ankrett, D.N.; Wilsher, N.E.; Butler, P.C.; Tan, H.L.; Lodhi, S.; Bhambra, A.S.; Potter, G.A.; et al. The Synthesis of Chalcones as Anticancer Prodrugs and their Bioactivation in CYP1 Expressing Breast Cancer Cells. Med. Chem. 2018, 14, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Yan, D.; Yan, H.; Yuan, J. Cytochrome P450: Implications for human breast cancer. Oncol. Lett. 2021, 22, 548. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Edson, K.Z.; Totah, R.A.; Rettie, A.E. Cytochrome P450 omega-Hydroxylases in Inflammation and Cancer. Adv. Pharmacol. 2015, 74, 223–262. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, Y.B.; Lee, S.J. Molecular Functionality of Cytochrome P450 4 (CYP4) Genetic Polymorphisms and Their Clinical Implications. Int. J. Mol. Sci. 2019, 20, 4274. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Alshagga, M.; Ong, C.E.; Chieng, J.Y.; Pan, Y. Cytochrome P450 4B1 (CYP4B1) as a target in cancer treatment. Hum. Exp. Toxicol. 2020, 39, 785–796. [Google Scholar] [CrossRef]
- Roos, P.H.; Bolt, H.M. Cytochrome P450 interactions in human cancers: New aspects considering CYP1B1. Expert. Opin. Drug Metab. Toxicol. 2005, 1, 187–202. [Google Scholar] [CrossRef]
- Horley, N.J.; Beresford, K.J.; Chawla, T.; McCann, G.J.; Ruparelia, K.C.; Gatchie, L.; Sonawane, V.R.; Williams, I.S.; Tan, H.L.; Joshi, P.; et al. Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur. J. Med. Chem. 2017, 129, 159–174. [Google Scholar] [CrossRef]
- Singh, M.S.; Francis, P.A.; Michael, M. Tamoxifen, cytochrome P450 genes and breast cancer clinical outcomes. Breast 2011, 20, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, B.; Faull, K.F.; Janzen, C.; Mascharak, P.K. Carbon Monoxide Inhibits Cytochrome P450 Enzymes CYP3A4/2C8 in Human Breast Cancer Cells, Increasing Sensitivity to Paclitaxel. J. Med. Chem. 2021, 64, 8437–8446. [Google Scholar] [CrossRef] [PubMed]
- Brueggemeier, R.W.; Richards, J.A.; Joomprabutra, S.; Bhat, A.S.; Whetstone, J.L. Molecular pharmacology of aromatase and its regulation by endogenous and exogenous agents. J. Steroid Biochem. Mol. Biol. 2001, 79, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Barros-Oliveira, M.D.C.; Costa-Silva, D.R.; Dos Santos, A.R.; Pereira, R.O.; Soares-Junior, J.M.; Silva, B.B.D. Influence of CYP19A1 gene expression levels in women with breast cancer: A systematic review of the literature. Clinics 2021, 76, e2846. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, S.M.; Shu, X.O.; Gao, Y.T.; Cai, Q.; Jin, F.; Zheng, W. Polymorphisms in CYP1A1 and breast carcinoma risk in a population-based case-control study of Chinese women. Cancer 2005, 103, 2228–2235. [Google Scholar] [CrossRef]
- Boyapati, S.M.; Shu, X.O.; Gao, Y.T.; Dai, Q.; Yu, H.; Cheng, J.R.; Jin, F.; Zheng, W. Correlation of blood sex steroid hormones with body size, body fat distribution, and other known risk factors for breast cancer in post-menopausal Chinese women. Cancer Causes Control. 2004, 15, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Al-Eitan, L.N.; Rababa’h, D.M.; Alghamdi, M.A.; Khasawneh, R.H. Association of CYP gene polymorphisms with breast cancer risk and prognostic factors in the Jordanian population. BMC Med. Genet. 2019, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Mu, Y.Q.; Fu, X.M.; Li, S.M.; Zhao, F.X. Association of CYP1B1 gene polymorphisms and the positive expression of estrogen alpha and estrogen beta with endometrial cancer risk. Eur. J. Gynaecol. Oncol. 2011, 32, 188–191. [Google Scholar] [PubMed]
- Androutsopoulos, V.P.; Spyrou, I.; Ploumidis, A.; Papalampros, A.E.; Kyriakakis, M.; Delakas, D.; Spandidos, D.A.; Tsatsakis, A.M. Expression profile of CYP1A1 and CYP1B1 enzymes in colon and bladder tumors. PLoS ONE 2013, 8, e82487. [Google Scholar] [CrossRef]
- Spyrou, I.; Sifakis, S.; Ploumidis, A.; Papalampros, A.E.; Felekouras, E.; Tsatsakis, A.M.; Spandidos, D.A.; Androutsopoulos, V.P. Expression profile of CYP1A1 and CYP1B1 enzymes in endometrial tumors. Tumour Biol. 2014, 35, 9549–9556. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Sharma, T.; Gupta, S.; Banerjee, B.D. CYP1A1 expression and organochlorine pesticides level in the etiology of bladder cancer in North Indian population. Hum. Exp. Toxicol. 2018, 37, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, W.; Shu, J.; Wang, D. Association of CYP1A1 and CYP1B1 Gene Polymorphisms with TypeIEndometrial Cancer in Chinese Women. Cancer Res. Prev. Treat. 2018, 45, 15–18. [Google Scholar] [CrossRef]
- Mrozikiewicz, P.M.; Grześkowiak, E.; Seremak-Mrozikiewicz, A.; Bogacz, A.; Barlik, M.; Semczuk, A.; Bartkowiak-Wieczorek, J.; Drews, K. Importance of CYP1A1 polymorphism and its transcriptional regulation in ovarian and endometrial cancer. Ginekol. Pol. 2011, 82, 925–932. [Google Scholar] [PubMed]
- Sergentanis, T.N.; Economopoulos, K.P.; Choussein, S.; Vlahos, N.F. Cytochrome P450 1A1 gene polymorphisms and endometrial cancer risk: A meta-analysis. Int. J. Gynecol. Cancer 2011, 21, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Economopoulos, K.P.; Choussein, S.; Vlahos, N.F. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and ovarian cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 9921–9930. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Economopoulos, K.P.; Choussein, S.; Vlahos, N.F. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and cervical cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 6647–6654. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wen, Q.; Li, S.F.; Zhang, Y.F.; Gao, N.; Tian, X.; Fang, Y.; Gao, J.; Cui, M.Z.; He, X.P.; et al. Significant change of cytochrome P450s activities in patients with hepatocellular carcinoma. Oncotarget 2016, 7, 50612–50623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Yan, L.N.; Dong, C.N.; Ma, N.; Yuan, M.N.; Zhou, J.; Gao, P. Cytochrome P450 family members are associated with fast-growing hepatocellular carcinoma and patient survival: An integrated analysis of gene expression profiles. Saudi J. Gastroenterol. 2019, 25, 167–175. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Z.Y.; Xu, W.; Fan, T.Y.; Li, J.; Lu, Y.F.; Cheng, M.L.; Liu, J. Expression of P450 and nuclear receptors in normal and end-stage Chinese livers. World J. Gastroenterol. 2014, 20, 8681–8690. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, G.G.; Lai, P.B.S. Targeting hepatocyte growth factor/c-mesenchymal-epithelial transition factor axis in hepatocellular carcinoma: Rationale and therapeutic strategies. Med. Res. Rev. 2021, 41, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xia, X.; Dong, Y.; Gong, Z.; Li, G.; Chen, G.G.; Lai, P.B.S. CYP1A2 suppresses hepatocellular carcinoma through antagonizing HGF/MET signaling. Theranostics 2021, 11, 2123–2136. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, X.; Feng, J.; Mao, J.; Zhang, Q.; He, M.; Mi, Y.; Mei, Y.; Jin, G.; Zhang, H. CYP2E1 plays a suppressive role in hepatocellular carcinoma by regulating Wnt/Dvl2/beta-catenin signaling. J. Transl. Med. 2022, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152–4163. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, N.; Gong, Z.; Liu, L.; Yang, S.; Chen, G.G.; Lai, P.B.S. Cytochrome P450 1A2 overcomes nuclear factor kappa B-mediated sorafenib resistance in hepatocellular carcinoma. Oncogene 2021, 40, 492–507. [Google Scholar] [CrossRef]
- Alexanian, A.; Sorokin, A. Targeting 20-HETE producing enzymes in cancer—Rationale, pharmacology, and clinical potential. Onco Targets Ther. 2013, 6, 243–255. [Google Scholar] [CrossRef]
- Eun, H.S.; Cho, S.Y.; Lee, B.S.; Seong, I.O.; Kim, K.H. Profiling cytochrome P450 family 4 gene expression in human hepatocellular carcinoma. Mol. Med. Rep. 2018, 18, 4865–4876. [Google Scholar] [CrossRef]
- Eun, H.S.; Cho, S.Y.; Lee, B.S.; Kim, S.; Song, I.S.; Chun, K.; Oh, C.H.; Yeo, M.K.; Kim, S.H.; Kim, K.H. Cytochrome P450 4A11 expression in tumor cells: A favorable prognostic factor for hepatocellular carcinoma patients. J. Gastroenterol. Hepatol. 2019, 34, 224–233. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Nakamura, H.; Ohara, N.; Naito, Y. Eicosanoids Derived from Arachidonic Acid and Their Family Prostaglandins and Cyclooxygenase in Psychiatric Disorders. Curr. Neuropharmacol. 2015, 13, 776–785. [Google Scholar] [CrossRef]
- Wang, K.; Shi, J.H.; Gao, J.; Sun, Y.; Wang, Z.; Shi, X.; Guo, W.; Jin, Y.; Zhang, S. Arachidonic acid metabolism CYP450 pathway is deregulated in hepatocellular carcinoma and associated with microvascular invasion. Cell Biol. Int. 2024, 48, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.V.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Metabolism and Excretion Prediction: Recent Advances, Challenges, and Future Perspectives. Pharmaceutics 2023, 15, 1260. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.F. Artificial Intelligence in Drug Safety and Metabolism. Methods Mol. Biol. 2022, 2390, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Cai, H.; Wei, J.; Zhao, D.; Chen, Y.; Wang, L. DEEPCYPs: A deep learning platform for enhanced cytochrome P450 activity prediction. Front. Pharmacol. 2023, 14, 1099093. [Google Scholar] [CrossRef] [PubMed]
| CYP Family | Main Function | Subfamily | Substrate/Endogenous Compound |
|---|---|---|---|
| CYP1 | Biotransformation | CYP1A1 | Granisetron and riociguat |
| CYP1A2 | Polycyclic aromatic hydrocarbons, caffeine, tizanidine, clozapine, olanzapine, theophylline, alosetron, duloxetine, melatonin, pirfenidone, ramelteon, tasimelteon, acetaminophen, antipyrine, bufuralol, ondansetron, phenacetin, and tacrine | ||
| CYP2 | Biotransformation | CYP2A6 | Nicotine, coumarin, and valproic acid |
| CYP2B6 | Efavirenz | ||
| CYP2C8 | Metabolizes over 60 clinically relevant drugs, including montelukast, pioglitazone, repaglinide, and rosiglitazone | ||
| CYP2C9 | Warfarin, carvedilol, celecoxib, glipizide, glimepiride, ibuprofen, irbesartan, losartan, phenytoin, and tolbutamide | ||
| CYP2C19 | Omeprazole, lansoprazole, and phenobarbital | ||
| CYP2D6 | Antidepressants, antipsychotics, beta-blockers, antiretroviral agents, antiarrhythmics, morphine derivatives, and Tamoxifen | ||
| CYP2E1 | Ethanol, acetaminophen, theophylline, and verapamil metabolism | ||
| CYP3 | Biotransformation | CYP3A4 | Alprazolam, amlodipine, buspirone, calcium channel blockers, caffeine, citalopram, clopidogrel, cocaine, cyclosporine, diazepam, erythromycin, drugs, montelukast, quetiapine, sertraline, sildenafil, statin drugs, tacrolimus, warfarin, zolpidem, estradiol, lidocaine, losartan, and many chemotherapeutic agents |
| CYP4 | Fatty acid metabolism | CYP4A | Arachidonic acid (AA) |
| CYP5 | Thromboxane A2 synthesis | - | |
| CYP7 | Bile acid biosynthesis | CYP7A1 | Converts cholesterol to 7α-hydroxycholesterol |
| CYP7B1 | Metabolizes dehydroepiandrosterone | ||
| CYP8 | Bile acid and prostacyclin biosynthesis | CYP8A1 | Converts prostaglandin H2 to thromboxane A |
| CYP11 | Steroid biosynthesis | CYP11A1 | Converts cholesterol to pregnenolone plus 4-methylpentanal |
| CYP17 | Steroid biosynthesis | CYP17A1 | Converts corticosterone to cortisol |
| CYP19 | Steroid biosynthesis | CYP19A1 | Metabolizes androstenedione and testosterone |
| CYP20 | Unknown function | - | |
| CYP21 | Steroid biosynthesis | CYP21A2 | Converts progesterone to deoxycortisone in pregnancy |
| CYP24 | Vitamin D degeneration | CYP24A1 | Converts 1,25-dihydroxyvitamin D3 (calcitriol) to 1α,24R,25-trihydroxyvitamin D3 |
| CYP26 | Retinoic acid hydroxylation | CYP26A1, CYP26B1, and CYP26C1 | Convert retinoic acid to 4-hydroxyretinoic acid |
| CYP27 | Vitamin D3 and bile acid biosynthesis | CYP27C1 | Converts retinol (vitamin A1) to 3,4-didehydroretinol (vitamin A2) |
| CYP39 | Cholesterol synthesis | CYP39A1 | Converts 24-hydroxycholesterol to 7α,24-dihydroxycholesterol |
| CYP46 | Cholesterol synthesis | CYP46A1 | Converts cholesterol to 24(S)-hydroxycholesterol |
| CYP51 | Cholesterol synthesis | CYP51A1 | Lanosterol |
| CYP450 Enzyme | Origin | Role |
|---|---|---|
| CYP11A1 | Mitochondria | Cholesterol side-chain cleavage enzyme- desmolase |
| CYP17A1 | sER (smooth endoplasmic reticulum) | 17 alpha-hydroxylase or 17,20-lyase |
| CYP21A2 | sER | 21-hydroxylase |
| CYP11B2 | Mitochondria | 11beta-hydroxylase |
| CYP18B2/18-HSD | Mitochondria | Aldosterone synthase |
| CYP19A1 | sER | Aromatase |
| CYP450 Gene | Variants | Impact |
|---|---|---|
| CYP19A1 | rs7176005 rs6493497 | Significantly associated with BC and creates a variable response to aromatase inhibitors at the initial stages of BC. |
| rs700519 | Associated with BC at menopause. Significantly associated with age at BC diagnosis and lymph node involvement. | |
| rs10046 rs4646 | The rs4646 conferred a beneficial effect in increasing metastatic time in BC patients. | |
| CYP2C19 | rs4244285 | Significantly associated with HER2 |
| CYP1A2 | CC genotype of rs762551 | Protective factor against progression and development. Significantly associated with age menopause, HER2, histology classification, and lymph involvement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossam Abdelmonem, B.; Abdelaal, N.M.; Anwer, E.K.E.; Rashwan, A.A.; Hussein, M.A.; Ahmed, Y.F.; Khashana, R.; Hanna, M.M.; Abdelnaser, A. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines 2024, 12, 1467. https://doi.org/10.3390/biomedicines12071467
Hossam Abdelmonem B, Abdelaal NM, Anwer EKE, Rashwan AA, Hussein MA, Ahmed YF, Khashana R, Hanna MM, Abdelnaser A. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines. 2024; 12(7):1467. https://doi.org/10.3390/biomedicines12071467
Chicago/Turabian StyleHossam Abdelmonem, Basma, Noha M. Abdelaal, Eman K. E. Anwer, Alaa A. Rashwan, Mohamed Ali Hussein, Yasmin F. Ahmed, Rana Khashana, Mireille M. Hanna, and Anwar Abdelnaser. 2024. "Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review" Biomedicines 12, no. 7: 1467. https://doi.org/10.3390/biomedicines12071467
APA StyleHossam Abdelmonem, B., Abdelaal, N. M., Anwer, E. K. E., Rashwan, A. A., Hussein, M. A., Ahmed, Y. F., Khashana, R., Hanna, M. M., & Abdelnaser, A. (2024). Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines, 12(7), 1467. https://doi.org/10.3390/biomedicines12071467






