Three-Dimensional Speckle-Tracking Echocardiography-Derived Tricuspid Annular Properties in Acromegaly—Results from the MAGYAR-Path Study
Abstract
1. Introduction
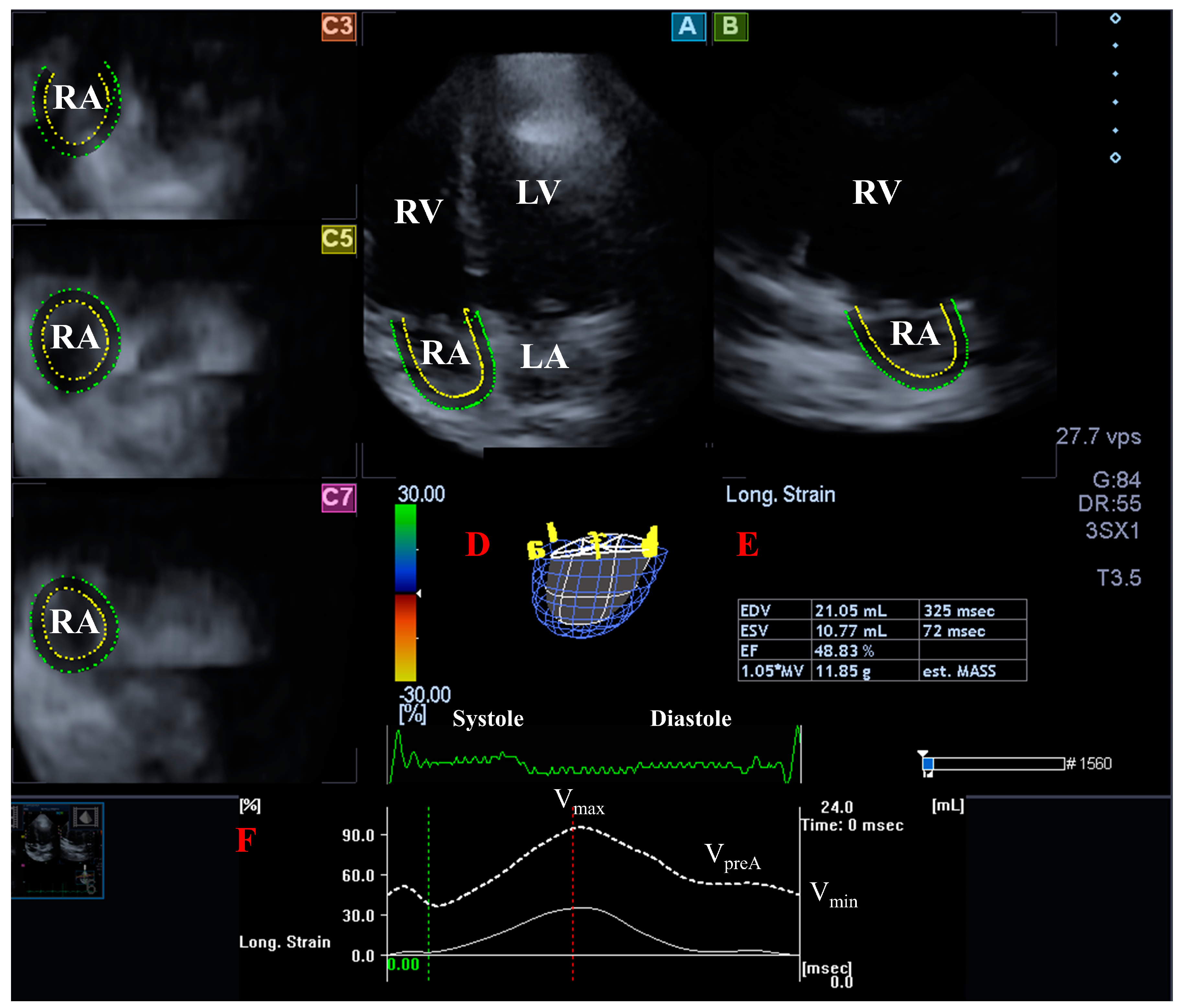
2. Materials and Methods
- TA diameter (TAD) was defined as the perpendicular line drawn from the peak of TA curvature to the opposite side of the TA border;
- TA area (TAA) was assessed by planimetry;
- TA perimeter (TAP) was evaluated by planimetry;
- TA fractional shortening (TAFS) = [end-diastolic TAD − end-systolic TAD]/end-diastolic TAD × 100;
- TA fractional area change (TAFAC) = [end-diastolic TAA − end-systolic TAA]/end-diastolic TAA × 100.
3. Results
4. Discussion
Limitation Section
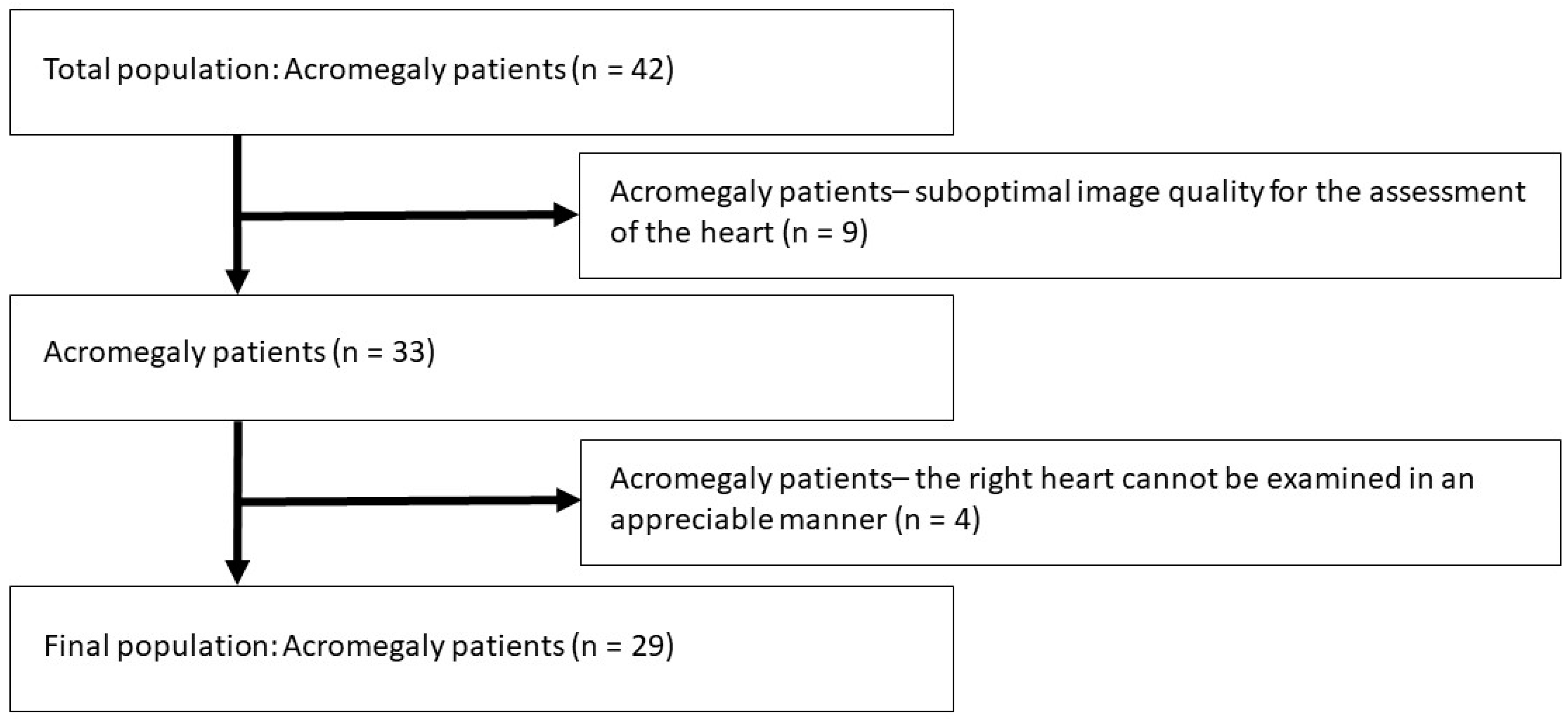
- A relatively low number of patients with acromegaly were involved in the present study due to the rare nature of the disease. In Hungary with a population of almost 10 million inhabitants, appr. 300 patients with acromegaly are alive at the same time. The total population of acromegaly patients involved in the present study was 42, from which several subjects had to be excluded due to inferior image quality. The remaining group consisted of 29 acromegaly patients, as mentioned in the text. That means that more than 14% of the total Hungarian acromegaly population has been involved. All the patients were recruited from a tertiary endocrine center responsible for treatment, care and management of such endocrine disorders like acromegaly. At the time of examinations, the involvement of more patients was not possible due to the absence of more subjects.
- During 3DSTE, no real 3D analysis was performed evaluating the saddle shape of TA. Only its 2D-projected evaluation was performed in selected 2D planes [25].
- Although validation of 3DSTE-derived TA parameters was not purposed, inter- and intra-observer variability of data for TA parameters have been given.
- Age and classic cardiovascular risk factors, including hypertension, diabetes mellitus, and hypercholesterolemia, were more frequent in acromegaly patients as compared with controls, which could influence findings.
- Abnormalities in transmitral flow velocities could be partly explained by the presence of acromegaly-related hypertension.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstenin, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Sanno, N.; Teramoto, A.; Osamura, R.Y.; Horvath, E.; Kovacs, K.; Lloyd, R.V.; Scheithauser, B.W. Pathology of pituitary tumors. Neurosurg. Clin. N. Am. 2003, 14, 25–39. [Google Scholar] [CrossRef]
- Clayton, R.N. Cardiovascular function in acromegaly. Endocr. Rev. 2003, 24, 272–277. [Google Scholar] [CrossRef]
- Ramos-Levi, A.M.; Marazuela, M. Cardiovascular comorbidities in acromegaly: An update on their diagnosis and management. Endocrine 2017, 55, 346–359. [Google Scholar] [CrossRef]
- Popielarz-Grygalewicz, A.; Gąsior, J.S.; Konwicka, A.; Grygalewicz, P.; Stelmachowska-Banaś, M.; Zgliczyński, W.; Dąbrowski, M. Heart in acromegaly: The echocardiographic characteristics of patients diagnosed with acromegaly in various stages of the disease. Int. J. Endocrinol. 2018, 2018, 6935054. [Google Scholar] [CrossRef]
- Uziȩbło-Życzkowska, B.; Jurek, A.; Witek, P.; Zieliński, G.; Gielerak, G.; Krzesiński, P. Left Heart Dysfunction in Acromegaly Revealed by Novel Echocardiographic Methods. Front. Endocrinol. (Lausanne) 2020, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Koca, H.; Koc, M.; Sumbul, H.E.; Icen, Y.K.; Gulumsek, E.; Koca, F.; Ozturk, H.A.; Baykan, A.O.; Kaypakli, O. Subclinical Left Atrial and Ventricular Dysfunction in Acromegaly Patients: A Speckle Tracking Echocardiography Study. Arq. Bras. Cardiol. 2022, 118, 634–645. [Google Scholar]
- Nemes, A.; Kormányos, Á.; Ambrus, N.; Lengyel, C.; Valkusz, Z. Myocardial, valvular and vascular structural and functional properties in acromegaly. J. Clin. Med. 2023, 12, 6857. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Lengyel, C.; Ambrus, N.; Valkusz, Z. Mitral annulus is dilated with preserved function in acromegaly regardless of its activity: Insights from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Rev. Port. Cardiol. (Engl. Ed.) 2021, 40, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Csanady, M.; Gáspár, L.; Hogye, M.; Gruber, N. The heart in acromegaly: An echocardiographic study. Int. J. Cardiol. 1983, 2, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Bihan, H.; Espinosa, C.; Valdes-Socin, H.; Salenave, S.; Young, J.; Levasseur, S.; Assayag, P.; Beckers, A.; Chanson, P. Long-term outcome of patients with acromegaly and congestive heart failure. J. Clin. Endocrinol. Metab. 2004, 89, 5308–5313. [Google Scholar] [CrossRef]
- Goldberg, M.D.; Vadera, N.; Yandrapalli, S.; Frishman, W.H. Acromegalic Cardiomyopathy: An Overview of Risk Factors, Clinical Manifestations, and Therapeutic Options. Cardiol. Rev. 2018, 26, 307–311. [Google Scholar] [CrossRef]
- Pirhan, O.; Ertuğrul, A.S.; Yıldız, C.; Karabulut, D.; Pehlivan, B.; Piskinpasa, H.; Dogansen, S.C.; Mert, M. Assessment of right ventricular functions in acromegaly: Comparison of active disease with remission. Kardiologiia 2022, 62, 52–58. [Google Scholar] [CrossRef]
- Pereira, A.M.; van Thiel, S.W.; Lindner, J.R.; Roelfsema, F.; van der Wall, E.E.; Morreau, H.; Smit, J.W.A.; Romijn, J.A.; Bax, J.J. Increased prevalence of regurgitant valvular heart disease in acromegaly. J. Clin. Endocrinol. Metab. 2004, 89, 71–75. [Google Scholar] [CrossRef]
- Ogedegbe, O.J.; Cheema, A.Y.; Khan, M.A.; Junaid, S.Z.S.; Erebo, J.K.; Ayirebi-Acquah, E.; Okpara, J.; Bofah, D.; Okon, J.G.; Munir, M.; et al. A Comprehensive Review of Four Clinical Practice Guidelines of Acromegaly. Cureus 2022, 14, e28722. [Google Scholar] [CrossRef]
- Melmed, S. Medical progress: Acromegaly. N. Engl. J. Med. 2006, 355, 2558–2573. [Google Scholar] [CrossRef]
- Vilar, L.; Vilar, C.F.; Lyra, R.; Lyra, R.; Naves, L.A. Acromegaly: Clinical features at diagnosis. Pituitary 2017, 20, 22–32. [Google Scholar] [CrossRef]
- Nemes, A.; Forster, T. Recent echocardiographic examination of the left ventricle–from M-mode to 3D speckle-tracking imaging. Orv. Hetil. 2015, 156, 1723–1740. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Ambrus, N.; Lengyel, C. Normal reference values of three-dimensional speckle-tracking echocardiography-derived right atrial volumes and volume-based functional properties in healthy adults (Insights from the MAGYAR-Healthy Study). J. Clin. Ultrasound 2020, 48, 263–268. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Rácz, G.; Ruzsa, Z.; Ambrus, N.; Lengyel, C. Normal reference values of tricuspid annular dimensions and functional properties in healthy adults using three-dimensional speckle-tracking echocardiography (insights from the MAGYAR-Healthy Study). Quant. Imaging Med. Surg. 2023, 13, 121–132. [Google Scholar] [CrossRef]
- Colao, A.; Marzullo, P.; Di Somma, C.; Lombardi, G. Growth hormone and the heart. Clin. Endocrinol. 2001, 54, 137–154. [Google Scholar] [CrossRef]
- Bogazzi, F.; Lombardi, M.; Strata, E.; Auqaro, G.; Di Bello, V.; Cosci, C.; Sardella, C.; Talini, E.; Martino, E. High prevalence of cardiac hypertrophy without detectable signs of fibrosis in patients with untreated active acromegaly: An in vivo study using magnetic resonance imaging. Clin. Endocrinol. 2008, 68, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sacca, L.; Napoli, R.; Cittadini, A. Growth hormone, acromegaly, and heart failure: An intricate triangulation. Clin. Endocrinol. (Oxf.) 2003, 59, 660–671. [Google Scholar] [CrossRef]
- Dahou, A.; Levin, D.; Reisman, M.; Hahn, R.T. Anatomy and physiology of the tricuspid valve. JACC Cardiovasc. Imaging 2019, 12, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Addetia, K.; Muraru, D.; Veronesi, F.; Jenei, C.; Cavalli, G.; Besser, S.A.; Mor-Avi, V.; Lang, R.M.; Badano, L.P. 3-Dimensional Echocardiographic Analysis of the Tricuspid Annulus Provides New Insights Into Tricuspid Valve Geometry and Dynamics. JACC Cardiovasc. Imaging 2019, 12, 401–412. [Google Scholar] [CrossRef]
- Badano, L.P.; Muraru, D.; Enriquez-Sarano, M. Assessment of functional tricuspid regurgitation. Eur. Heart J. 2013, 34, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Addetia, K.; Guta, A.C.; Ochoa-Jimenez, R.C.; Genovese, D.; Veronesi, F.; Basso, C.; Iliceto, S.; Badano, L.P.; Lang, R.M. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: A three-dimensional echocardiography study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Kormányos, Á.; Rácz, G.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Tricuspid annular and right atrial volume changes are associated in healthy adults–insights from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Front. Cardiovasc. Med. 2023, 10, 1140599. [Google Scholar] [CrossRef] [PubMed]

| Controls | All Acromegaly Patients | p vs. Controls | Active Acromegalic Patients | p vs. Controls | Inactive Acromegalic Patients | p vs. Controls | p vs. Active Acromegaly | |
|---|---|---|---|---|---|---|---|---|
| (n = 57) | (n = 29) | (n = 13) | (n = 16) | |||||
| Clinical and demographic data | ||||||||
| Age (years) | 53.2 ± 8.4 | 55.9 ± 14.5 | 0.0001 | 61.3 ± 11.1 | 0.03 | 51.4 ± 15.8 | 0.55 | 0.07 |
| Male sex (%) | 38 (67) | 21 (72) | 0.63 | 9 (69) | 0.52 | 12 (75) | 0.76 | 1.00 |
| Hypertension (%) | 0 (0) | 18 (62) | 0.0001 | 9 (69) | 0.0001 | 9 (56) | 0.0001 | 0.70 |
| Diabetes mellitus (%) | 0 (0) | 6 (21) | 0.001 | 4 (31) | 0.0008 | 2 (13) | 0.05 | 0.38 |
| Hypercholesterolemia (%) | 0 (0) | 14 (48) | 0.0001 | 7 (54) | 0.0001 | 7 (44) | 0.0001 | 0.71 |
| Laboratory findings | ||||||||
| Serum hGH (ng/mL) | - | 4.49 ± 5.83 | - | 5.12 ± 3.76 | - | 3.79 ± 7.01 | - | 0.54 |
| Serum IGF-1 (ng/mL) | - | 345.4 ± 301.5 | - | 417.9 ± 192.3 | - | 286.6 ± 363.1 | - | 0.25 |
| Serum IGF-1 index | - | 1.39 ± 1.01 | - | 1.84 ± 0.86 | - | 1.02 ± 0.99 | - | 0.03 |
| Therapy | ||||||||
| Somatostatin analogue (%) | 0 (0) | 10 (34) | 0.0001 | 6 (46) | 0.0001 | 4 (25) | 0.002 | 0.27 |
| Bromocriptine (%) | 0 (0) | 10 (34) | 0.0001 | 7 (54) | 0.0001 | 3 (19) | 0.009 | 0.06 |
| Pegvisomant (%) | 0 (0) | 1 (3) | 0.34 | 1 (8) | 0.19 | 0 (0) | 1.00 | 0.45 |
| Hypophysectomy (%) | 0 (0) | 11 (38) | 0.0001 | 7 (54) | 0.0001 | 4 (25) | 0.002 | 0.14 |
| Controls | All Acromegaly Patients | p vs. Controls | Active Acromegaly Patients | p vs. Controls | Inactive Acromegaly Patients | p vs. Controls | p vs. Active Acromegaly | |
|---|---|---|---|---|---|---|---|---|
| (n = 57) | (n = 29) | (n = 13) | (n = 16) | |||||
| LA and LV dimensions | ||||||||
| LA diameter (mm) | 37.8 ± 4.9 | 42.7 ± 6.2 | 0.001 | 42.5 ± 7.5 | 0.009 | 42.8 ± 5.1 | 0.001 | 0.89 |
| LV-EDD (mm) | 48.5 ± 3.9 | 51.7 ± 5.4 | 0.003 | 51.0 ± 5.7 | 0.07 | 52.4 ± 5.3 | 0.003 | 0.50 |
| LV-EDV (mL) | 111.5 ± 33.5 | 132.2 ± 29.7 | 0.009 | 128.0 ± 29.0 | 0.11 | 135.9 ± 30.9 | 0.02 | 0.50 |
| LV-ESD (mm) | 32.5 ± 13.0 | 32.0 ± 4.9 | 0.70 | 30.9 ± 4.6 | 0.25 | 33.0 ± 4.6 | 0.69 | 0.26 |
| LV-ESV (mL) | 39.2 ± 13.0 | 43.2 ± 16.0 | 0.24 | 39.1 ± 13.6 | 0.94 | 46.7 ± 17.5 | 0.08 | 0.22 |
| IVS (mm) | 9.2 ± 1.4 | 10.3 ± 1.8 | 0.005 | 10.9 ± 2.0 | 0.001 | 9.8 ± 1.5 | 0.18 | 0.09 |
| LV-PW (mm) | 9.2 ± 1.6 | 10.9 ± 1.9 | 0.001 | 11.1 ± 2.0 | 0.001 | 10.8 ± 1.8 | 0.002 | 0.76 |
| LV-EF (%) | 64.6 ± 3.9 | 67.3 ± 7.3 | 0.04 | 68.8 ± 8.2 | 0.01 | 66.1 ± 6.5 | 0.28 | 0.33 |
| E (cm/s) | 71.5 ± 14.6 | 67.4 ± 15.3 | 0.25 | 58.3 ± 8.0 | 0.004 | 74.2 ± 16.1 | 0.55 | 0.004 |
| A (cm/s) | 64.7 ± 16.3 | 77.1 ± 15.9 | 0.002 | 76.0 ± 15.4 | 0.04 | 78.0 ± 16.6 | 0.007 | 0.75 |
| Mitral regurgitation | ||||||||
| Mean MR grade | 0.07 ± 0.26 | 0.58 ± 0.40 | 0.002 | 0.64 ± 0.41 | 0.001 | 0.25 ± 0.38 | 0.001 | 0.38 |
| Grade 0 (%) | 56 (98) | 16 (55) | 0.0001 | 8 (62) | 0.0006 | 8 (50) | 0.0001 | 0.71 |
| Grade 1 (%) | 1 (2) | 11 (38) | 0.0001 | 6 (46) | 0.0001 | 5 (31) | 0.002 | 0.47 |
| Grades 2–4 (%) | 0 (0) | 2 (7) | 0.11 | 2 (15) | 0.03 | 0 (0) | 1.00 | 0.19 |
| Tricuspid regurgitation | ||||||||
| Mean TR grade | 0.07 ± 0.26 | 0.58 ± 0.40 | 0.002 | 0.64 ± 0.41 | 0.001 | 0.25 ± 0.38 | 0.001 | 0.38 |
| Grade 0 (%) | 53 (93) | 17 (59) | 0.0002 | 6 (46) | 0.0003 | 11 (69) | 0.02 | 0.27 |
| Grade 1 (%) | 4 (7) | 12 (41) | 0.0002 | 7 (54) | 0.0003 | 5 (31) | 0.02 | 0.27 |
| Grades 2–4 (%) | 0 (0) | 0 (0) | 1.000 | 0 (0) | 1.000 | 0 (0) | 1.000 | 1.00 |
| Controls | All Acromegaly Patients | p vs. Controls | Active Acromegaly Patients | p vs. Controls | Inactive Acromegaly Patients | p vs. Controls | p vs. Acromegaly | |
|---|---|---|---|---|---|---|---|---|
| (n = 57) | (n = 29) | (n = 13) | (n = 16) | |||||
| TA morphological parameters | ||||||||
| TAD-D (cm) | 2.23 ± 0.27 | 2.47 ± 0.27 | 0.001 | 2.51 ± 0.21 | 0.001 | 2.44 ± 0.32 | 0.010 | 0.378 |
| TAA-D (cm2) | 6.67 ± 1.40 | 8.73 ± 1.77 | 0.001 | 9.06 ± 1.48 | <0.001 | 8.47 ± 1.98 | <0.001 | 0.867 |
| TAP-D (cm) | 10.20 ± 1.10 | 11.56 ± 1.34 | 0.001 | 11.51 ± 1.02 | <0.001 | 11.59 ± 1.59 | <0.001 | 0.456 |
| TAD-S (cm) | 1.77 ± 0.28 | 1.97 ± 0.27 | 0.001 | 1.93 ± 0.25 | 0.059 | 2.00 ± 0.29 | 0.005 | 0.907 |
| TAA-S (cm2) | 5.01 ± 1.42 | 6.24 ± 1.61 | 0.001 | 6.28 ± 1.49 | 0.005 | 6.21 ± 1.76 | 0.006 | 0.736 |
| TAP-S (cm) | 8.72 ± 1.10 | 9.80 ± 1.35 | 0.001 | 9.90 ± 1.07 | 0.001 | 9.72 ± 1.58 | 0.005 | 0.500 |
| TA functional parameters | ||||||||
| TAFAC (%) | 27.64 ± 15.34 | 28.77 ± 9.8 | 0.720 | 30.98 ± 10.12 | 0.459 | 26.98 ± 9.81 | 0.872 | 0.292 |
| TAFS (%) | 20.51 ± 8.81 | 20.60 ± 9.08 | 0.822 | 22.98 ± 10.22 | 0.380 | 17.69 ± 7.53 | 0.246 | 0.120 |
| Right Atrial Volumes | ||||||||
| RA-Vmax (mL) | 45.37 ± 13.69 | 53.19 ± 13.95 | 0.044 | 51.06 ± 6.35 | 0.233 | 54.94 ± 18.17 | 0.063 | 0.550 |
| RA-VpreA (mL) | 36.03 ± 11.07 | 44.09 ± 11.01 | 0.010 | 44.01 ± 7.09 | 0.046 | 44.17 ± 13.78 | 0.047 | 0.975 |
| RA-Vmin (mL) | 26.12 ± 8.27 | 34.20 ± 9.60 | 0.001 | 34.20 ± 7.68 | 0.011 | 34.20 ± 11.31 | 0.012 | 1.000 |
| Controls | All Acromegaly Patients | p vs. Controls | Acromegaly Patients with Hypertension | p vs. Controls | Acromegaly Patients without Hypertension | p vs. Controls | p vs. Acromegaly Patients with Hypertension | |
|---|---|---|---|---|---|---|---|---|
| (n = 57) | (n = 29) | (n = 18) | (n = 11) | |||||
| TA morphological parameters | ||||||||
| TAD-D (cm) | 2.23 ± 0.27 | 2.47 ± 0.27 | 0.001 | 2.53 ± 0.27 | <0.001 | 2.38 ± 0.26 | 0.085 | 0.168 |
| TAA-D (cm2) | 6.67 ± 1.40 | 8.73 ± 1.77 | 0.001 | 9.26 ± 1.89 | <0.001 | 7.87 ± 1.16 | 0.019 | 0.037 |
| TAP-D (cm) | 10.20 ± 1.10 | 11.56 ± 1.34 | 0.001 | 11.86 ± 1.51 | <0.001 | 11.05 ± 0.84 | 0.019 | 0.111 |
| TAD-S (cm) | 1.77 ± 0.28 | 1.97 ± 0.27 | 0.001 | 1.98 ± 0.31 | 0.006 | 1.93 ± 0.18 | 0.062 | 0.618 |
| TAA-S (cm2) | 5.01 ± 1.42 | 6.24 ± 1.61 | 0.001 | 6.57 ± 1.69 | <0.001 | 5.70 ± 1.39 | 0.144 | 0.159 |
| TAP-S (cm) | 8.72 ± 1.10 | 9.80 ± 1.35 | 0.001 | 10.08 ± 1.41 | <0.001 | 9.35 ± 1.18 | 0.087 | 0.166 |
| TA functional parameters | ||||||||
| TAFAC (%) | 27.64 ± 15.34 | 28.77 ± 9.8 | 0.720 | 29.17 ± 9.74 | 0.694 | 28.13 ± 10.79 | 0.920 | 0.792 |
| TAFS (%) | 20.51 ± 8.81 | 20.60 ± 9.08 | 0.822 | 21.18 ± 9.56 | 0.788 | 18.24 ± 8.34 | 0.431 | 0.408 |
| Right atrial volumes | ||||||||
| RA-Vmax (mL) | 45.37 ± 13.69 | 53.19 ± 13.95 | 0.044 | 54.13 ± 16.60 | 0.071 | 51.78 ± 9.59 | 0.215 | 0.723 |
| RA-VpreA (mL) | 36.03 ± 11.07 | 44.09 ± 11.01 | 0.010 | 45.51 ± 12.12 | 0.014 | 41.97 ± 9.47 | 0.165 | 0.496 |
| RA-Vmin (mL) | 26.12 ± 8.27 | 34.20 ± 9.60 | 0.001 | 35.17 ± 10.41 | 0.003 | 32.76 ± 8.72 | 0.047 | 0.584 |
| Intra-Observer Agreement | Inter-Observer Agreement | |||
|---|---|---|---|---|
| Mean ± 2 SD Difference in Values Obtained by 2 Measurements of the Same Observer | ICC between Measurements of the Same Observer | Mean ± 2 SD Difference in Values Obtained by 2 Observers | ICC between Independent Measurements of 2 Observers | |
| End-diastolic TAD | 0.01 ± 0.18 cm | 0.95 (p < 0.0001) | 0.03 ± 0.22 cm | 0.95 (p < 0.0001) |
| End-diastolic TAA | −0.03 ± 1.11 cm2 | 0.95 (p < 0.0001) | 0.03 ± 0.49 cm2 | 0.95 (p < 0.0001) |
| End-diastolic TAP | −0.02 ± 0.65 cm | 0.96 (p < 0.0001) | −0.12 ± 0.62 cm | 0.97 (p < 0.0001) |
| End-systolic TAD | −0.04 ± 0.29 cm | 0.97 (p < 0.0001) | 0.03 ± 0.42 cm | 0.96 (p < 0.0001) |
| End-systolic TAA | −0.03 ± 0.27 cm2 | 0.96 (p < 0.0001) | −0.04 ± 0.59 cm2 | 0.97 (p < 0.0001) |
| End-systolic TAP | 0.06 ± 0.49 cm | 0.96 (p < 0.0001) | 0.03 ± 0.51 cm | 0.96 (p < 0.0001) |
| Vmax | 1.1 ± 6.1 mL | 0.97 (p < 0.0001) | 1.2 ± 4.9 mL | 0.94 (p < 0.0001) |
| VpreA | −1.3 ± 7.9 mL | 0.89 (p < 0.0001) | −1.4 ± 8.1 mL | 0.89 (p < 0.0001) |
| Vmin | 0.7 ± 5.4 mL | 0.93 (p < 0.0001) | 0.8 ± 4.1 mL | 0.93 (p < 0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Rácz, G.; Kormányos, Á.; Gyenes, N.; Ambrus, N.; Lengyel, C.; Valkusz, Z. Three-Dimensional Speckle-Tracking Echocardiography-Derived Tricuspid Annular Properties in Acromegaly—Results from the MAGYAR-Path Study. Biomedicines 2024, 12, 1464. https://doi.org/10.3390/biomedicines12071464
Nemes A, Rácz G, Kormányos Á, Gyenes N, Ambrus N, Lengyel C, Valkusz Z. Three-Dimensional Speckle-Tracking Echocardiography-Derived Tricuspid Annular Properties in Acromegaly—Results from the MAGYAR-Path Study. Biomedicines. 2024; 12(7):1464. https://doi.org/10.3390/biomedicines12071464
Chicago/Turabian StyleNemes, Attila, Gergely Rácz, Árpád Kormányos, Nándor Gyenes, Nóra Ambrus, Csaba Lengyel, and Zsuzsanna Valkusz. 2024. "Three-Dimensional Speckle-Tracking Echocardiography-Derived Tricuspid Annular Properties in Acromegaly—Results from the MAGYAR-Path Study" Biomedicines 12, no. 7: 1464. https://doi.org/10.3390/biomedicines12071464
APA StyleNemes, A., Rácz, G., Kormányos, Á., Gyenes, N., Ambrus, N., Lengyel, C., & Valkusz, Z. (2024). Three-Dimensional Speckle-Tracking Echocardiography-Derived Tricuspid Annular Properties in Acromegaly—Results from the MAGYAR-Path Study. Biomedicines, 12(7), 1464. https://doi.org/10.3390/biomedicines12071464







