Abstract
Around 30–60% of patients with rheumatoid arthritis (RA) present treatment failure to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). Chitinase-like proteins (CLPs) (YKL-40, YKL-39, SI-CLP) might play a role, as they are associated with the inflammatory process. This study aimed to evaluate CLP utility as a biomarker in the treatment failure of csDMARDs. A case–control study included 175 RA patients classified into two groups based on therapeutic response according to DAS28-ESR: responders (DAS28 < 3.2); non-responders (DAS28 ≥ 3.2). CLP serum levels were determined by ELISA. Multivariable logistic regression and receiver operating characteristic (ROC) curves were used to evaluate CLPs’ utility as biomarkers of treatment failure. Non-responders presented higher levels of YKL-40, YKL-39, and SI-CLP compared with responders (all: p < 0.001). YKL-40 correlated positively with YKL-39 (rho = 0.39, p < 0.001) and SI-CLP (rho = 0.23, p = 0.011) and YKL-39 with SI-CLP (rho = 0.34, p < 0.001). The addition of CLPs to the regression models improves diagnostic accuracy (AUC 0.918) compared to models including only clinical classical variables (AUC 0.806) p < 0.001. Non-responders were positive for all CLPs in 35.86%. Conclusions: CLPs could be considered as a useful biomarker to assess treatment failure, due to their association with clinical variables and improvement to the performance of regression models.
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease of unknown etiology that involves genetic, environmental, immunologic, and hormonal factors [1,2]. The primary goal of RA treatment is to achieve and maintain disease remission or disease activity levels as low as possible [3]. Disease-modifying antirheumatic drugs (DMARDs) have been used to achieve these objectives [1]. In Latin American countries, pharmacological treatment is mainly based on conventional synthetic DMARDs (csDMARDs) [4,5,6]. However, despite significant improvements in the majority of RA patients with csDMARDs, a notable proportion (30–60%) experienced treatment failure with csDMARDs [7,8,9]. Therefore, it is crucial to have tools that help identify patients with early treatment failure in order to prevent irreversible structural damage, disability, decreased quality of life, and persistent inflammatory states [10,11,12]. Although traditionally used as treatment failure biomarkers, rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPAs), and anti-peptidyl arginine deiminase 4 antibodies (anti-PAD4) have not proven to be useful in identifying patients with DMARD treatment failure [13,14,15]. In this regard, chitinase-like proteins (CLPs) might play a role, as they are associated with the inflammatory process in RA patients [16]. CLPs are proteins without enzymatic activity that lack six cysteine residues, resulting in conformational changes in their active site [17]. Three main CLPs are known in humans: YKL-40 (CHI3L1: chitinase-3-like-1), YKL-39 (CHI3L2: chitinase-3-like-2), and SI-CLP: Stabilin-interacting chitinase-like protein (CHID1: Chitinase domain-containing protein 1) [18]. YKL-40 has been primarily studied in RA and is associated with increased clinical disease activity and higher serum levels in patients with high disease activity [19,20]. To the best of our knowledge, YKL-39 and SI-CLP have not been extensively studied in RA patients. However, information from other diseases suggests that both YKL-39 and SI-CLP play crucial roles in inflammation and joint damage [21,22].
YKL-40, YKL-39, and SI-CLP present a structural relationship between them [16,23,24,25]. However, the in vivo relationships among these three structurally related CLPs are not well understood. Furthermore, the role of these three CLPs in RA and treatment failure based on csDMARDs is not known. Therefore, this study aimed to evaluate the utility of CLPs as biomarkers to identify RA patients with treatment failure.
2. Materials and Methods
2.1. Study Design
Case–control study. The study included 175 RA patients classified according to the 1987 ACR criteria [26] who voluntarily agreed to participate. The inclusion criteria were as follows: (a) females, (b) over 18 years old, and (c) pharmacological treatment based on csDMARDs. Exclusion criteria included: (a) overlap syndrome; (b) use of vitamins, antioxidants, and dietary supplements known to affect YKL-40 production [19]; (c) pregnant or lactating women; (d) treatment with biologic DMARDs. Patients with RA were recruited from the outpatient clinic of the Research Department of the University Center of Health Sciences, University of Guadalajara.
2.2. Clinical Assessments
All patients were evaluated by a rheumatologist using a structured questionnaire to collect sociodemographic variables, personal pathological history, and disease activity. Disease activity was assessed using the Disease Activity Score 28 combined with erythrocyte sedimentation rate (DAS28) [27]. Sedentary behavior was considered as any waking behavior, such as sitting, lying down, or reclining, characterized by an energy expenditure of 1.5 METs (metabolic equivalent of task) or lower, according to the definition of the Sedentary Behavior Research Network and World Health Organization [28].
2.3. Study Groups
Patients were divided into two groups based on their therapeutic response according to the DAS28-ESR: responders to csDMARDs and non-responders to csDMARDs. The responder group included patients with RA who met one of the following criteria: (1) a DAS28 score of less than 3.2 for at least six months prior to inclusion in the study without any changes to their csDMARD pharmacological regimen; or (2) a DAS28 score of 3.2 or greater who achieved a 50% reduction in their DAS28 score over a six-month period without any changes to their csDMARD pharmacological regimen. The non-responder group comprised patients with RA who met one or both of the following criteria: (1) active patients with a DAS28 score of 3.2 or greater who did not achieve a 50% reduction in their DAS28 score over the six-month period prior to study inclusion, despite treatment with csDMARDs; or (2) active patients with a DAS28 score of 3.2 or greater who remained active and required the addition of another csDMARD, as determined by the treating physician, within the six-month period prior to study inclusion. Supplementary Figure S1 shows the conformation of responders and non-responders, based on previous clinical characteristics.
2.4. Determination of Serum Levels of Chitinase-like Proteins (YKL-40, YKL-39, and SI-CLP)
Fasting blood samples were collected, and serum was stored at −80 °C until laboratory analysis. ELISA kits from MyBioSource, San Diego, CA, USA, with catalog numbers MBS765293 (YKL-40), MBS450589 (YKL-39), and MBS764700 (SI-CLP) were used to determine CLP serum levels. All samples were analyzed in duplicate by investigators blinded to the patient’s clinical characteristics, following the manufacturer’s instructions.
2.5. Other Laboratory Parameters
Additional samples were obtained for ESR (mm/h), C-reactive protein (CRP; mg/dL), rheumatoid factor (RF; IU/mL), and ACPAs (UI/mL). These determinations were performed according to the manufacturer’s instructions by investigators who were blinded to the patient’s clinical characteristics.
2.6. Statistical Analysis
Data normality for the main study variables (CLPs) was assessed using the Kolmogorov–Smirnov–Lilliefors test and graphs, indicating a non-normal distribution. Quantitative variables were expressed as median and interquartile range (IQR), and qualitative variables were expressed as frequencies and percentages (%). The Mann–Whitney U test was used for the comparison of quantitative variables between two independent groups, and the chi-square test (or Fisher’s exact test for small expected counts) for the comparison of proportions. Spearman’s correlation test was used to identify the strength of correlation between serum levels of CLPs and other quantitative clinical variables. To understand the influence of different variables on CLPs at various points in their distribution, quantile regression was performed, one for each CLP. These regressions were adjusted for variables associated with CLPs in the bivariate correlation analysis, as well as for variables with biological plausibility. In these regressions, it was validated that the models were adequate by verifying the independence and normality of the residuals and the linearity of the relationships between the independent and dependent variables in each analyzed quantile. Additionally, multicollinearity between the variables included in the models was examined. Receiver operating characteristic (ROC) curves were used to evaluate the CLP concentrations that best identified RA patients with treatment failure. The Youden index was used to select the optimal cut-off point. The area under the curve (AUC), 95% confidence intervals, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratios for these serum levels were calculated to assess their utility in identifying RA patients with csDMARD treatment failure (non-responders). Logistic regression models were used to adjust for associated and confounding variables related to treatment failure. Initially, a model that included only clinical variables, excluding CLPs, was selected. Variables evaluated in this model were those with a p-value < 0.20 in univariate analysis, and biologically plausible. The final reference model was chosen considering Akaike information criterion (AIC) and Bayesian Information criterion (BIC) values. Additionally, the normality of the residual error and multicollinearity were used to validate the logistic regression models. Subsequently, to assess whether the addition of chitinases improved the performance of the reference model in identifying treatment failure, 14 new models were created, including each CLP and all possible combinations. ROC curves for each model were compared using the non-parametric DeLong test [29]. For all statistical analyses, the R programming language, R Core Team, Viena, Austria, version 4.3 was used, employing the following R packages: tidyverse v2.0.0; [30], ggcorrplot v0.1.4.1; [31], ggpubr v0.6.0; [32], rstatix v0.7.2; [33], pROC v1.18.4; [34], cutpointr v1.1.2; [35], epiR v2.0.65; [36], ggalluvial v0.12.5; [37] and quantreg v5.97; [38]. Statistical significance was set at p < 0.05.
3. Results
3.1. Comparison of Clinical Characteristics of RA Responders and Non-Responders
A total of 175 patients with RA were included: 87 in the responder group and 88 in the non-responder group. Table 1 describes and compares the clinical and sociodemographic characteristics of the two RA patient groups (responders and non-responders). The groups were similar in terms of sex, age, tobacco use, comorbidities, and rheumatoid factor levels. Additionally, the proportion of patients using csDMARDs (methotrexate, leflunomide, azathioprine, sulfasalazine, and mycophenolate) and glucocorticoids was similar in both groups. However, the responder group used chloroquine more frequently than the non-responder group (17% vs. 6.6%, respectively; p = 0.034). Non-responders had higher ACPA concentrations (156 IU/mL (60; 277 IU/mL)) than responders (46 IU/mL (6; 111 IU/mL)) (p < 0.001).

Table 1.
Comparison between RA patients with failure to DMARDs (non-responders) and RA patients with response to DMARDs (responders).
3.2. Comparison of CLP Levels between RA Responders and Non-Responders
Figure 1 compares the CLP levels between RA responders and non-responder patients. YKL-40 levels (Figure 1A) were higher in non-responders (1.82 ng/mL (1.47 ng/mL; 2.15 ng/mL)) than in responders (1.20 ng/mL (1.00 ng/mL; 1.38 ng/mL)) (p < 0.001). YKL-39 levels (Figure 1B) were also higher in non-responders (4.14 ng/mL (2.67 ng/mL; 6.17 ng/mL)) compared to responders (2.15 ng/mL (1.43 ng/mL; 2.73 ng/mL)) (p < 0.001). Furthermore, SI-CLP levels (Figure 1C) were higher in non-responders (4.65 ng/mL (2.68 ng/mL; 6.10 ng/mL)) compared to responders (2.10 ng/mL (1.11 ng/mL; 3.33 ng/mL)) (p < 0.001).
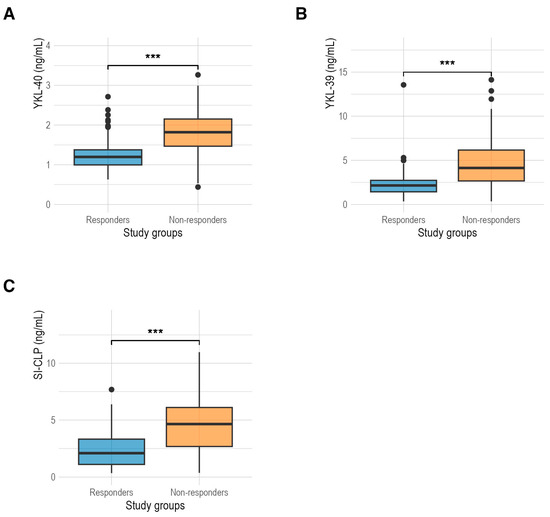
Figure 1.
(A) Comparison of serum levels of YKL-40 protein between responders (1.20 ng/mL) and non-responders (1.82 ng/mL) in RA patients; (B) comparison of serum levels of YKL-39 protein between responders (2.15 ng/mL) and non-responders (4.14 ng/mL) in RA patients; (C) comparison of serum levels of SI-CLP protein between responders (2.10 ng/mL) and non-responders (4.65 ng/mL). ***: p < 0.001.
3.3. Correlations between CLP Concentrations and Selected Clinical Characteristics of Patients with RA
Positive correlations were found between the serum levels of YKL-40, YKL-39 (rho = 0.39, p < 0.001), and SI-CLP (rho = 0.23, p = 0.011), as well as between the serum levels of YKL-39 and SI-CLP (rho = 0.34, p < 0.001). Additionally, a positive correlation was detected between the YKL-39 protein and stiffness assessment using the visual analog scale (rho = 0.22, p = 0.002) and between the SI-CLP protein and the total dose of glucocorticoids (rho = 0.14, p = 0.040). Other statistically significant correlations between CLPs and clinical variables such as HAQ-Di, DAS28 score, erythrocyte sedimentation rate, and anti-citrullinated peptide antibodies are shown in Figure 2.
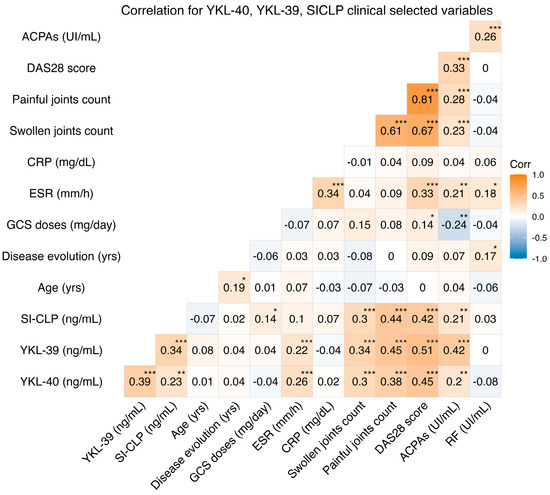
Figure 2.
Correlations between YKL-40, YKL-39, SI-CLP, clinical variables, and laboratory values. ACPAs: anticyclic citrullinated protein antibodies; DAS28: disease activity score 28; CRP: C reactive protein; ESR: erythrocyte sedimentation rate; GCS: glucocorticoids; RF: rheumatoid factor. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
3.4. Quantile Regressions
Quantile regression models were performed for the 25th, 50th, and 75th percentiles of the YKL-40, YKL-39, and SI-CLP distributions. Three models were performed: the first included YKL-40 as an independent variable, the second included YKL-39 as an independent variable, and the third included SI-CLP as an independent variable. The independent variables included in all three models were patient age, disease duration, anti-CCP antibody titers, rheumatoid factor titers, and total cumulative glucocorticoid dose. Additionally, the two CLPs that were not considered dependent variables in each model were included as independent variables.
For YKL-40, it was observed that the DAS28 score was positively associated in the 0.25 and 0.75 quantiles, indicating a varied relationship depending on YKL-40 concentrations. Additionally, YKL-39 and SI-CLP showed a significant positive effect on YKL-40 in the 0.25 and 0.75 quantiles, but not in the median quantiles, suggesting a relationship between CLPs, especially at high and low values. Other variables, such as age, disease progression, anti-CCP titers, and rheumatoid factor, were not associated with YKL-40 in the quantile regression analysis.
In the case of YKL-39, quantile regression results indicated that both YKL-40 and DAS28 scores were predictors of higher levels of YKL-39 in all three quantiles evaluated. However, YKL-39 and SI-CLP were only associated with the 0.25 quantile. Conversely, the anti-CCP coefficients indicated a positive effect in all quantiles, being significant in the 0.25 and 0.75 quantiles. Variables such as age and disease duration did not show a significant impact on YKL-39 levels, suggesting that these factors are not determinants of the variability of this biomarker in patients with RA.
The quantile regression model for SI-CLP showed that for the DAS28 score, in all quantiles, there was a significant increase in SI-CLP levels associated with an increase in the DAS28 score. For YKL-39, a significant relationship was observed only in the 0.5 and 0.75 percentiles, indicating that the relationship between YKL-39 and SI-CLP is present at medium and high levels. Anti-CCP titers were associated with SI-CLP only in the 0.75 quantile. In contrast, other variables such as YKL-40, age, disease progression, and rheumatoid factor titers did not show an effect on SI-CLP.
Supplementary Tables S1–S3 show the coefficients and their confidence intervals for quantile regressions of YKL-40, YKL-39, and SI-CLP, respectively.
3.5. ROC Curves of CLPs
Table 2 displays the AUC, 95% confidence interval, sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratio of the selected cut-off value according to the Youden index for CLPs to identify non-responder patients. The ROC curves for RA responders and non-responders showed AUC values for YKL-40, YKL-39, and SI-CLP, 0.776, 0.805, and 0.759, respectively (all p < 0.001). The optimal cut-off values according to the Youden index for YKL-40, YKL-39, and SI-CLP to differentiate RA responders from non-responders to csDMARDs were 1.38 ng/mL, 3.26 ng/mL, and 4.10 ng/mL, respectively. ROC curves are provided in Figure 3.

Table 2.
Responders and non-responders values of YKL-40, YKL-39, and SI-CLP in RA patients.
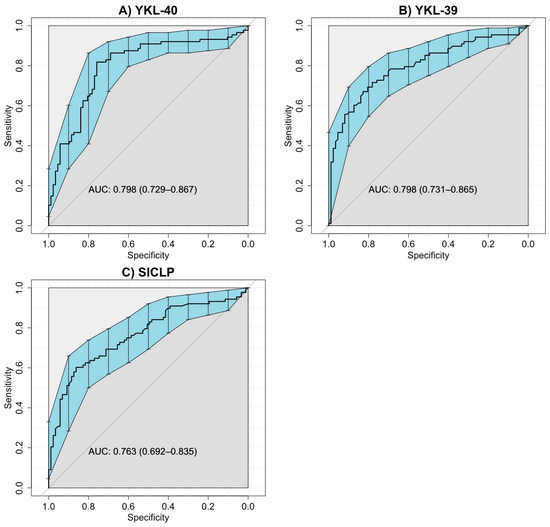
Figure 3.
ROC curves of CLPs for RA patients included in both study groups. (A) ROC curve for serum levels of YKL-40 in RA patients responders and non-responders (AUC = 0.798, IC 95% 0.729–0.867). (B) ROC curve for serum levels YKL-39 in RA patients responders and non-responders (AUC = 0.798, IC 95% 0.731–0.865). (C). ROC curve for serum levels SI-CLP in RA patients responders and non-responders (AUC = 0.763, IC 95% 0.692–0.835). The blue area represents the confidence intervals for the ROC curve.
3.6. Logistic Regression Models
To select the reference model, the disease duration, combined DMARD therapy, leflunomide use, total dose of glucocorticoids, and anti-citrullinated peptide antibodies were evaluated as independent variables. However, in the final reference model, only HAQ-Di, ESR, ACPA titles, and total dose of glucocorticoids were included as these variables were identified as the only variables associated with treatment failure. This final model was used to compare the performances of the remaining models. Table S4 presents the results of the final model (reference model) and previous models.
3.7. Models for AUC Comparison Using the DeLong Test
Table 3 shows the performance evaluation of the logistic regression models 1, 2, 3, 4, and 5 to identify factors associated with treatment failure based on csDMARDs, and their comparison with the reference model. Model 2, which included variables associated with treatment failure from the reference model and the three CLPs, achieved the highest AUC and was statistically significant compared to the reference model (0.808 vs. 0.911; p < 0.001). Furthermore, other models that assessed the addition of CLPs individually or in combination with the reference model showed improved performance compared with the reference model (model 3, p = 0.019; model 4, p = 0.027; model 5, p = 0.055; model 6, p = 0.001; model 7, p < 0.001; model 8, p = 0.012; and model 9, p = 0.037). The comparison of models 6 to 15 is presented in Table S5 of the Supplementary Material.

Table 3.
Selected models. Models 1 to 5 of the variables associated with treatment failure and CLPs and their comparison using DeLong test.
3.8. Chitinase Combinations (Positivity–Negativity)
Among the non-responder patients, 35.87% were positive for all three evaluated chitinases; approximately half of this percentage (17.39%) was positive for both YKL-40 and YKL-39. The third highest percentage of patients with positivity was observed in those who only tested positive for YKL-40, accounting for 14.13%. These percentages represented 18.13%, 8.79%, and 7.14% of the total patients included in this study, respectively. In the responder group, more than half of the patients did not have chitinase positivity (56.67%). Responder patients positive for YKL-40 and SI-CLP accounted for 14.44% and 11.11% of the total patients in this group, respectively. Table S6 presents the total results for both patient groups and the positivity or negativity for various chitinases as well as all possible combinations, along with Figure 4 depicting an alluvial plot.
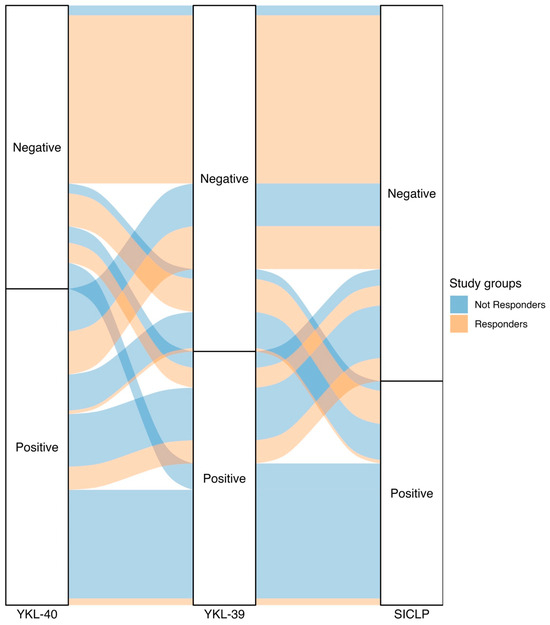
Figure 4.
The alluvial plot depicting the classification of patients from the study groups (responders and non-responders) into positive or negative for chitinase-like proteins. Patients with RA who respond to treatment are represented in pink, and patients with RA who do not respond to treatment are represented in blue. We observe that one-third of the patients with RA who do not respond to treatment (35.87%) have elevated levels of all 3 CLPs. Additionally, more than half of the patients with RA who respond to treatment (56.67%) do not have elevated levels of all 3 CLPs.
4. Discussion
In the present study, the possible use of serum levels of YKL-40, YKL-39, and SI-CLP proteins as biomarkers to identify patients with csDMARD treatment failure is described for the first time. These proteins demonstrated suitable sensitivity, specificity, and predictive value for the identification of patients with RA who do not respond to csDMARDs after 6 months of use. Furthermore, the performance of regression models that included CLPs suggests that measuring CLPs is better for characterizing non-responding patients with RA to csDMARDs than not including them. Interestingly, we observed, for the first time, a positive correlation between serum levels of YKL-40 and YKL-39 and SI-CLP in patients with RA.
Of the three CLPs evaluated as biomarkers in this study, YKL-40 is the most studied in both RA and other diseases [17,39]. In this study, we observed that RA patients non-responding to csDMARDs had higher YKL-40 concentrations than responding patients. In RA, treatment failure is defined as a lack of response or limited efficacy in achieving remission or low disease activity for at least three consecutive months, requiring treatment adjustment [15,40]. Therefore, RA patients with treatment failure are characterized by a persistent inflammatory status due to high concentrations of acute-phase reactants (CRP and ESR), proinflammatory cytokines (IL-1, IL-6, TNF-α), and autoantibodies (ACPAs, RF) [10,41,42]. Therefore, YKL-40 is produced by macrophages [43], chondrocytes [44], synoviocytes [45], synovial fibroblasts [46], and neutrophils [47], and its elevation in non-responding patients may be explained by the inflammatory state. Moreover, there is evidence demonstrating a correlation between YKL-40 levels, disease activity, and persistent inflammatory status in patients with RA [19,20,48,49]. A previous study has reported elevated serum levels of YKL-40 in patients with RA with high and moderate disease activity compared to those with low disease activity and remission based on the DAS28 score [19]. Furthermore, existing information suggests that YKL-40 could have an active role in the pathogenesis of RA stimulating the production of inflammatory mediators such as chemokines (CCL2 and CXCL2), proinflammatory cytokines, and metalloproteinases (MMP-3 and MMP-9), and activating connective tissue growth factor (CTGF), favoring the formation of pannus, a determinant element in the development of erosions and joint disorganization characteristic of this disease [50,51,52,53,54]. Collectively, this evidence supports the hypothesis that YKL-40 is elevated during inflammatory states.
In our study, we also observed that elevated serum levels of YKL-40 were positively correlated with patient disability (evaluated by the HAQ-Di score), and concentrations of ACPAs that are considered classic parameters of deterioration in patients with RA [55,56,57,58,59,60]. Moreover, RA patients who do not respond to treatment often experience increased disease progression, greater loss of functionality, worse prognosis, and elevated ACPA concentrations, consistent with our findings [10,28,29,61,62,63,64,65].
In the case of YKL-39, we also observed that non-responding patients treated with csDMARDs had higher serum levels than responding patients. To our knowledge, this is the first study to correlate elevated serum levels of YKL-39 in patients with RA with treatment failure. Moreover, we found positive correlations among YKL-39, HAQ-Di, and ACPAs. The involvement of YKL-39 in diseases other than RA has been reviewed and described [22,66]. In osteoarthritis, YKL-39 is involved in the initial degeneration of cartilage and tissue remodeling [17,67]. A study observed higher serum levels of YKL-39 in patients with early-stage multiple sclerosis compared to healthy subjects [68]. Similarly, YKL-39 levels in cerebrospinal fluid were higher in patients with amyotrophic lateral sclerosis (ALS) than in controls or healthy subjects [69]. Therefore, our study strengthens the hypothesis that YKL-39 can be used as a biomarker associated with treatment failure, as there is evidence of its role in the pathogenesis of various diseases. In addition, in our study, we observed positive correlations with laboratory parameters commonly used in RA, such as ACPAs and ESR, and clinical parameters, such as HAQ-Di and DAS28-ESR, suggesting that elevated YKL-39 could be associated with persistent inflammatory status in RA patients who do not respond to treatment and their prognosis.
Regarding SI-CLP, information related to RA is very limited. However, in murine models with induced RA, it has been described that SI-CLP is attached to the surface of macrophages, increases the expression of IL-1β, IL-6, and IL-12, and worsens the inflammatory state of this disease [70]. SI-CLP is secreted by monocytic cells [70] and promotes the production of proinflammatory cytokines [70]. This information is consistent with what we found in our study, where we observed an elevation of SI-CLP in RA patients who did not respond to treatment and a correlation with some RA prognostic markers (ACPAs). The evidence thus far suggests that this relationship may be due to an increase in macrophage activity and the establishment of an inflammatory state with the help of SI-CLP, which favors the production of proinflammatory cytokines [70,71]. Another interesting contribution was the correlation between SI-CLP and the total dose of glucocorticoids. This could be explained by a cell culture study of macrophages, where it was shown that SI-CLP is regulated and stimulated by glucocorticoids by inhibiting the stabilin-1 receptor through the regulation of a lysosomal pathway [71].
Interestingly, in our study, we observed a direct relationship between the serum concentrations of these three CLPs. This positive correlation among the three CLPs can be explained by the structural relationship between YKL-40, YKL-39, and SI-CLP [16,23,24,25,72,73]. Furthermore, the structural relationship between YKL-40 and YKL-39 has been described in a gene interaction analysis performed using a web prediction tool, where the interaction between the two proteins was co-expressed [16]. In a study of patients with optic neuritis and CIS, YKL-39 levels were positively correlated with YKL-40 levels in cerebrospinal fluid [74]. Even though the Møllgaard et al. study was conducted in another autoimmune disease, to our knowledge it is the only evidence where a correlation between YKL-40 and YKL-39 has been described. The relationship between YKL-40, YKL-39, and SI-CLP described in this study, has been reported for the first time in patients and may raise new hypotheses regarding the relationship between the pathophysiology of these three proteins. However, further studies specifically designed for this purpose are necessary.
The sensitivity and specificity values obtained for CLPs were generally good [75]. For the YKL-40 protein, the sensitivity and specificity values (sensitivity: 80.43%, specificity: 74.44%) indicated its ability to detect 76.04% of patients who did not respond to csDMARDs. However, the LR values (LR+ (3.15) and LR− (0.26)) are not optimal [75,76,77]; therefore, the clinical utility of evaluating this marker should be performed with other types of studies that can measure changes in YKL-40 and control other factors related to the elevation of this protein.
Regarding the clinical utility results of the YKL-39 and SI-CLP proteins, the sensitivity and specificity values of the cutoff point for both proteins indicate that they have a good capacity [75] to detect patients with and without treatment failure. However, the estimation of LR+ and LR− indicates that more studies are needed to understand the clinical value of measuring these proteins in clinical practice.
In this study, in addition to evaluating sensitivity, specificity, predictive value, and likelihood ratio, the utility of CLPs as potential biomarkers for treatment failure was assessed using logistic regression models. Since we did not have other biomarkers, variables commonly used in RA patients (HAQ-Di, ESR, total dose of glucocorticoids, and ACPAs) were used as a reference model. Compared with this model, the measurement of CLPs adds diagnostic precision, and improves the accuracy of the model, increasing the AUC from 0.808 to 0.911 (DeLong test; p < 0.001) [78] and could lead to potential clinical benefits in patients with csDMARDs, such as the possibility of early and personalized interventions. This improvement could translate into a better diagnosis and prognosis for patients with RA, allowing a more precise identification of those likely to not respond to standard treatment. Additionally, this improvement in discrimination could lead to a reduction in the administration of ineffective treatments, enhance the quality of life of the patient, and reduce healthcare costs. The results of the models, as well as the positive correlations between the three CLPs evaluated in this research, suggest that the three CLPs may have an impact on RA patients not responding to csDMARDs. Furthermore, these results suggest that the joint measurement of the three proteins improves performance more than the individual addition to the models.
This study has several limitations. First, this study does not prove that the elevation of CLPs is a phenomenon exclusive to patients with DMARD failure. Therefore, it is necessary to generate more information to determine which other biological and pathological conditions elevate CLPS. Another limitation of our study is its design and temporality. Our study was not designed to evaluate the changes in CLP concentrations in patients with RA. Therefore, we could not determine whether the increase in CLPs occurred before or after treatment failure. To validate the clinical usefulness of these biomarkers, it is necessary to carry out cohort studies. Therefore, new studies are required to understand the temporal relationship between CLPs and treatment failure. Furthermore, although our results on sensitivity, specificity, and predictive values suggest that measuring CLPs in patients with RA could have clinical utility, the values of LR+ and LR− are not optimal. We believe that other variables may influence the concentrations of CLPs. These suboptimal LR values are also reflected in elevated CLPs in some patients responding to csDMARDs. Additionally, in this study, we did not measure pro-inflammatory cytokines or other markers of inflammation other than ESR; therefore, we cannot assert that the elevation of CLPs is due to persistent inflammation in non-responders. The validation of our hypothesis related to inflammation and the elevation of CLPs requires new studies that ideally should be proposed with other longitudinal epidemiological designs, the measurement of other variables, the measurement of other variables such as renal function and other clinical variables, including patients who begin treatment with csDMARDs as well as the inclusion of patients with biological DMARDs. In this way, it will be possible to understand the mechanisms underlying the elevation of CLPs in patients with RA. In this war, another limitation of this study is that we did not include a comparison group of patients receiving biological treatment. Therefore, we do not know whether the elevation of CLPs is observed only in patients undergoing treatment with csDMARDs or if treatment with biological DMARDs could potentially alter it. In this study, although we statistically demonstrated the direct relationship between the levels of YKL-40, YKL-39, and SI-CLP, our results do not allow us to determine whether this statistical relationship is biologically significant. Further studies are needed to evaluate the cellular mechanisms that could explain the elevation of these three CLPs.
This study had several strengths. Evaluation of CLPs as biomarkers was performed using different methods (ROC curves, logistic regressions, sensitivity, specificity, PPV, NPV, and LR). This offers greater diagnostic precision because the combined use of these indicators provides a more comprehensive and accurate view of the performance of CLPs in identifying non-responders to csDMARDs, as each of these measures provides a unique perspective on how CLPs behave in different situations. The use of this approach could provide guidelines for subsequent studies to improve therapeutic strategies. Additionally, the measurement of CLPs could be easily implemented in most laboratories. In this study, we used quantile regression models to identify the factors associated with CLP elevation. This approach allows us to understand the relationship between variables and CLPs at different points in the distribution, thereby providing a more detailed and comprehensive view of the relationship between the variables. Another strength is that the evaluation of CLPs was conducted using a multivariate approach utilizing logistic regression models, which allowed for the adjustment of confounding effects to obtain a clearer and more direct relationship between CLPs and response to treatment. Additionally, multivariate analysis allows for a more comprehensive understanding of how other variables might influence treatment failure and the elevation of CLP concentrations. Lastly, we consider another strength of this study to be our observation of correlations between YKL-40, YKL-39, and SI-CLP with clinical and laboratory parameters commonly used in patients with RA, which allows for a comparison of CLPs with variables commonly used in RA.
5. Conclusions
The measurement of CLPs can be a useful biomarker for identifying patients who do not respond to csDMARDs, as the inclusion of CLPs also enhances the performance of models that only incorporate clinical variables and laboratory results. However, further studies with a different epidemiological approach are required to validate these findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12071406/s1, Table S1: Quantile regression for YKL-40; Table S2: Quantile regression for YKL-39, Table S3: Quantile regression for SI-CLP; Table S4: Factors associated with failure to treatment with DMARDs (logistic regression); Table S5: Models 6 to 15 of the variables associated with treatment failure and CLPs and their comparison using DeLong test; Table S6: Patients with RA included in the study groups (responders and non-responders) who tested positive and negative for CLPs (YKL-40, YKL-39, and SI-CLP) according to the cut-off point of the ROC curves. Supplementary Figure S1: Conformation of responders and non-responders; Supplementary Figures S2–S4: comparisons of YKL-40, YKL-39, and SI-CLP according to degrees of DAS28: Supplementary Figure S5: scatter plot for DAS28 and CLPs.
Author Contributions
Conceptualization, J.D.T.E.-D., J.I.G.-N. and E.E.P.-G.; methodology, J.D.T.E.-D., J.I.G.-N., L.G.-L., A.M.S.-C. and A.C.M.-S.; software, A.B.-R. and M.R.G.-M.; validation, J.D.T.E.-D., M.R.G.-M. and A.G.F.-V.; formal analysis, A.B.-R. and E.E.P.-G.; investigation, J.D.T.E.-D., J.I.G.-N. and L.G.-L.; resources, E.E.P.-G.; data curation, JA.B.-R. and E.E.P.-G.; writing—original draft preparation, J.D.T.E.-D., J.I.G.-N., L.G.-L., A.M.S.-C., A.C.M.-S., A.B.-R., M.R.G.-M., A.G.F.-V. and E.E.P.-G.; writing—review and editing, J.D.T.E.-D., J.I.G.-N., L.G.-L., A.M.S.-C., A.C.M.-S., A.B.-R., M.R.G.-M., A.G.F.-V. and E.E.P.-G.; supervision, J.I.G.-N. and E.E.P.-G.; project administration, E.E.P.-G.; funding acquisition, E.E.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the “Programa de Apoyo a la Mejora en las Condiciones de Producción de los Miembros del SNI y SNCA-PROSNI” and “Programa de Incorporación y Permanencia del Posgrado en el PNPC (PROINPEP) 2021”. Both funds were provided by Universidad de Guadalajara, and assigned to E.E.P.-G.
Institutional Review Board Statement
This study adhered to the guidelines of the 64th Declaration of Helsinki and was approved by the Research, Ethics in Research, and Biosafety Committees of the University Center of Health Sciences (protocol number: CI-00121 on 18 January 2021). The confidentiality of sensitive data for all participants was preserved.
Informed Consent Statement
All participants voluntarily signed an informed consent form before inclusion in the study.
Data Availability Statement
The data presented in this study will be available upon request to the corresponding author. The data are not publicly available due to privacy issues.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Cardiel, M.H.; Carrillo, S.; Pérez, M.; Andrade, L.; Pacheco Tena, C.; Silveira, L.H.; Limón, L.; Cerpa, S.; Gutiérrez Ureña, S.; Durán, S.; et al. Actualización de las guías del tratamiento farmacológico de la artritis reumatoide del Colegio Mexicano de Reumatología 2018. Reumatol. Clínica 2021, 17, 215–228. [Google Scholar] [CrossRef]
- Burgos Vargas, R.; Cardiel, M.H. Rheumatoid Arthritis in Latin America. Important Challenges to Be Solved. Clin. Rheumatol. 2015, 34, 1–3. [Google Scholar] [CrossRef][Green Version]
- Goycochea-Robles, M.V.; Arce-Salinas, C.A.; Guzmán-Vázquez, S.; Cardiel-Ríos, M.H. Prescription Rheumatology Practices among Mexican Specialists. Arch. Med. Res. 2007, 38, 354–359. [Google Scholar] [CrossRef]
- Pineda, C.; Caballero-Uribe, C.V. Challenges and Opportunities for Diagnosis and Treatment of Rheumatoid Arthritis in Latin America. Clin. Rheumatol. 2015, 34, 5–7. [Google Scholar] [CrossRef]
- Braun, J.; Kästner, P.; Flaxenberg, P.; Währisch, J.; Hanke, P.; Demary, W.; Von Hinüber, U.; Rockwitz, K.; Heitz, W.; Pichlmeier, U.; et al. Comparison of the Clinical Efficacy and Safety of Subcutaneous versus Oral Administration of Methotrexate in Patients with Active Rheumatoid Arthritis: Results of a Six-month, Multicenter, Randomized, Double-blind, Controlled, Phase IV Trial. Arthritis Rheumatol. 2008, 58, 73–81. [Google Scholar] [CrossRef]
- Perez-Guerrero, E.E.; Gonzalez-Lopez, L.; Muñoz-Valle, J.F.; Vasquez-Jimenez, J.C.; Ramirez-Villafaña, M.; Sanchez-Rodriguez, E.N.; Gutierrez-Ureña, S.R.; Cerpa-Cruz, S.; Aguilar-Chavez, E.A.; Cardona-Muñoz, E.G.; et al. Serum P-Glycoprotein Level: A Potential Biomarker of DMARD Failure in Patients with Rheumatoid Arthritis. Inflammopharmacology 2018, 26, 1375–1381. [Google Scholar] [CrossRef]
- Plant, D.; Maciejewski, M.; Smith, S.; Nair, N.; the Maximising Therapeutic Utility in Rheumatoid Arthritis Consortium, the RAMS Study Group; Hyrich, K.; Ziemek, D.; Barton, A.; Verstappen, S. Profiling of Gene Expression Biomarkers as a Classifier of Methotrexate Nonresponse in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Buch, M.H.; Eyre, S.; McGonagle, D. Persistent Inflammatory and Non-Inflammatory Mechanisms in Refractory Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2021, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.A. MRI and Ultrasound of the Hands and Wrists in Rheumatoid Arthritis. I. Imaging Findings. Skelet. Radiol. 2019, 48, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Alten, R.; Gomez-Reino, J.J.; Caporali, R.; Bertin, P.; Sullivan, E.; Wood, R.; Piercy, J.; Vasilescu, R.; Spurden, D.; et al. Clinical Characteristics and Patient-Reported Outcomes in Patients with Inadequately Controlled Rheumatoid Arthritis despite Ongoing Treatment. RMD Open 2018, 4, e000615. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, D.; Baker, T.; Galscow, A. Biomarkers for the Diagnosis and Treatment of Rheumatoid Arthritis—A Systematic Review. Postgrad. Med. 2021, 135, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kolarz, B.; Podgorska, D.; Podgorski, R. Insights of Rheumatoid Arthritis Biomarkers. Biomarkers 2021, 26, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, J.; Xie, D.; He, D.; Lu, A.; Liang, C. Toward Overcoming Treatment Failure in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 755844. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.J.; Lee, C.-K.; Han, S.-B.; Yun, J.; Hong, J.T. Roles of Chitinase 3-like 1 in the Development of Cancer, Neurodegenerative Diseases, and Inflammatory Diseases. Pharmacol. Ther. 2019, 203, 107394. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Distefano, G.; Zorena, K.; Malaguarnera, L. Chitinases and Immunity: Ancestral Molecules with New Functions. Immunobiology 2016, 221, 399–411. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Yin, S.; Liu, T.; Riabov, V.; Mitrofanova, I. Role of Chitinase-like Proteins in Cancer. Biol. Chem. 2016, 397, 231–247. [Google Scholar] [CrossRef]
- Jafari-Nakhjavani, M.R.; Ghorbanihaghjo, A.; Bagherzadeh-Nobari, B.; Malek-Mahdavi, A.; Rashtchizadeh, N. Serum YKL-40 Levels and Disease Characteristics in Patients with Rheumatoid Arthritis. Casp. J. Intern. Med. 2019, 10, 92–97. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. YKL-40 Levels in Rheumatoid Arthritis and Their Correlation with Disease Activity: A Meta-Analysis. J. Rheum. Dis. 2019, 26, 257. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Larionova, I.; Liu, T. YKL-39 as a Potential New Target for Anti-Angiogenic Therapy in Cancer. Front. Immunol. 2020, 10, 2930. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, L. Chitinases as Biomarkers in Bone Studies. In Biomarkers in Bone Disease; Preedy, V.R., Ed.; Biomarkers in Disease: Methods, Discoveries and Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 1–27. ISBN 978-94-007-7745-3. [Google Scholar]
- Johansen, J.S. Studies on Serum YKL-40 as a Biomarker in Diseases with Inflammation, Tissue Remodelling, Fibroses and Cancer. Dan. Med. Bull. 2006, 53, 172–209. [Google Scholar]
- Meng, G.; Zhao, Y.; Bai, X.; Liu, Y.; Green, T.J.; Luo, M.; Zheng, X. Structure of Human Stabilin-1 Interacting Chitinase-like Protein (SI-CLP) Reveals a Saccharide-Binding Cleft with Lower Sugar-Binding Selectivity. J. Biol. Chem. 2010, 285, 39898–39904. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mcshane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheumatol. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Prevoo, M.L.L.; Van’T Hof, M.A.; Kuper, H.H.; Van Leeuwen, M.A.; Van De Putte, L.B.A.; Van Riel, P.L.C.M. Modified Disease Activity Scores That Include Twenty-Eight-Joint Counts Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Ggcorrplot: Visualization of a Correlation Matrix Using “Ggplot2”, R Package Version 0.1.4.1; 2023. Available online: https://cran.r-project.org/web/packages/ggcorrplot/index.html (accessed on 19 May 2024).
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots, R Package Version 0.6.0; 2023. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 19 May 2024).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests, R Package Version 0.7.2; 2023. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 19 May 2024).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Thiele, C.; Hirschfeld, G. Cutpointr: Improved Estimation and Validation of Optimal Cutpoints in R. J. Stat. Softw. 2021, 98, 1–27. [Google Scholar] [CrossRef]
- Stevenson, M.; Sergeant, E.; Firestone, S. epiR: Tools for the Analysis of Epidemiological Data, R Package Version 2.0.65; 2024. Available online: https://cran.r-project.org/web/packages/epiR/index.html (accessed on 19 May 2024).
- Brunson, J. Ggalluvial: Layered Grammar for Alluvial Plots. J. Open Source Softw. 2020, 5, 2017. [Google Scholar] [CrossRef] [PubMed]
- Koenker, R. Quantreg: Quantile Regression, R Package Version 5.97; 2023. Available online: https://cran.r-project.org/web/packages/quantreg/index.html (accessed on 19 May 2024).
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-Protein-1 Function and Its Role in Diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Conigliaro, P.; D’Antonio, A.; D’Erme, L.; Lavinia Fonti, G.; Triggianese, P.; Bergamini, A.; Chimenti, M.S. Failure and Multiple Failure for Disease Modifying Antirheumatic Drugs in Rheumatoid Arthritis: Real-Life Evidence from a Tertiary Referral Center in Italy. PLoS ONE 2023, 18, e0281213. [Google Scholar] [CrossRef]
- Albrecht, K.; Zink, A. Poor Prognostic Factors Guiding Treatment Decisions in Rheumatoid Arthritis Patients: A Review of Data from Randomized Clinical Trials and Cohort Studies. Arthritis Res. Ther. 2017, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Wijbrandts, C.A.; Tak, P.P. Prediction of Response to Targeted Treatment in Rheumatoid Arthritis. Mayo Clin. Proc. 2017, 92, 1129–1143. [Google Scholar] [CrossRef]
- Kirkpatrick, R.; Emery, J.; Connor, J.; Dodds, R.; Lysko, P.; Rosenberg, M. Induction and Expression of Human Cartilage Glycoprotein 39 in Rheumatoid Inflammatory and Peripheral Blood Monocyte-Derived Macrophages. Exp. Cell Res. 1997, 237, 46–54. [Google Scholar] [CrossRef]
- Recklies, A.D.; Ling, H.; White, C.; Bernier, S.M. Inflammatory Cytokines Induce Production of CHI3L1 by Articular Chondrocytes. J. Biol. Chem. 2005, 280, 41213–41221. [Google Scholar] [CrossRef]
- Hakala, B.E.; White, C.; Recklies, A.D. Human Cartilage Gp-39, a Major Secretory Product of Articular Chondrocytes and Synovial Cells, Is a Mammalian Member of a Chitinase Protein Family. J. Biol. Chem. 1993, 268, 25803–25810. [Google Scholar] [CrossRef]
- Volck, B.; Johansen, J.S.; Stoltenberg, M.; Garbarsch§, C.; Price, P.A.; Østergaard, M.; Ostergaard, K.; Løvgreen-Nielsen, P.; Sonne-Holm, S.; Lorenzen, I. Studies on YKL-40 in Knee Joints of Patients with Rheumatoid Arthritis and Osteoarthritis. Involvement of YKL-40 in the Joint Pathology. Osteoarthr. Cartil. 2001, 9, 203–214. [Google Scholar] [CrossRef]
- Nordenbaek, C.; Johansen, J.S.; Junker, P.; Borregaard, N.; Sørensen, O.; Price, P.A. YKL-40, a Matrix Protein of Specific Granules in Neutrophils, Is Elevated in Serum of Patients with Community-Acquired Pneumonia Requiring Hospitalization. J. Infect. Dis. 1999, 180, 1722–1726. [Google Scholar] [CrossRef]
- Johansen, J. Serum YKL-40 Concentrations in Patients with Rheumatoid Arthritis: Relation to Disease Activity. Rheumatology 1999, 38, 618–626. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tsurumoto, T. Serum YKL-40 Levels in Rheumatoid Arthritis: Correlations between Clinical and Laborarory Parameters. Clin. Exp. Rheumatol. 2001, 19, 655–660. [Google Scholar] [PubMed]
- Chen, X.; Jiao, J.; He, X.; Zhang, J.; Wang, H.; Xu, Y.; Jin, T. CHI3L1 Regulation of Inflammation and the Effects on Osteogenesis in a Staphylococcus Aureus Induced Murine Model of Osteomyelitis. FEBS J. 2017, 284, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Szychlinska, M.A.; Tibullo, D.; Malaguarnera, L.; Musumeci, G. Expression of CHI3L1 and CHIT1 in Osteoarthritic Rat Cartilage Model. A Morphological Study. Eur. J. Histochem. 2014, 58, 2423. [Google Scholar] [CrossRef]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic Acid and Ellagic Acid Ameliorate Adjuvant-Induced Arthritis in Rats via Targeting Inflammatory Signals, Chitinase-3-like Protein-1 and Angiogenesis. Biomed. Pharmacother. 2019, 110, 878–886. [Google Scholar] [CrossRef]
- Johansen, J.S.; Jensen, H.S.; Price, P.A. A New Biochemical Marker for Joint Injury. Analysis of YKL-40 in Serum and Synovial Fluid. Rheumatology 1993, 32, 949–955. [Google Scholar] [CrossRef]
- Nielsen, K.R.; Steffensen, R.; Boegsted, M.; Baech, J.; Lundbye-Christensen, S.; Hetland, M.L.; Krintel, S.B.; Johnsen, H.E.; Nyegaard, M.; Johansen, J.S. Promoter Polymorphisms in the Chitinase 3-like 1 Gene Influence the Serum Concentration of YKL-40 in Danish Patients with Rheumatoid Arthritis and in Healthy Subjects. Arthritis Res. Ther. 2011, 13, R109. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Bruce, B.; Fries, J. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23, 14–18. [Google Scholar]
- Li, K.; Wang, M.; Zhao, L.; Liu, Y.; Zhang, X. ACPA-Negative Rheumatoid Arthritis: From Immune Mechanisms to Clinical Translation. eBioMedicine 2022, 83, 104233. [Google Scholar] [CrossRef] [PubMed]
- Maska, L.; Anderson, J.; Michaud, K. Measures of Functional Status and Quality of Life in Rheumatoid Arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res. 2011, 63, S4–S13. [Google Scholar] [CrossRef]
- Wolfe, F. The Determination and Measurement of Functional Disability in Rheumatoid Arthritis. Arthritis Res. 2002, 4, 11–15. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yang, H.-Y.; Lai, J.-H. Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis. Int. J. Mol. Sci. 2020, 21, 4015. [Google Scholar] [CrossRef]
- Koga, T.; Okada, A.; Fukuda, T.; Hidaka, T.; Ishii, T.; Ueki, Y.; Kodera, T.; Nakashima, M.; Takahashi, Y.; Honda, S.; et al. Anti-Citrullinated Peptide Antibodies Are the Strongest Predictor of Clinically Relevant Radiographic Progression in Rheumatoid Arthritis Patients Achieving Remission or Low Disease Activity: A Post Hoc Analysis of a Nationwide Cohort in Japan. PLoS ONE 2017, 12, e0175281. [Google Scholar] [CrossRef]
- Kroot, E.-J.J.A.; de Jong, B.A.W.; Van Leeuwen, M.A.; Swinkels, H.; van den Hoogen, F.H.J. The Prognostic Value of Anti-Cyclic Citrullinated Peptide Antibody in Patients with Recent Onset Rheumatoid Arthritis. Arthritis Rheumatol. 2000, 43, 1831–1835. [Google Scholar] [CrossRef]
- Rantapää-Dahlqvist, S.; De Jong, B.A.W.; Berglin, E.; Hallmans, G.; Wadell, G.; Stenlund, H.; Sundin, U.; Van Venrooij, W.J. Antibodies against Cyclic Citrullinated Peptide and IgA Rheumatoid Factor Predict the Development of Rheumatoid Arthritis. Arthritis Rheumatol. 2003, 48, 2741–2749. [Google Scholar] [CrossRef]
- Sobhy, N.; Ghoniem, S.A.; Eissa, B.M.; Kamal, A.; Medhat, A.; Elsaid, N.Y. Disease Characteristics in High versus Low Titers of Rheumatoid Factor or Anti-Citrullinated Peptide Antibody in Rheumatoid Arthritis Patients. Egypt. Rheumatol. 2022, 44, 325–328. [Google Scholar] [CrossRef]
- Van Den Broek, M.; Dirven, L.; Klarenbeek, N.; Molenaar, T.; Han, K.; Kerstens, P.; Huizinga, T.; Dijkmans, B.; Allaart, C. The Association of Treatment Response and Joint Damage with ACPA-Status in Recent-Onset RA: A Subanalysis of the 8-Year Follow-up of the BeSt Study. Ann. Rheum. Dis. 2012, 71, 245–248. [Google Scholar] [CrossRef]
- Xue, L.; Chu, W.; Wan, F.; Wu, P.; Zhao, X.; Ma, L.; She, Y.; Li, C.; Li, Y. YKL-39 Is an Independent Prognostic Factor in Gastric Adenocarcinoma and Is Associated with Tumor-Associated Macrophage Infiltration and Angiogenesis. World J. Surg. Oncol. 2022, 20, 362. [Google Scholar] [CrossRef]
- Ceuninck, F.D.; Berger, S.; Caliez, A.; Dumont, V.; Raes, M.; Anract, P.; Leclerc, G.; Boutin, J.A.; Ferry, G. Assessment of Some Tools for the Characterization of the Human Osteoarthritic Cartilage Proteome. J. Biomol. Tech. 2005, 16, 256. [Google Scholar]
- Petržalka, M.; Meluzínová, E.; Libertínová, J.; Mojžišová, H.; Hanzalová, J.; Ročková, P.; Elišák, M.; Kmetonyová, S.; Šanda, J.; Sobek, O.; et al. IL-2, IL-6 and Chitinase 3-like 2 Might Predict Early Relapse Activity in Multiple Sclerosis. PLoS ONE 2022, 17, e0270607. [Google Scholar] [CrossRef]
- Costa, J.; Gromicho, M.; Pronto-Laborinho, A.; Almeida, C.; Gomes, R.A.; Guerreiro, A.C.L.; Oliva, A.; Pinto, S.; De Carvalho, M. Cerebrospinal Fluid Chitinases as Biomarkers for Amyotrophic Lateral Sclerosis. Diagnostics 2021, 11, 1210. [Google Scholar] [CrossRef]
- Xiao, W.; Meng, G.; Zhao, Y.; Yuan, H.; Li, T.; Peng, Y.; Zhao, Y.; Luo, M.; Zhao, W.; Li, Z.; et al. Human Secreted Stabilin-1−Interacting Chitinase-like Protein Aggravates the Inflammation Associated with Rheumatoid Arthritis and Is a Potential Macrophage Inflammatory Regulator in Rodents. Arthritis Rheumatol. 2014, 66, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Mamidi, S.; Gratchev, A.; Kremmer, E.; Schmuttermaier, C.; Krusell, L.; Haus, G.; Utikal, J.; Schledzewski, K.; Scholtze, J.; et al. Novel Stabilin-1 Interacting Chitinase-like Protein (SI-CLP) Is up-Regulated in Alternatively Activated Macrophages and Secreted via Lysosomal Pathway. Blood 2006, 107, 3221–3228. [Google Scholar] [CrossRef]
- Fusetti, F.; Pijning, T.; Kalk, K.H.; Bos, E.; Dijkstra, B.W. Crystal Structure and Carbohydrate-Binding Properties of the Human Cartilage Glycoprotein-39. J. Biol. Chem. 2003, 278, 37753–37760. [Google Scholar] [CrossRef]
- Rusak, A.; Jabłońska, K.; Dzięgiel, P. Rola białka YKL-40 w procesie nowotworowym. Postępy Hig. Med. Doświadczalnej 2016, 70, 1286–1299. [Google Scholar]
- Møllgaard, M.; Degn, M.; Sellebjerg, F.; Frederiksen, J.L.; Modvig, S. Cerebrospinal Fluid Chitinase-3-like 2 and Chitotriosidase Are Potential Prognostic Biomarkers in Early Multiple Sclerosis. Eur. J. Neurol. 2016, 23, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Houle, T.T. Statistical Evaluation of a Biomarker. Anesthesiology 2010, 112, 1023–1040. [Google Scholar] [CrossRef]
- Perneger, T.V. How to Use Likelihood Ratios to Interpret Evidence from Randomized Trials. J. Clin. Epidemiol. 2021, 136, 235–242. [Google Scholar] [CrossRef]
- Ranganathan, P.; Aggarwal, R. Understanding the Properties of Diagnostic Tests—Part 2: Likelihood Ratios. Perspect. Clin. Res. 2018, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).