The Gastric Cancer Immune Prognostic Score (GCIPS) Shows Potential in Predicting an Unfavorable Prognosis for Gastric Cancer Patients Undergoing Immune Checkpoint Inhibitor Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Follow-Up
2.3. Immune Checkpoint Inhibitors
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Establishment of the GCIPS in the Test Set
3.3. The Prognostic Value of the GCIPS
3.4. Survival Analysis of the GCIPS in the Test Set
3.4.1. Cox Regression Analysis
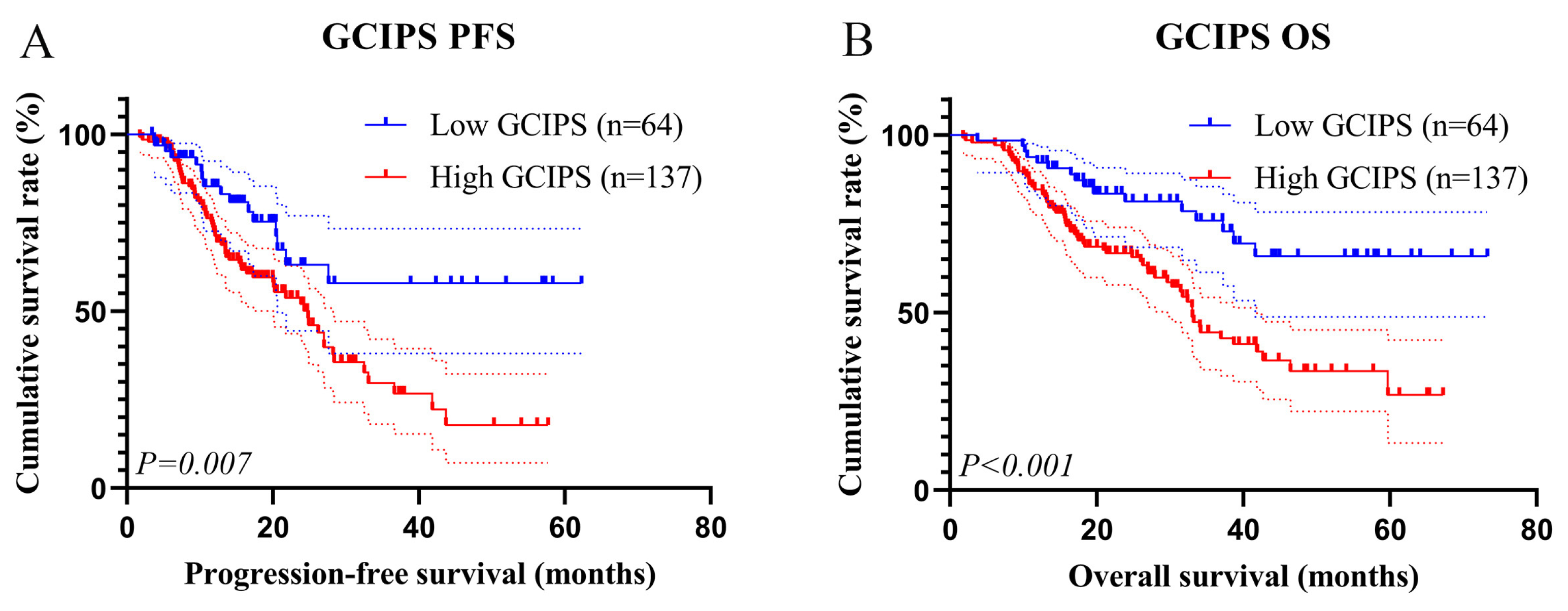
3.4.2. Kaplan–Meier Survival Analysis
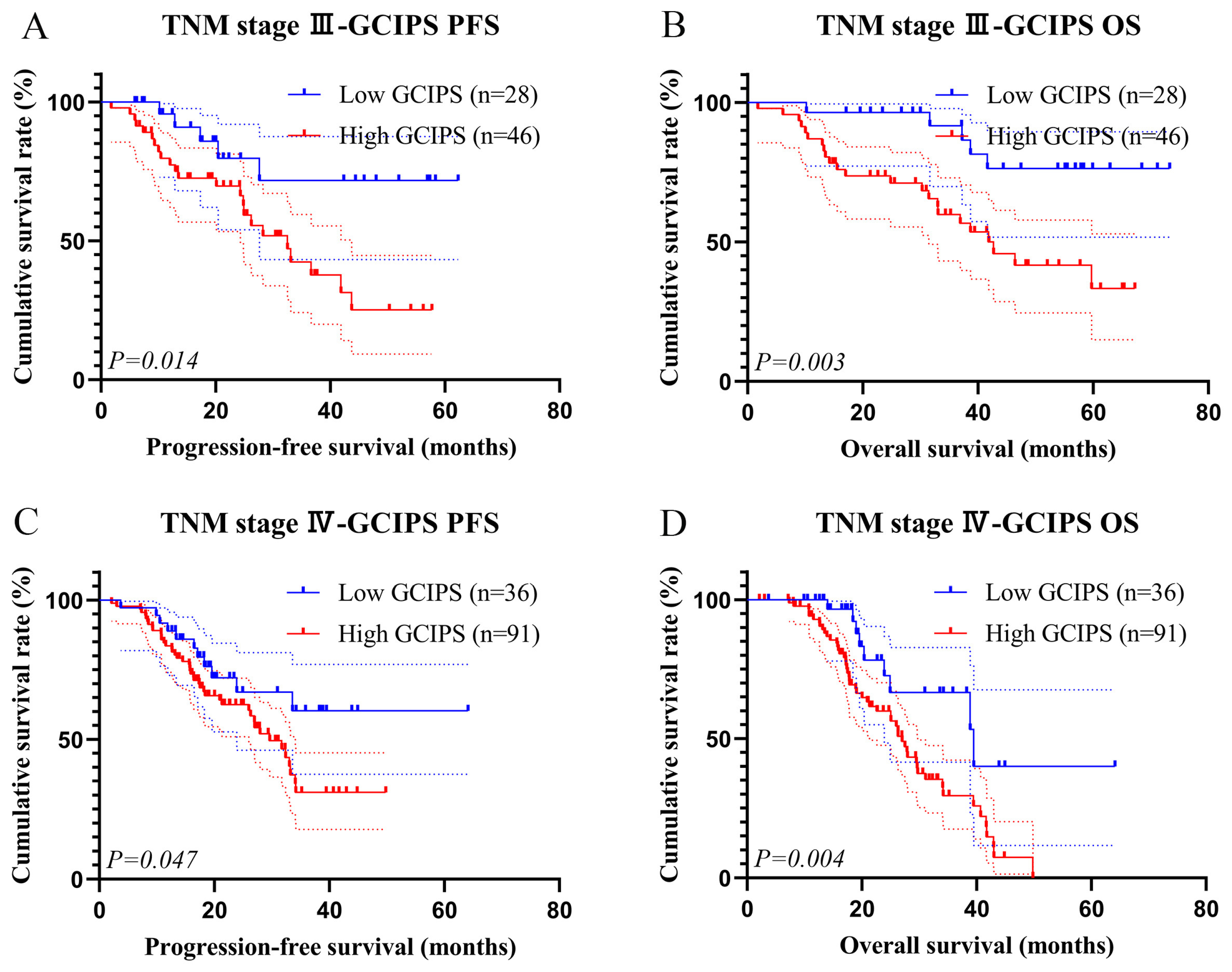
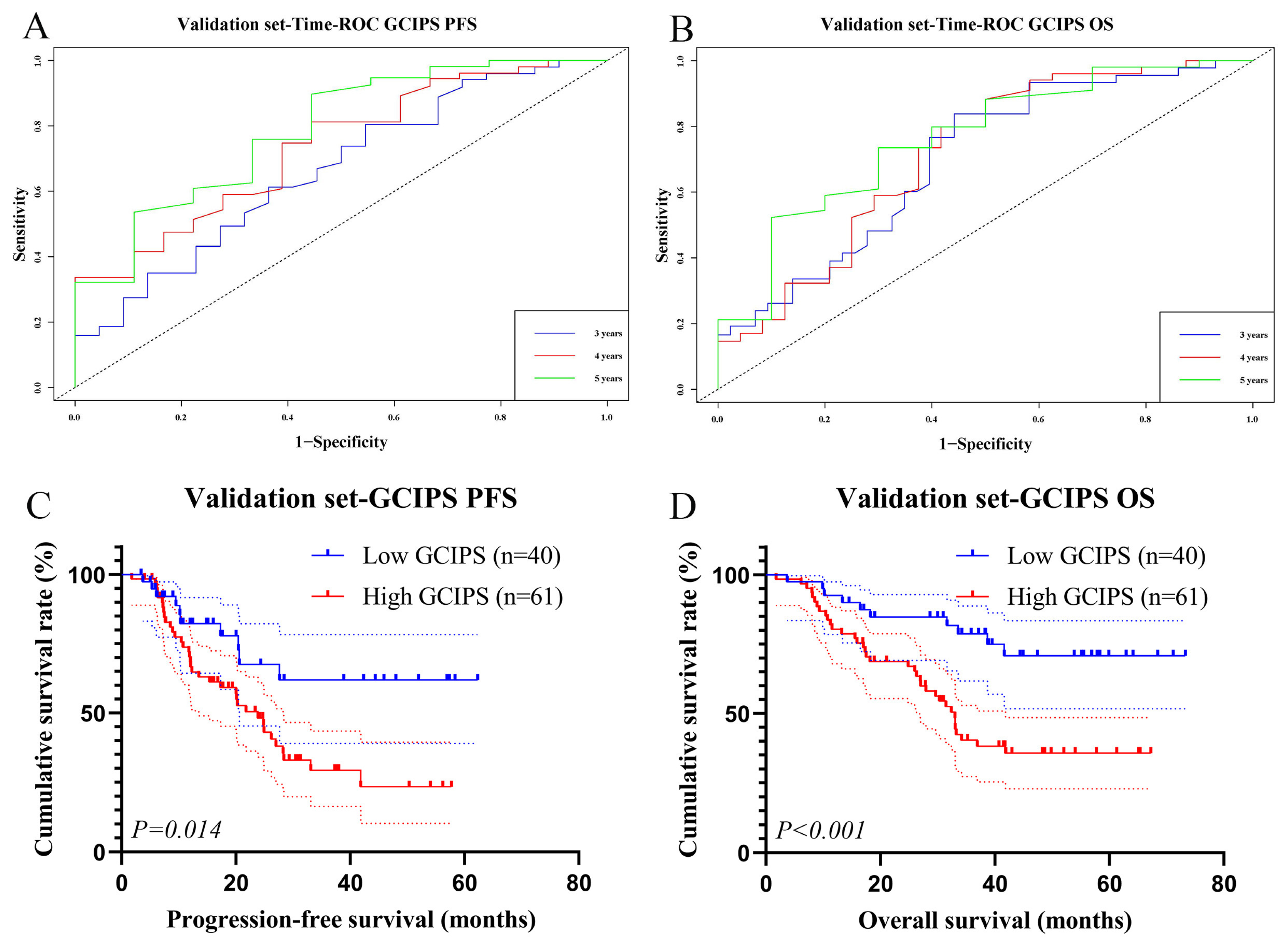
3.5. Survival Analysis of the GCIPS in the Validation Set
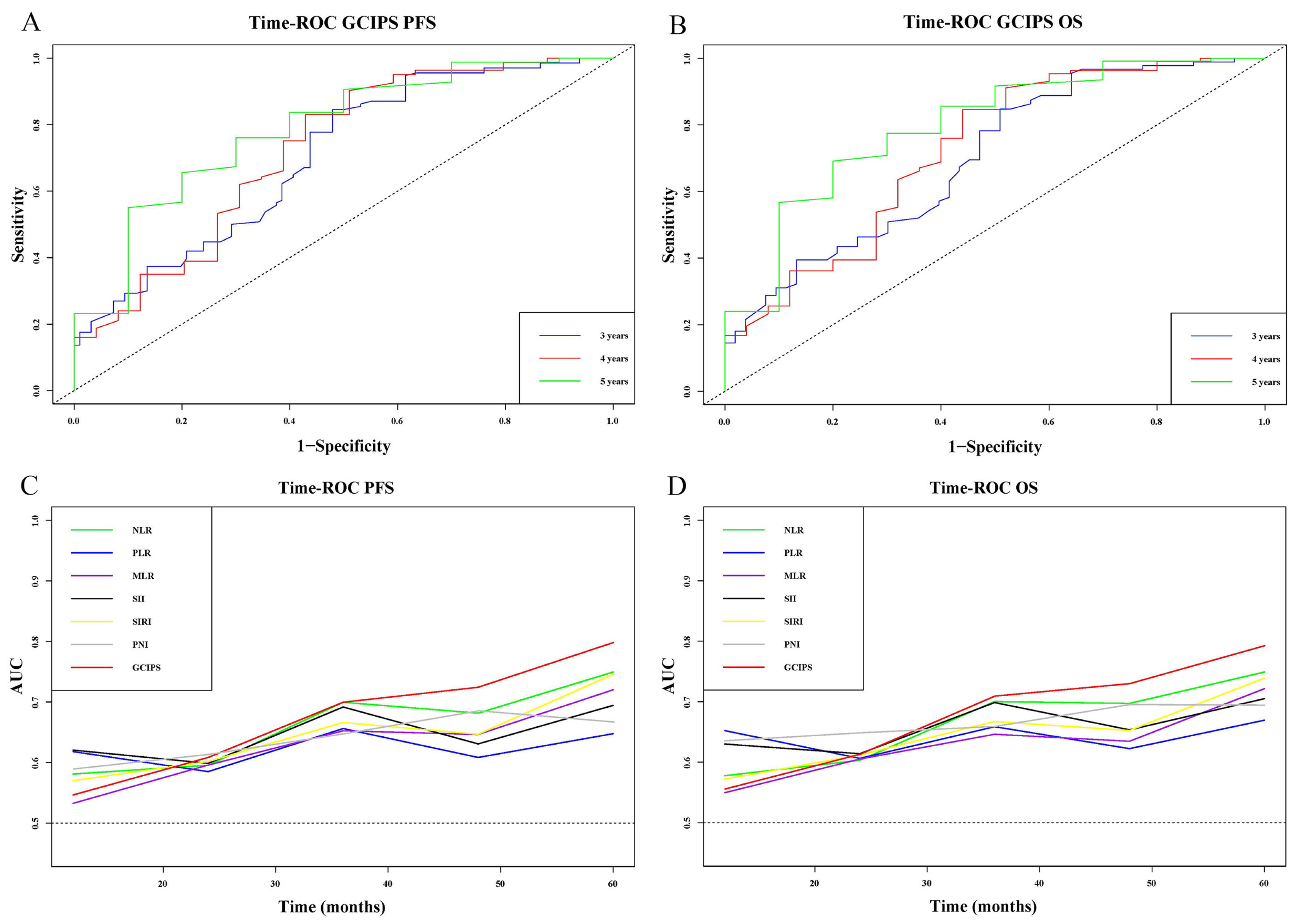
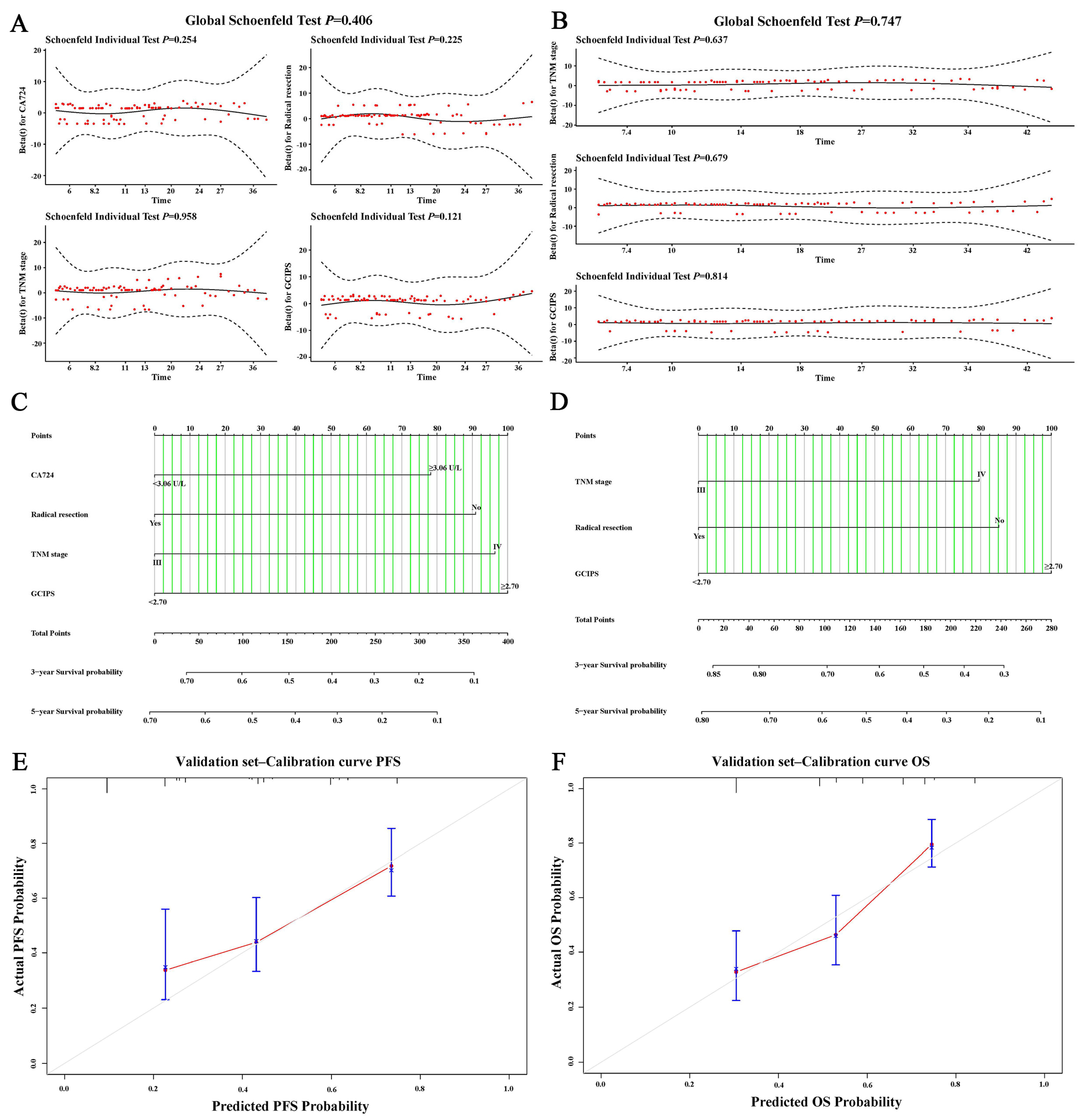
3.6. Nomograms of the GCIPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- López, M.J.; Carbajal, J.; Alfaro, A.L.; Saravia, L.G.; Zanabria, D.; Araujo, J.M.; Quispe, L.; Zevallos, A.; Buleje, J.L.; Cho, C.E.; et al. Characteristics of gastric cancer around the world. Crit. Rev. Oncol. Hematol. 2022, 181, 103841. [Google Scholar] [CrossRef]
- Kono, K.; Nakajima, S.; Mimura, K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric. Cancer 2020, 23, 565–578. [Google Scholar] [CrossRef]
- Zhao, Q.; Cao, L.; Guan, L.; Bie, L.; Wang, S.; Xie, B.; Chen, X.; Shen, X.; Cao, F. Immunotherapy for gastric cancer: Dilemmas and prospect. Brief. Funct. Genom. 2019, 18, 107–112. [Google Scholar] [CrossRef]
- Jin, X.; Liu, Z.; Yang, D.; Yin, K.; Chang, X. Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front. Immunol. 2022, 13, 948647. [Google Scholar] [CrossRef]
- Li, S.; Yu, W.; Xie, F.; Luo, H.; Liu, Z.; Lv, W.; Shi, D.; Yu, D.; Gao, P.; Chen, C.; et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat. Commun. 2023, 14, 8. [Google Scholar] [CrossRef]
- Li, K.; Zhang, A.; Li, X.; Zhang, H.; Zhao, L. Advances in clinical immunotherapy for gastric cancer. BBA-Rev. Cancer 2021, 1876, 188615. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandala, M.; Ryu, M.H.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric. Cancer 2021, 25, 197–206. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Caglevic, C.; Chung, H.C.; Muro, K.; Van Cutsem, E.; et al. Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 1127–1136. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and Therapeutic Significance of Heat Shock Proteins in Cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef] [PubMed]
- Röcken, C. Predictive biomarkers in gastric cancer. J. Cancer Res. Clin. 2022, 149, 467–481. [Google Scholar] [CrossRef]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. EJSO-Eur. J. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Mouchli, M.; Reddy, S.; Gerrard, M.; Boardman, L.; Rubio, M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Review article. Ann. Hepatol. 2020, 22, 100249. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Guo, H.; Sun, C.; Yang, P.; Kim, N.H.; Tian, Y.; Liu, Y.; Liu, P.; Li, Y.; Zhao, Q. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: A prospective study. BMC Gastroenterol. 2022, 22, 121. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhai, E.T.; Yuan, Y.J.; Wu, K.M.; Xu, J.B.; Peng, J.J.; Chen, C.Q.; He, Y.L.; Cai, S.R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef]
- Rebuzzi, S.E.; Prelaj, A.; Friedlaender, A.; Cortellini, A.; Addeo, A.; Genova, C.; Naqash, A.R.; Auclin, E.; Mezquita, L.; Banna, G.L. Prognostic scores including peripheral blood-derived inflammatory indices in patients with advanced non-small-cell lung cancer treated with immune checkpoint inhibitors. Crit. Rev. Oncol. Hematol. 2022, 179, 103806. [Google Scholar] [CrossRef]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Zhang, R.; Guo, Z.; Wang, P.; Qu, Z. Prognostic value of nutritional and inflammatory markers in patients with hepatocellular carcinoma who receive immune checkpoint inhibitors. Oncol. Lett. 2023, 26, 437. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2019, 18, 534–542. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef]
- Nose, Y.; Saito, T.; Yamamoto, K.; Yamashita, K.; Tanaka, K.; Yamamoto, K.; Makino, T.; Takahashi, T.; Kawashima, A.; Haruna, M.; et al. The tissue-resident marker CD103 on peripheral blood T cells predicts responses to anti-PD-1 therapy in gastric cancer. Cancer Immunol. Immunother. 2022, 72, 169–181. [Google Scholar] [CrossRef]
- Yang, H.; Zou, X.; Yang, S.; Zhang, A.; Li, N.; Ma, Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Front. Immunol. 2023, 14, 1149989. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Huang, R.; Pan, H.; Zuo, Y.; Zhao, R.; Xue, Y.; Song, H. Prognostic nutritional index for predicting the clinical outcomes of patients with gastric cancer who received immune checkpoint inhibitors. Front. Nutr. 2022, 9, 1038118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, W.; Qiu, Z.; Kuang, T.; Wang, K.; Hu, B.; Wang, W. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 2023, 14, 1219929. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Ding, Y.; Mao, C.; Ma, X.; Li, N.; Xiao, C.; Qian, J.; Jiang, H.; Zheng, Y.; Wu, L.; et al. Association of inflammatory markers with survival in patients with advanced gastric cancer treated with immune checkpoint inhibitors combined with chemotherapy as first line treatment. Front. Oncol. 2022, 12, 1029960. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, W.; Duan, Z.; Li, J.; Liu, Y.; Liu, C.; Zhang, L.; Zhang, Q.; Zhou, S.; Zhang, K.; et al. Development and validation of a nomogram for predicting varices needing treatment in compensated advanced chronic liver disease: A multicenter study. Saudi J. Gastroenterol. 2021, 27, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zheng, G.; Wang, Q.; Liu, S.; Liu, Z.; Xu, G.; Wang, F.; Guo, M.; Lian, X.; Zhang, H. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. 2018, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Sun, Q. Prognostic and clinicopathological significance of prognostic nutritional index (PNI) in patients with oral cancer: A meta-analysis. Aging 2023, 15, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Cheng, X.; Li, T.; Xiao, Y.; Peng, L.; Feng, Y.; He, J.; Hao, P.; Deng, H.; et al. White Blood Cell Count Predicts Mortality in Patients with Spontaneous Intracerebral Hemorrhage. Neurocrit. Care 2023, 39, 445–454. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2021, 22, 173–187. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. BBA-Rev. Cancer 2022, 1877, 188762. [Google Scholar] [CrossRef]
- Ocana, A.; Nieto-Jiménez, C.; Pandiella, A.; Templeton, A.J. Neutrophils in cancer: Prognostic role and therapeutic strategies. Mol. Cancer 2017, 16, 137. [Google Scholar] [CrossRef]
- Ng, M.S.F.; Tan, L.; Wang, Q.; Mackay, C.R.; Ng, L.G. Neutrophils in cancer-unresolved questions. Sci. China Life Sci. 2021, 64, 1829–1841. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin. Cancer Res. 2020, 27, 2383–2393. [Google Scholar] [CrossRef]
- Patysheva, M.; Frolova, A.; Larionova, I.; Afanas’ev, S.; Tarasova, A.; Cherdyntseva, N.; Kzhyshkowska, J. Monocyte programming by cancer therapy. Front. Immunol. 2022, 13, 994319. [Google Scholar] [CrossRef] [PubMed]
- Ugel, S.; Canè, S.; De Sanctis, F.; Bronte, V. Monocytes in the Tumor Microenvironment. Annu. Rev. Pathol.-Mech. 2021, 16, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.J.; Ma, J.Y.; Hu, G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin. Chim. Acta 2018, 481, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Fendl, B.; Berghoff, A.S.; Preusser, M.; Maier, B. Macrophage and monocyte subsets as new therapeutic targets in cancer immunotherapy. ESMO Open 2023, 8, 100776. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- MacPherson, S.; Kilgour, M.; Lum, J.J. Understanding lymphocyte metabolism for use in cancer immunotherapy. FEBS J. 2018, 285, 2567–2578. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 19, 75–90. [Google Scholar] [CrossRef]
- Asrir, A.; Tardiveau, C.; Coudert, J.; Laffont, R.; Blanchard, L.; Bellard, E.; Veerman, K.; Bettini, S.; Lafouresse, F.; Vina, E.; et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell 2022, 40, 318–334.e9. [Google Scholar] [CrossRef] [PubMed]
- Grisaru-Tal, S.; Rothenberg, M.E.; Munitz, A. Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat. Immunol. 2022, 23, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, H.; Pan, H.; Zuo, Y.; Zhao, R.; Huang, R.; Xue, Y.; Song, H. CD19 (+) B Cell Combined with Prognostic Nutritional Index Predicts the Clinical Outcomes of Patients with Gastric Cancer Who Undergoing Surgery. Cancers 2023, 15, 2531. [Google Scholar] [CrossRef] [PubMed]
- Chornenki, N.L.J.; Fralick, M.; Sholzberg, M. International normalized ratio and activated partial thromboplastin time testing. Can. Med. Assoc. J. 2022, 194, E1135. [Google Scholar] [CrossRef] [PubMed]
- Hiraide, M.; Minowa, Y.; Nakano, Y.; Suzuki, K.; Shiga, T.; Nishio, M.; Miyoshi, J.; Takahashi, H.; Hama, T. Drug interactions between tyrosine kinase inhibitors (gefitinib and erlotinib) and warfarin: Assessment of international normalized ratio elevation characteristics and in vitro CYP2C9 activity. J. Oncol. Pharm. Pract. 2018, 25, 1599–1607. [Google Scholar] [CrossRef]
- Haruki, K.; Shiba, H.; Saito, N.; Horiuchi, T.; Shirai, Y.; Fujiwara, Y.; Furukawa, K.; Sakamoto, T.; Yanaga, K. Risk stratification using a novel liver functional reserve score of combination prothrombin time-international normalized ratio to albumin ratio and albumin in patients with hepatocellular carcinoma. Surgery 2018, 164, 404–410. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, C.; Fang, L.; Yao, S.K. Increased international normalized ratio level in hepatocellular carcinoma patients with diabetes mellitus. World J. Gastroenterol. 2013, 19, 2395–2403. [Google Scholar] [CrossRef]


| Total Set | Test Set | Validation Set | p | |
|---|---|---|---|---|
| Items | n = 302 | n = 201 | n = 101 | |
| Age (years), mean (SD) | 63.73 (10.56) | 57.94 (9.97) | 57.72 (9.94) | 0.861 |
| Sex, n (%) | 0.316 | |||
| Male | 200 (66.2) | 137 (68.2) | 63 (62.4) | |
| Female | 102 (33.8) | 64 (31.8) | 38 (37.6) | |
| BMI (Kg/m2), mean (SD) | 21.93 (3.28) | 21.95 (3.26) | 21.90 (3.34) | 0.906 |
| SLN, n (%) | 0.443 | |||
| Positive | 36 (11.9) | 26 (12.9) | 10 (9.9) | |
| Negative | 266 (88.1) | 175 (87.1) | 91 (90.1) | |
| Surgery, n (%) | 0.092 | |||
| Yes | 177 (58.6) | 111 (55.2) | 66 (65.3) | |
| No | 125 (41.4) | 90 (44.8) | 35 (34.7) | |
| Radical resection, n (%) | 0.080 | |||
| Yes | 108 (35.8) | 65 (32.3) | 43 (42.6) | |
| No | 194 (64.2) | 136 (67.7) | 58 (57.4) | |
| Primary tumor site, n (%) | 0.912 | |||
| Upper 1/3 | 44 (14.6) | 30 (14.9) | 14 (13.9) | |
| Middle 1/3 | 66 (21.9) | 45 (22.4) | 21 (20.8) | |
| Low 1/3 | 184 (60.9) | 120 (59.7) | 64 (63.4) | |
| Whole | 8 (2.6) | 6 (3.0) | 2 (2.0) | |
| Borrmann type, n (%) | 0.173 | |||
| I | 38 (12.6) | 25 (12.4) | 13 (12.9) | |
| II | 6 (2.0) | 4 (2.0) | 2 (2.0) | |
| III | 193 (63.9) | 136 (67.7) | 57 (56.4) | |
| IV | 65 (21.5) | 36 (17.9) | 29 (28.7) | |
| Tumor size, n (%) | 0.952 | |||
| <20 mm | 55 (18.2) | 36 (17.9) | 19 (18.8) | |
| 20–50 mm | 32 (10.6) | 22 (10.9) | 10 (9.9) | |
| >50 mm | 215 (71.2) | 143 (71.1) | 72 (71.3) | |
| Differentiation, n (%) | 0.157 | |||
| Poor | 121 (40.1) | 74 (36.8) | 47 (46.5) | |
| Moderate | 26 (8.6) | 15 (7.5) | 11 (10.9) | |
| Good | 2 (0.7) | 1 (0.5) | 1 (1.0) | |
| Unknown | 153 (50.7) | 111 (55.2) | 42 (41.6) | |
| Lauren type, n (%) | 0.237 | |||
| Intestinal | 27 (8.9) | 16 (8.0) | 11 (10.9) | |
| Diffuse | 27 (8.9) | 16 (8.0) | 11 (10.9) | |
| Mixed | 34 (11.3) | 19 (9.5) | 15 (14.9) | |
| Unknown | 214 (70.9) | 150 (74.6) | 64 (63.4) | |
| TNM stage, n (%) | 0.104 | |||
| III | 121 (40.1) | 74 (36.8) | 47 (46.5) | |
| IV | 181 (59.9) | 127 (63.2) | 54 (53.5) | |
| AFP, n (%) | 0.272 | |||
| <2.96 ng/mL | 151 (50.0) | 105 (52.2) | 46 (45.5) | |
| ≥2.96 ng/mL | 151 (50.0) | 96 (47.8) | 55 (54.5) | |
| CEA, n (%) | ||||
| <2.43 ng/mL | 151 (50.0) | 91 (45.3) | 60 (59.4) | 0.051 |
| ≥2.43 ng/mL | 151 (50.0) | 110 (54.7) | 41 (40.6) | |
| CA199, n (%) | 0.080 | |||
| <14.40 U/L | 149 (49.3) | 92 (45.8) | 57 (56.4) | |
| ≥14.40 U/L | 153 (50.7) | 109 (54.2) | 44 (43.6) | |
| CA724, n (%) | 0.155 | |||
| <3.06 U/L | 150 (49.7) | 94 (46.8) | 56 (55.4) | |
| ≥3.06 U/L | 152 (50.3) | 107 (53.2) | 45 (44.6) | |
| CA125Ⅱ, n (%) | 0.653 | |||
| <25.19 U/L | 144 (47.7) | 94 (46.8) | 50 (49.5) | |
| ≥25.19 U/L | 158 (52.3) | 107 (53.2) | 51 (50.5) |
| Total Set | Test Set | Validation Set | p | |
|---|---|---|---|---|
| Items | n = 302 | n = 201 | n = 101 | |
| ALT (U/L, median (IQR)) | 14.00 (10.00, 26.00) | 14.00 (10.00, 25.95) | 14.00 (10.00, 26.00) | 0.910 |
| AST (U/L, median (IQR)) | 20.00 (16.00, 27.00) | 20.00 (16.00, 28.00) | 20.00 (16.00, 25.00) | 0.559 |
| γ-GGT (U/L, median (IQR)) | 23.00 (16.00, 45.50) | 24.00 (16.00, 46.00) | 21.00 (16.00, 45.50) | 0.661 |
| LDH (U/L, median (IQR)) | 171.00 (147.00, 215.00) | 174.00 (148.00, 216.50) | 167.00 (144.50, 208.50) | 0.164 |
| TBIL (μmol/L, median (IQR)) | 12.00 (9.20, 15.60) | 12.00 (9.20, 15.70) | 12.00 (9.15, 15.31) | 0.653 |
| DBIL (μmol/L, median (IQR)) | 2.70 (1.93, 3.66) | 2.60 (1.85, 3.60) | 2.80 (2.03, 3.86) | 0.218 |
| IDBIL (μmol/L, median (IQR)) | 9.00 (7.00, 12.10) | 9.30 (7.10, 12.17) | 8.79 (6.63, 11.85) | 0.235 |
| TP (g/L, mean (SD)) | 69.13 (7.10) | 69.32 (7.11) | 68.73 (7.08) | 0.494 |
| ALB (g/L, mean (SD)) | 38.98 (4.43) | 38.85 (4.46) | 39.26 (4.40) | 0.447 |
| GLOB (g/L, mean (SD)) | 30.06 (5.11) | 30.36 (5.22) | 29.46 (4.85) | 0.153 |
| A/G, mean (SD) | 1.33 (0.25) | 1.31 (0.25) | 1.35 (0.26) | 0.129 |
| PALB (mg/L, mean (SD)) | 199.60 (65.32) | 202.30 (65.66) | 194.24 (64.63) | 0.313 |
| BUN (mmol/L, mean (SD)) | 5.52 (1.60) | 5.61 (1.62) | 5.33 (1.56) | 0.158 |
| CREA (μmol/L, mean (SD)) | 76.14 (16.56) | 76.08 (16.15) | 76.27 (17.44) | 0.926 |
| UA (μmol/L, mean (SD)) | 290.70 (83.67) | 296.76 (87.07) | 278.64 (75.44) | 0.076 |
| ALP (U/L, median (IQR)) | 90.00 (73.00, 121.00) | 89.00 (71.50, 121.00) | 91.00 (73.50, 121.00) | 0.631 |
| Glu (mmol/L, median (IQR)) | 5.10 (4.57, 5.80) | 5.10 (4.60, 5.80) | 5.10 (4.50, 5.80) | 0.513 |
| WBC (109/L, median (IQR)) | 6.40 (5.07, 8.09) | 6.46 (5.13, 8.33) | 6.31 (4.90, 7.93) | 0.232 |
| NEU (109/L, median (IQR)) | 3.90 (2.84, 5.38) | 3.97 (2.92, 5.44) | 3.76 (2.72, 5.03) | 0.247 |
| LYM (109/L, median (IQR)) | 1.65 (1.24, 1.99) | 1.65 (1.24, 2.03) | 1.63 (1.22, 1.93) | 0.601 |
| MON (109/L, median (IQR)) | 0.48 (0.32, 0.62) | 0.49 (0.33, 0.64) | 0.44 (0.29, 0.60) | 0.085 |
| RBC (109/L, mean (SD)) | 4.27 (0.62) | 4.29 (0.62) | 4.23 (0.62) | 0.492 |
| HGB (109/L, mean (SD)) | 122.15 (21.75) | 122.70 (22.44) | 121.07 (20.39) | 0.539 |
| HCT (109/L, mean (SD)) | 37.86 (6.38) | 38.10 (6.81) | 37.40 (5.41) | 0.337 |
| PLT (109/L, median (IQR)) | 236.50 (184.00, 312.00) | 241.00 (186.50, 314.00) | 232.00 (180.50, 308.50) | 0.546 |
| INR, mean (SD) | 1.00 (0.13) | 1.01 (0.12) | 0.98 (0.15) | 0.135 |
| Fbg (g/L, median (IQR)) | 3.47 (2.80, 4.36) | 3.47 (2.80, 4.37) | 3.47 (2.79, 4.34) | 0.612 |
| Ddi (ng/L, median (IQR)) | 0.75 (0.41, 1.58) | 0.67 (0.41, 1.19) | 0.78 (0.46, 1.80) | 0.211 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Items | HR | 95% CI | p | HR | 95% CI | p |
| ALT (U/L) | 0.996 | 0.985–1.008 | 0.509 | |||
| AST (U/L) | 1.008 | 0.999–1.017 | 0.070 | |||
| γ-GGT (U/L) | 1.002 | 1.000–1.004 | 0.027 | 1.001 | 0.998–1.003 | 0.543 |
| LDH (U/L) | 1.001 | 1.000–1.002 | 0.068 | |||
| TBIL (μmol/L) | 1.004 | 0.999–1.008 | 0.106 | |||
| DBIL (μmol/L) | 1.007 | 0.999–1.016 | 0.089 | |||
| IDBIL (μmol/L) | 1.007 | 0.998–1.016 | 0.141 | |||
| TP (g/L) | 0.977 | 0.955–0.999 | 0.044 | 0.978 | 0.954–1.002 | 0.077 |
| ALB (g/L) | 0.942 | 0.908–0.978 | 0.002 | |||
| GLOB (g/L) | 0.997 | 0.964–1.031 | 0.862 | |||
| A/G | 0.452 | 0.219–0.932 | 0.032 | 0.553 | 0.243–1.260 | 0.159 |
| PALB (mg/L) | 0.995 | 0.992–0.998 | 0.001 | 0.998 | 0.994–1.001 | 0.174 |
| BUN (mmol/L) | 0.973 | 0.862–1.097 | 0.651 | |||
| CREA (μmol/L) | 0.993 | 0.981–1.004 | 0.216 | |||
| UA (μmol/L) | 0.999 | 0.996–1.001 | 0.172 | |||
| ALP (U/L) | 1.002 | 1.000–1.004 | 0.022 | 1.001 | 0.999–1.003 | 0.399 |
| Glu (mmol/L) | 0.943 | 0.813–1.094 | 0.439 | |||
| WBC (109/L) | 1.084 | 1.046–1.123 | <0.001 | 1.073 | 1.035–1.113 | <0.001 |
| NEU (109/L) | 1.083 | 1.006–1.166 | 0.034 | 0.951 | 0.871–1.037 | 0.256 |
| LYM (109/L) | 0.637 | 0.475–0.854 | 0.003 | 0.720 | 0.535–0.969 | 0.030 |
| MON (109/L) | 0.830 | 0.355–1.940 | 0.667 | |||
| RBC (109/L) | 0.798 | 0.600–1.061 | 0.120 | |||
| HGB (109/L) | 0.997 | 0.989–1.005 | 0.425 | |||
| HCT (109/L) | 0.992 | 0.965–1.020 | 0.567 | |||
| PLT (109/L) | 1.002 | 1.000–1.004 | 0.026 | 1.002 | 1.000–1.004 | 0.127 |
| INR | 2.788 | 1.748–3.335 | <0.001 | 1.732 | 1.034–3.812 | 0.007 |
| Fbg (g/L) | 1.017 | 0.921–1.124 | 0.739 | |||
| Ddi (ng/L) | 1.066 | 0.967–1.175 | 0.198 | |||
| Items | β Value | HR | 95% CI | p |
|---|---|---|---|---|
| WBC (109/L) | 0.071 | 1.074 | 1.038–1.111 | <0.001 |
| LYM (109/L) | −0.375 | 0.687 | 0.512–0.922 | 0.012 |
| INR | 2.986 | 2.809 | 1.882–3.132 | 0.002 |
| Items | Calculation Formulas |
|---|---|
| NLR | Neutrophils (109/L)/lymphocytes (109/L) |
| PLR | Platelets (109/L) /lymphocytes (109/L) |
| MLR | Monocytes (109/L)/lymphocytes (109/L) |
| SII | Platelets (109/L) × neutrophils (109/L)/lymphocytes (109/L) |
| SIRI | Monocytes (109/L) × neutrophils (109/L)/lymphocytes (109/L) |
| PNI | Albumin (g/dL) + 5 × lymphocytes (109/L) |
| Items | AUC | 95% CI |
|---|---|---|
| NLR | 0.581 | 0.501–0.661 |
| PLR | 0.540 | 0.459–0.621 |
| MLR | 0.513 | 0.431–0.595 |
| SII | 0.544 | 0.463–0.625 |
| SIRI | 0.507 | 0.425–0.588 |
| PNI | 0.591 | 0.511–0.671 |
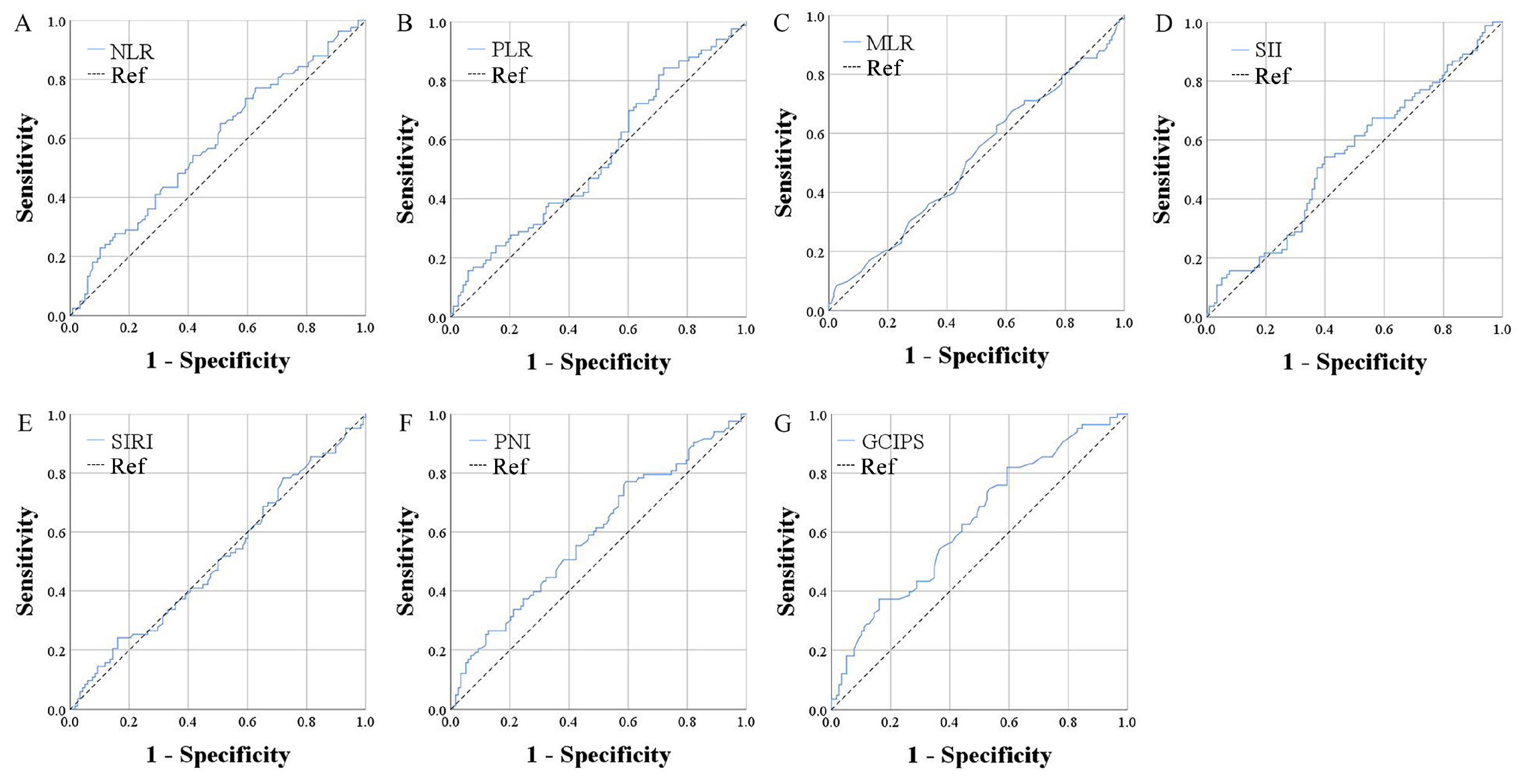
| GCIPS | 0.634 | 0.557–0.712 |
| PFS | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Items | HR (95% CI) | p | HR (95% CI) | p |
| Age (years) | 1.001 (0.984–1.019) | 0.879 | ||
| Sex | ||||
| Male | Ref | |||
| Female | 1.017 (0.708–1.461) | 0.927 | ||
| BMI (Kg/m2) | 0.977 (0.927–1.029) | 0.376 | ||
| GCIPS | ||||
| <2.70 | Ref | Ref | ||
| ≥2.70 | 2.197 (1.425–3.387) | <0.001 | 2.020 (1.092–2.708) | 0.009 |
| SLN | ||||
| Negative | Ref | Ref | ||
| Positive | 1.661 (1.037–2.662) | 0.035 | 1.372 (0.842–2.235) | 0.204 |
| Surgery | ||||
| Yes | Ref | Ref | ||
| No | 1.836 (1.275–2.643) | 0.001 | 1.321 (0.811–2.152) | 0.264 |
| Radical resection | ||||
| Yes | Ref | Ref | ||
| No | 2.504 (1.692–3.705) | <0.001 | 1.922 (1.171–3.156) | 0.010 |
| Primary tumor site | ||||
| Low 1/3 | Ref | |||
| #Other | 1.004 (0.698–1.444) | 0.984 | ||
| Borrmann type | ||||
| Ⅰ + Ⅱ | Ref | |||
| III + IV | 1.062 (0.675–1.671) | 0.795 | ||
| Tumor size | ||||
| <50 mm | Ref | Ref | ||
| ≥50 mm | 1.513 (1.015–2.254) | 0.042 | 1.087 (0.642–1.842) | 0.756 |
| TNM stage | ||||
| III | Ref | Ref | ||
| IV | 2.810 (1.891–4.175) | <0.001 | 1.800 (1.005–3.224) | 0.048 |
| AFP | ||||
| <2.96 ng/mL | Ref | |||
| ≥2.96 ng/mL | 1.230 (0.869–1.740) | 0.242 | ||
| CEA | ||||
| <2.43 ng/mL | Ref | Ref | ||
| ≥2.43 ng/mL | 1.801 (1.256–2.582) | 0.001 | 1.322 (0.892–1.960) | 0.164 |
| CA199 | ||||
| <14.40 U/L | Ref | Ref | ||
| ≥14.40 U/L | 1.326 (0.936–1.879) | 0.013 | 1.079 (0.754–1.546) | 0.676 |
| CA724 | ||||
| <3.06 U/L | Ref | Ref | ||
| ≥3.06 U/L | 2.210 (1.535–3.180) | <0.001 | 1.494 (1.007–2.218) | 0.046 |
| CA125Ⅱ | ||||
| <25.19 U/L | Ref | |||
| ≥25.19 U/L | 1.229 (0.868–1.739) | 0.245 |
| OS | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Items | HR (95% CI) | p | HR (95% CI) | p |
| Age (years) | 0.999 (0.982–1.016) | 0.880 | ||
| Sex | ||||
| Male | Ref | |||
| Female | 1.078 (0.750–1.549) | 0.684 | ||
| BMI (Kg/m2) | 0.973 (0.922–1.026) | 0.312 | ||
| GCIPS | ||||
| <2.70 | Ref | Ref | ||
| ≥2.70 | 2.776 (1.801–4.278) | <0.001 | 2.272 (1.464–3.524) | <0.001 |
| SLN | ||||
| Negative | Ref | Ref | ||
| Positive | 2.011 (1.254–3.223) | 0.004 | 1.612 (0.995–2.612) | 0.053 |
| Surgery | ||||
| Yes | Ref | Ref | ||
| No | 1.637 (1.144–2.342) | 0.007 | 1.469 (0.925–2.418) | 0.101 |
| Radical resection | ||||
| Yes | Ref | Ref | ||
| No | 2.384 (1.614–3.521) | <0.001 | 1.901 (1.161–3.113) | 0.011 |
| Primary tumor site | ||||
| Low 1/3 | Ref | |||
| #Other | 1.001 (0.696–1.439) | 0.997 | ||
| Borrmann type | ||||
| Ⅰ + Ⅱ | Ref | |||
| III + IV | 1.229 (0.783–1.930) | 0.370 | ||
| Tumor size | ||||
| <50 mm | Ref | |||
| ≥50 mm | 1.428 (0.960–2.125) | 0.078 | ||
| TNM stage | ||||
| III | Ref | Ref | ||
| IV | 2.279 (1.560–3.330) | <0.001 | 1.755 (1.201–2.565) | 0.004 |
| AFP | ||||
| <2.96 ng/mL | Ref | |||
| ≥2.96 ng/mL | 1.148 (0.812–1.623) | 0.435 | ||
| CEA | ||||
| <2.43 ng/mL | Ref | Ref | ||
| ≥2.43 ng/mL | 2.081 (1.454–2.979) | <0.001 | 1.481 (1.000–2.194) | 0.050 |
| CA199 | ||||
| <14.40 U/L | Ref | Ref | ||
| ≥14.40 U/L | 1.550 (1.092–2.201) | 0.014 | 1.080 (0.751–1.553) | 0.678 |
| CA724 | ||||
| <3.06 U/L | Ref | Ref | ||
| ≥3.06 U/L | 2.464 (1.713–3.546) | <0.001 | 1.475 (0.884–2.461) | 0.137 |
| CA125Ⅱ | ||||
| <25.19 U/L | Ref | |||
| ≥25.19 U/L | 1.109 (0.784–1.567) | 0.560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Sun, H.; Pan, H.; Zhao, R.; Xue, Y.; Song, H. The Gastric Cancer Immune Prognostic Score (GCIPS) Shows Potential in Predicting an Unfavorable Prognosis for Gastric Cancer Patients Undergoing Immune Checkpoint Inhibitor Treatment. Biomedicines 2024, 12, 491. https://doi.org/10.3390/biomedicines12030491
Zuo Y, Sun H, Pan H, Zhao R, Xue Y, Song H. The Gastric Cancer Immune Prognostic Score (GCIPS) Shows Potential in Predicting an Unfavorable Prognosis for Gastric Cancer Patients Undergoing Immune Checkpoint Inhibitor Treatment. Biomedicines. 2024; 12(3):491. https://doi.org/10.3390/biomedicines12030491
Chicago/Turabian StyleZuo, Yanjiao, Hao Sun, Hongming Pan, Ruihu Zhao, Yingwei Xue, and Hongjiang Song. 2024. "The Gastric Cancer Immune Prognostic Score (GCIPS) Shows Potential in Predicting an Unfavorable Prognosis for Gastric Cancer Patients Undergoing Immune Checkpoint Inhibitor Treatment" Biomedicines 12, no. 3: 491. https://doi.org/10.3390/biomedicines12030491
APA StyleZuo, Y., Sun, H., Pan, H., Zhao, R., Xue, Y., & Song, H. (2024). The Gastric Cancer Immune Prognostic Score (GCIPS) Shows Potential in Predicting an Unfavorable Prognosis for Gastric Cancer Patients Undergoing Immune Checkpoint Inhibitor Treatment. Biomedicines, 12(3), 491. https://doi.org/10.3390/biomedicines12030491






