Interleukin-1α as a Potential Prognostic Biomarker in Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.2.1. TCGA Data
2.2.2. ICGC Data
2.2.3. Single-Cell RNA-Seq Data
2.3. Single-Cell RNA-Seq Data Analysis
2.4. IL-1 Pathway Analysis
2.5. Statistical Analysis
3. Results
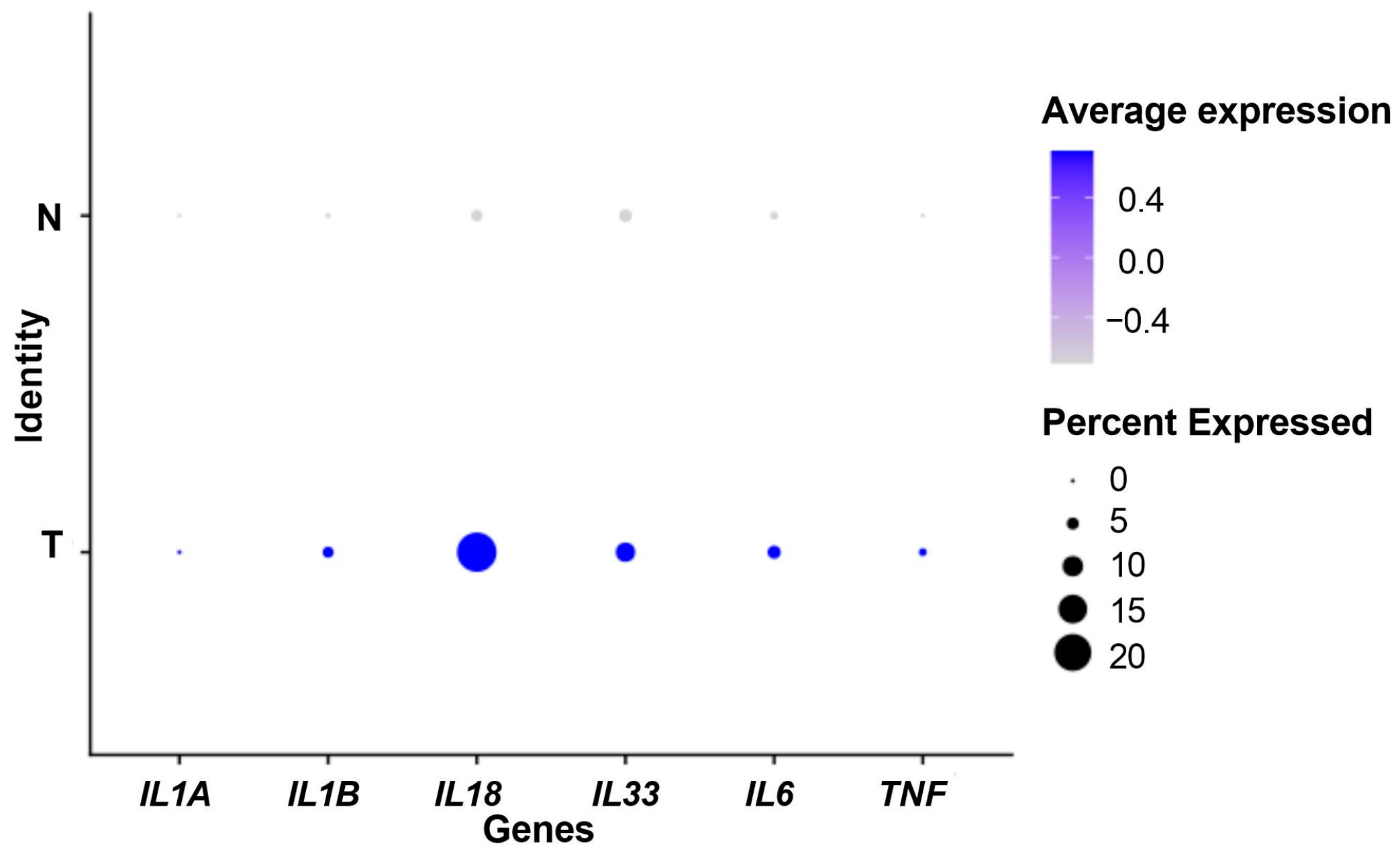
3.1. IL-1-Pathway-Related Gene Expression: Tumor versus Controls
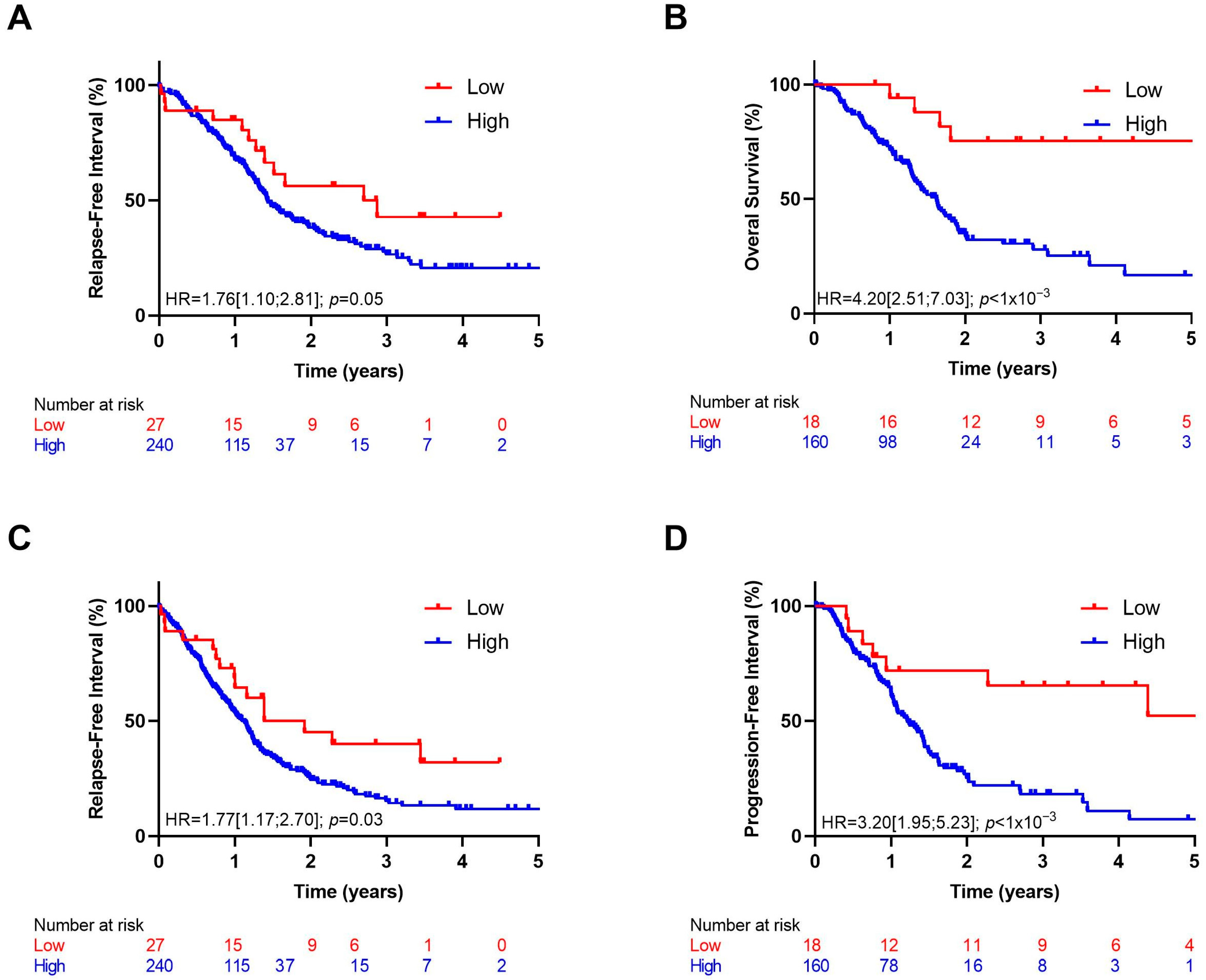
3.2. Survival Analysis
3.2.1. Patient Characteristics
3.2.2. IL-1 Pathway Score as a Prognostic Marker
3.2.3. IL-1α as a Prognostic Marker
3.2.4. Bailey’s Molecular Subtypes and IL-1α Expression
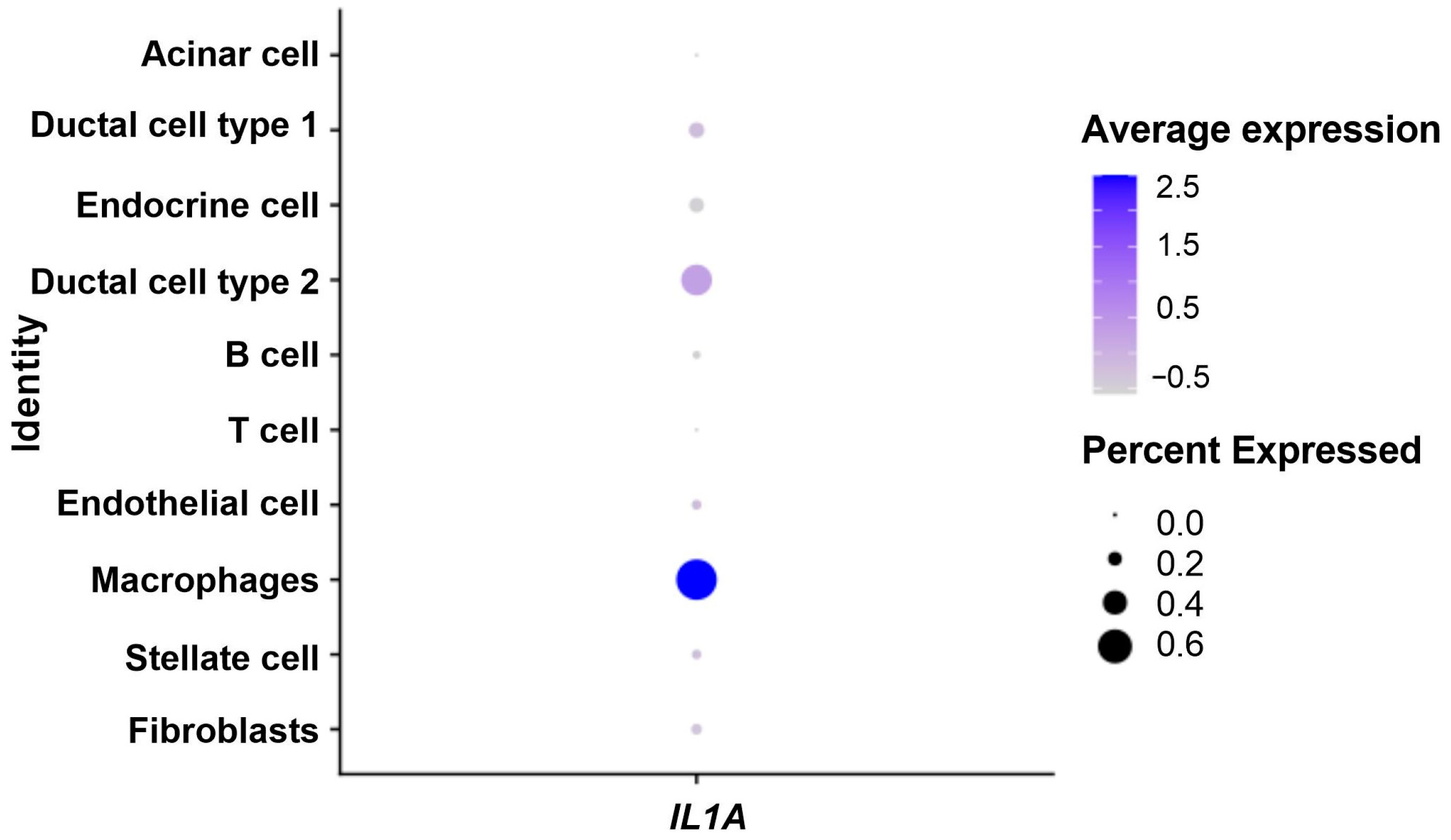
3.3. IL1A Overexpression in the Tumor Microenvironment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Du Pancréas|SNFGE.Org—Société Savante Médicale Française d’Hépato-Gastroentérologie et d’Oncologie Digestive. Available online: https://www.snfge.org/grand-public/maladies-digestives/cancer-du-pancreas (accessed on 29 January 2022).
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More Deaths from Pancreatic Cancer than Breast Cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Yarchoan, M.; Lee, V.; Laheru, D.A.; Jaffee, E.M. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin. Cancer Res. 2017, 23, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, K.; Bailey, P.; Springfeld, C.; Roth, S.; Kurilov, R.; Brors, B.; Gress, T.; Buchholz, M.; An, J.; et al. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 743908. [Google Scholar] [CrossRef]
- Australian Pancreatic Cancer Genome Initiative; Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; et al. Genomic Analyses Identify Molecular Subtypes of Pancreatic Cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, P.; Caygill, C.; Wall, C.; Bhatacharjee, S.; Ramus, J.; Watson, A.; Winslet, M. Lifetime Risk of Esophageal Adenocarcinoma in Patients with Barrett’s Esophagus. World, J. Gastroenterol. WJG 2014, 20, 9611–9617. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-Analyses of Colorectal Cancer Risk Factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Arend, W.P.; Palmer, G.; Gabay, C. IL-1, IL-18, and IL-33 Families of Cytokines. Immunol. Rev. 2008, 223, 20–38. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The Interleukin-1 Family: Back to the Future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.N.; Voronov, E. Immunotherapeutic Approaches of IL-1 Neutralization in the Tumor Microenvironment. J. Leukoc. Biol. 2017, 102, 293–306. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Projects. Available online: https://portal.gdc.cancer.gov/projects/TCGA-PAAD (accessed on 29 January 2022).
- Zhang, J.; Baran, J.; Cros, A.; Guberman, J.M.; Haider, S.; Hsu, J.; Liang, Y.; Rivkin, E.; Wang, J.; Whitty, B.; et al. International Cancer Genome Consortium Data Portal—A One-Stop Shop for Cancer Genomics Data. Database 2011, 2011, bar026. [Google Scholar] [CrossRef]
- NGDC—BioProject. Available online: https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA001063 (accessed on 29 January 2022).
- Peng, J.; Sun, B.-F.; Chen, C.-Y.; Zhou, J.-Y.; Chen, Y.-S.; Chen, H.; Liu, L.; Huang, D.; Jiang, J.; Cui, G.-S.; et al. Single-Cell RNA-Seq Highlights Intra-Tumoral Heterogeneity and Malignant Progression in Pancreatic Ductal Adenocarcinoma. Cell Res. 2019, 29, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial Reconstruction of Single-Cell Gene Expression Data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Greene, C. Signal Transduction Pathways Activated by the IL-1 Receptor Family: Ancient Signaling Machinery in Mammals, Insects, and Plants. J. Leukoc. Biol. 1998, 63, 650–657. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Barbie, D.A.; Tamayo, P.; Boehm, J.S.; Kim, S.Y.; Moody, S.E.; Dunn, I.F.; Schinzel, A.C.; Sandy, P.; Meylan, E.; Scholl, C.; et al. Systematic RNA Interference Reveals That Oncogenic KRAS-Driven Cancers Require TBK1. Nature 2009, 462, 108–112. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef] [PubMed]
- Donor Clinical Data Guidelines—ICGC DCC Docs. Available online: https://docs.icgc.org/submission/guide/donor-clinical-data-guidelines/#description-of-script-restrictions-for-donor-clinical-fields (accessed on 9 March 2022).
- Hothorn, T.; Lausen, B. On the Exact Distribution of Maximally Selected Rank Statistics. Comput. Stat. Data Anal. 2002, 43, 121–137. [Google Scholar] [CrossRef]
- Xu, D.; Matsuo, Y.; Ma, J.; Koide, S.; Ochi, N.; Yasuda, A.; Funahashi, H.; Okada, Y.; Takeyama, H. Cancer Cell-Derived IL-1α Promotes HGF Secretion by Stromal Cells and Enhances Metastatic Potential in Pancreatic Cancer Cells. J. Surg. Oncol. 2010, 102, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Niu, J.; Chang, Z.; Xia, Q.; Peng, B.; Ishiyama, S.; Evans, D.B.; Chiao, P.J. Secreted Interleukin-1α Induces a Metastatic Phenotype in Pancreatic Cancer by Sustaining a Constitutive Activation of Nuclear Factor-κB. Mol. Cancer Res. 2009, 7, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Tjomsland, V.; Spångeus, A.; Välilä, J.; Sandström, P.; Borch, K.; Druid, H.; Falkmer, S.; Falkmer, U.; Messmer, D.; Larsson, M. Interleukin 1α Sustains the Expression of Inflammatory Factors in Human Pancreatic Cancer Microenvironment by Targeting Cancer-Associated Fibroblasts. Neoplasia 2011, 13, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Ju, H.-Q.; Aguilar, M.; Gocho, T.; Li, H.; Iida, T.; Lee, H.; Fan, X.; Zhou, H.; Ling, J.; et al. IL1 Receptor Antagonist Inhibits Pancreatic Cancer Growth by Abrogating NF-ΚB Activation. Clin. Cancer Res. 2016, 22, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Nagathihalli, N.S.; Castellanos, J.A.; VanSaun, M.N.; Dai, X.; Ambrose, M.; Guo, Q.; Xiong, Y.; Merchant, N.B. Pancreatic Stellate Cell Secreted IL-6 Stimulates STAT3 Dependent Invasiveness of Pancreatic Intraepithelial Neoplasia and Cancer Cells. Oncotarget 2016, 7, 65982–65992. [Google Scholar] [CrossRef] [PubMed]
- Poli, V.; Camporeale, A. STAT3-Mediated Metabolic Reprograming in Cellular Transformation and Implications for Drug Resistance. Front. Oncol. 2015, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of Cancer Relapse and Metastasis by Inhibiting Cancer Stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef]
- Dosch, A.R.; Singh, S.; Dai, X.; Mehra, S.; Silva, I.D.C.; Bianchi, A.; Srinivasan, S.; Gao, Z.; Ban, Y.; Chen, X.; et al. Targeting Tumor-Stromal IL6/STAT3 Signaling through IL1 Receptor Inhibition in Pancreatic Cancer. Mol. Cancer Ther. 2021, 20, 2280–2290. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Knott, M.M.L.; Vetter, V.K.; Rapp, M.; Haubner, S.; Fesseler, J.; Kühnemuth, B.; Layritz, P.; Thaler, R.; Kruger, S.; et al. Cancer Cell-Derived IL-1α Induces CCL22 and the Recruitment of Regulatory T Cells. Oncoimmunology 2016, 5, e1175794. [Google Scholar] [CrossRef] [PubMed]
- Somerville, T.D.; Biffi, G.; Daßler-Plenker, J.; Hur, S.K.; He, X.-Y.; Vance, K.E.; Miyabayashi, K.; Xu, Y.; Maia-Silva, D.; Klingbeil, O.; et al. Squamous Trans-Differentiation of Pancreatic Cancer Cells Promotes Stromal Inflammation. eLife 2020, 9, e53381. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Guan, R.; Hong, W.; Zhou, Y.; Lin, Y.; Jin, H.; Hou, B.; Jian, Z. Prognostic Value of Tumor-Associated Macrophages in Pancreatic Cancer: A Meta-Analysis. Cancer Manag. Res. 2019, 11, 4041–4058. [Google Scholar] [CrossRef] [PubMed]

| Variables | ICGC Cohort (N = 267) | TCGA Cohort (N = 178) | p-Value |
|---|---|---|---|
| Age, N (%) | 0.124 | ||
| <60 years | 69 (25.9) | 59 (33.1) | |
| ≥60 years | 197 (74.1) | 119 (66.9) | |
| Sex, N (%) | 0.771 | ||
| Women | 125 (46.8) | 80 (44.9) | |
| Men | 142 (53.2) | 98 (55.1) | |
| Tumor stage, N (%) | 0.489 | ||
| IIB + III + IV | 176 (75.5) | 126 (72) | |
| IA + IB + IIA | 57 (24.5) | 49 (28) | |
| Missing values | 34 | 3 | |
| Grading, N (%) | 0.282 | ||
| Poorly differentiated + undifferentiated | 79 (34.2) | 50 (28.4) | |
| Moderately differentiated + well differentiated | 152 (65.8) | 126 (71.6) | |
| Missing values | 36 | 2 | |
| Bailey’s subtypes, N (%) | 0.077 | ||
| ADEX | 14 (15.9) | 38 (25.5) | |
| Immunogenic | 25 (28.4) | 27 (18.1) | |
| Pancreatic progenitor | 25 (28.4) | 53 (35.6) | |
| Squamous | 24 (27.3) | 31 (20.8) | |
| Missing values | 179 | 29 | |
| Overall survival, median (IQR) | 1.5 (2.5) | 1.7 (4.6) | |
| Progression-free interval, median (IQR) | - | 1.3 (2.8) | |
| Relapse-free survival, median (IQR) | 1.1 (1.6) | - |
| Overall Survival | ||||
|---|---|---|---|---|
| Variables | ICGC | TCGA | ||
| HR [95%CI] | p-Value | HR [95%CI] | p-Value | |
| Stage | ||||
| IA + IB + IIA (ref) | ||||
| IIB + III + IV | 1.47 [0.96–2.25] | 0.074 | 1.85 [1.07–3.20] | 0.028 |
| Grading | ||||
| G1 + G2 (ref) | ||||
| G3 + G4 | 1.63 [1.15–2.30] | 0.006 | 1.27 [0.82–1.97] | 0.278 |
| IL1 pathway score | 4.74 [1.00–22.43] | 0.050 | 5.18 [0.85–31.52] | 0.074 |
| Relapse-Free Survival | Progression-Free Interval | |||
|---|---|---|---|---|
| Variables | ICGC | TCGA | ||
| HR [95%CI] | p-Value | HR [95%CI] | p-Value | |
| Stage | ||||
| IA + IB + IIA (ref) | ||||
| IIB + III + IV | 1.70 [1.15–2.52] | 0.008 | 1.45 [0.90–2.35] | 0.124 |
| Grading | ||||
| G1 + G2 (ref) | ||||
| G3 + G4 | 1.42 [1.03–1.96] | 0.031 | 1.40 [0.92–2.11] | 0.112 |
| IL1 pathway score | 5.19 [1.27–21.23] | 0.022 | 14.88 [2.35–94.21] | 0.004 |
| Overall Survival | ||||
|---|---|---|---|---|
| Variables | ICGC | TCGA | ||
| HR [95%CI] | p-Value | HR [95%CI] | p-Value | |
| Stage | ||||
| IA + IB + IIA (ref) | ||||
| IIB + III + IV | 1.46 [0.95–2.23] | 0.082 | 1.68 [0.97–2.92] | 0.065 |
| Grade | ||||
| G1 + G2 (ref) | ||||
| G3 + G4 | 1.72 [1.22–2.42] | 0.002 | 1.17 [0.75–1.82] | 0.482 |
| IL-1A | ||||
| <10% (ref) | ||||
| ≥10% | 1.99 [1.01–3.93] | 0.046 | 3.00 [1.14–7.90] | 0.026 |
| Relapse-Free Survival | Progression-Free Interval | |||
|---|---|---|---|---|
| Variables | ICGC | TCGA | ||
| HR [95%CI] | p-Value | HR [95%CI] | p-Value | |
| Stage | ||||
| IA + IB + IIA (ref) | ||||
| IIB + III + IV | 1.70 [1.15–2.52] | 0.008 | 1.38 [0.85–2.26] | 0.196 |
| Grade | ||||
| G1 + G2 (ref) | ||||
| G3 + G4 | 1.54 [1.13–2.11] | 0.006 | 1.31 [0.86–1.99] | 0.212 |
| IL-1A | ||||
| <10% (ref) | ||||
| ≥10% | 1.85 [1.02–3.34] | 0.041 | 3.11 [1.24–7.80] | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigante, L.; Gaudillière-Le Dain, G.; Bertaut, A.; Truntzer, C.; Ghiringhelli, F. Interleukin-1α as a Potential Prognostic Biomarker in Pancreatic Cancer. Biomedicines 2024, 12, 1216. https://doi.org/10.3390/biomedicines12061216
Gigante L, Gaudillière-Le Dain G, Bertaut A, Truntzer C, Ghiringhelli F. Interleukin-1α as a Potential Prognostic Biomarker in Pancreatic Cancer. Biomedicines. 2024; 12(6):1216. https://doi.org/10.3390/biomedicines12061216
Chicago/Turabian StyleGigante, Leonardo, Gwladys Gaudillière-Le Dain, Aurélie Bertaut, Caroline Truntzer, and François Ghiringhelli. 2024. "Interleukin-1α as a Potential Prognostic Biomarker in Pancreatic Cancer" Biomedicines 12, no. 6: 1216. https://doi.org/10.3390/biomedicines12061216
APA StyleGigante, L., Gaudillière-Le Dain, G., Bertaut, A., Truntzer, C., & Ghiringhelli, F. (2024). Interleukin-1α as a Potential Prognostic Biomarker in Pancreatic Cancer. Biomedicines, 12(6), 1216. https://doi.org/10.3390/biomedicines12061216







