The Specific ROCK2 Inhibitor KD025 Alleviates Glycolysis through Modulating STAT3-, CSTA- and S1PR3-Linked Signaling in Human Trabecular Meshwork Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Trabecular Meshwork (HTM) Cells
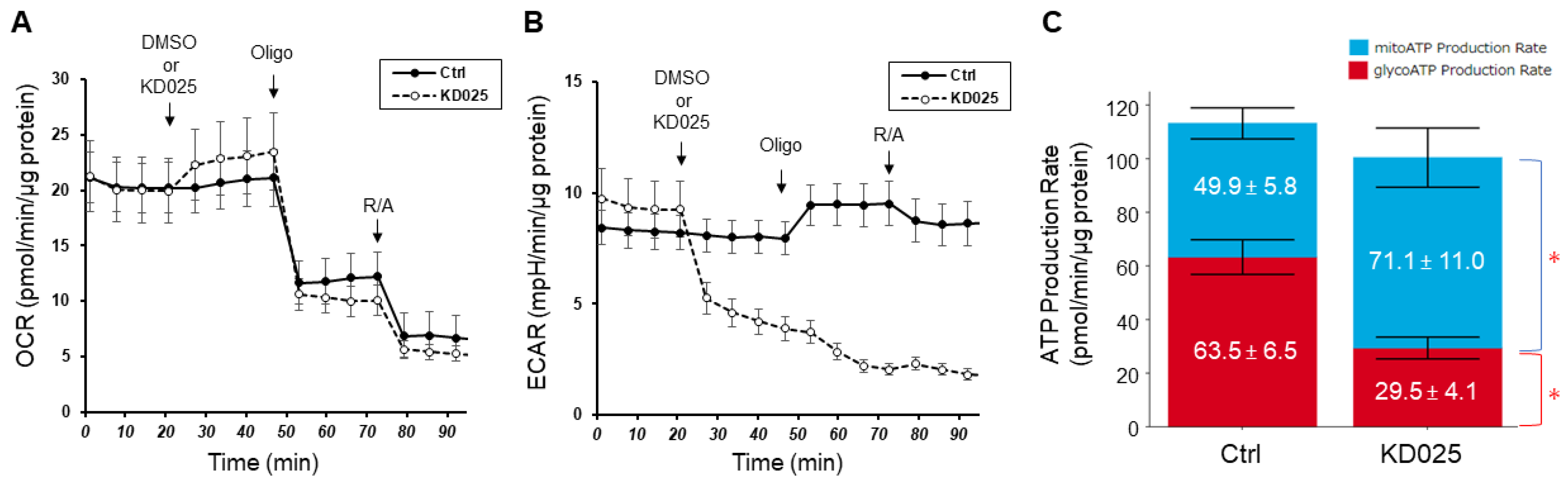
2.2. Real-Time ATP Rate Assay in Planar 2D Cultured HTM Cells by Using a Seahorse XF96e Analyzer
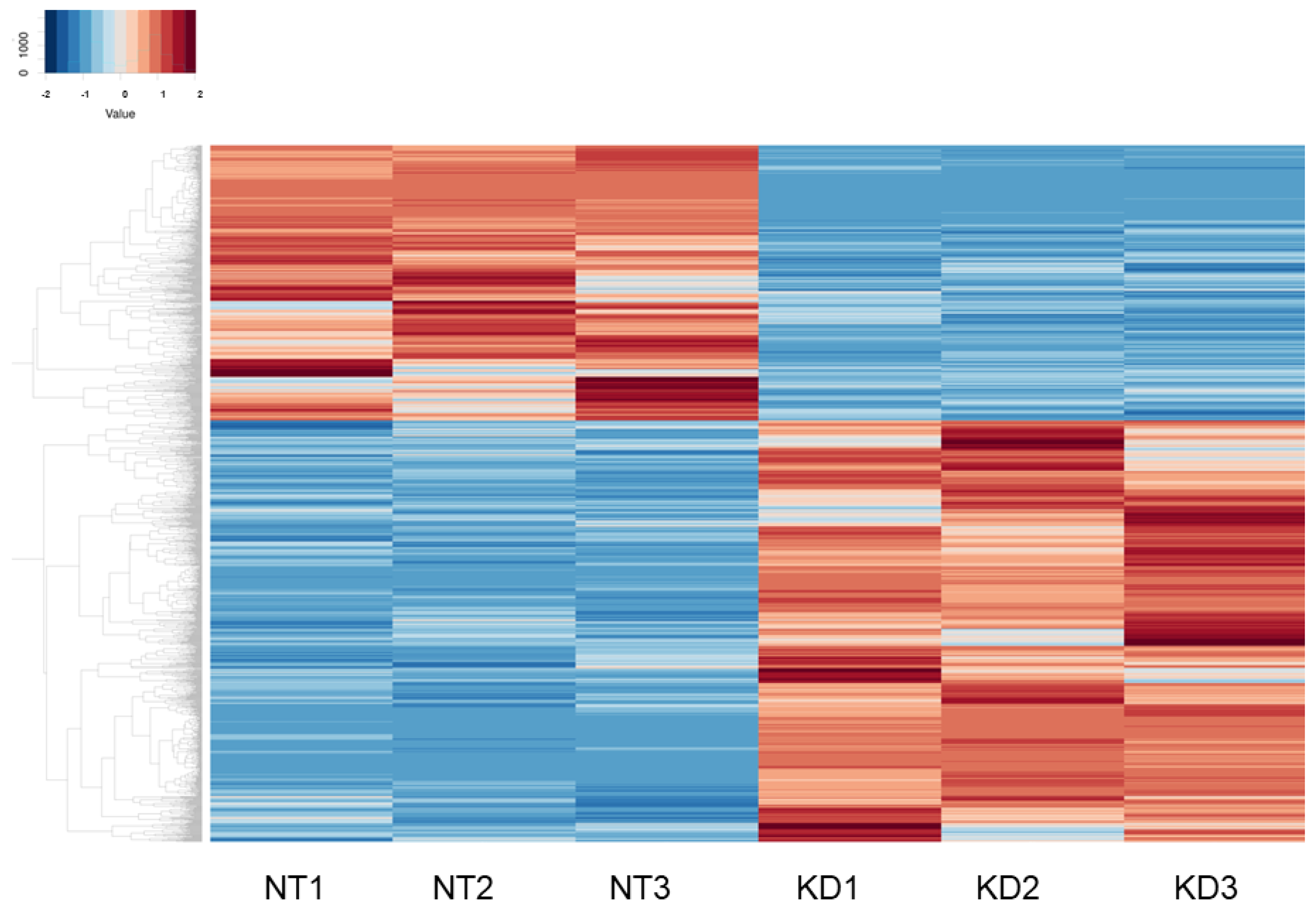
2.3. RNA Sequencing Analysis and IPA Gene Function and Pathway Analysis
2.4. Other Analytical Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertrand, J.; Winton, M.J.; Rodriguez-Hernandez, N.; Campenot, R.B.; McKerracher, L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 2005, 25, 1113–1121. [Google Scholar] [CrossRef]
- Tan, H.-B.; Zhong, Y.-S.; Cheng, Y.; Shen, X. Rho/ROCK pathway and neural regeneration: A potential therapeutic target for central nervous system and optic nerve damage. Int. J. Ophthalmol. 2011, 4, 652–657. [Google Scholar] [CrossRef]
- Sagawa, H.; Terasaki, H.; Nakamura, M.; Ichikawa, M.; Yata, T.; Tokita, Y.; Watanabe, M. A novel ROCK inhibitor, Y-39983, promotes regeneration of crushed axons of retinal ganglion cells into the optic nerve of adult cats. Exp. Neurol. 2007, 205, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Watabe, H.; Abe, S.; Yoshitomi, T. Effects of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on isolated rabbit ciliary arteries. Jpn. J. Ophthalmol. 2011, 55, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.M.; Kurisetty, V.; Mitchell, D.C.; Bryan, B.A. Rho Kinase Proteins Regulate Global miRNA Expression in Endothelial Cells. Cancer Genom. Proteom. 2013, 10, 251–263. [Google Scholar]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Araki, K.; Jee, Y.; Lee, D.H.; Oh, B.C.; Huang, H.; Park, K.S.; Lee, S.W.; Zabolotny, J.M.; Kim, Y.B. Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology 2012, 153, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef]
- Fukiage, C.; Mizutani, K.; Kawamoto, Y.; Azuma, M.; Shearer, T.R. Involvement of Phosphorylation of Myosin Phosphatase by ROCK in Trabecular Meshwork and Ciliary Muscle Contraction. Biochem. Biophys. Res. Commun. 2001, 288, 296–300. [Google Scholar] [CrossRef]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Utsunomiya, K.; Taniguchi, K.; Gojo, A.; Kurata, H.; Tajima, N. Involvement of the Rho/Rho Kinase Signaling Pathway in Platelet-Derived Growth Factor BB-induced Vascular Endothelial Growth Factor Expression in Diabetic Rat Retina. Jpn. J. Ophthalmol. 2007, 51, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Tayyari, F.; Shimokawa, H.; et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes 2009, 58, 215–226. [Google Scholar] [CrossRef]
- Hollanders, K.; Van Bergen, T.; Kindt, N.; Castermans, K.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. The Effect of AMA0428, a Novel and Potent ROCK Inhibitor, in a Model of Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1335–1348. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Hamuro, J.; Kinoshita, S. The New Therapeutic Concept of Using a Rho Kinase Inhibitor for the Treatment of Corneal Endothelial Dysfunction. Cornea 2011, 30, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-associated kinase pathway in glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Inoue, T.; Inatani, M.; Fujimoto, T.; Honjo, M.; Kasaoka, N.; Inoue-Mochita, M.; Yoshimura, N.; Tanihara, H. The Effect of Rho-Associated Protein Kinase Inhibitor on Monkey Schlemm’s Canal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3092–3103. [Google Scholar] [CrossRef]
- Van de Velde, S.; Van Bergen, T.; Sijnave, D.; Hollanders, K.; Castermans, K.; Defert, O.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. AMA0076, a Novel, Locally Acting Rho Kinase Inhibitor, Potently Lowers Intraocular Pressure in New Zealand White Rabbits with Minimal Hyperemia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanihara, H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Progress Retin. Eye Res. 2013, 37, 1–12. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M.; K-115 Clinical Study Group. Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: A randomized, open-label, crossover study. Acta Ophthalmol. 2015, 93, e254–e260. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Establishment of appropriate glaucoma models using dexamethasone or TGFβ2 treated three-dimension (3D) cultured human trabecular meshwork (HTM) cells. Sci. Rep. 2021, 11, 19369. [Google Scholar] [CrossRef]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ruan, F.; Zheng, Z. Ripasudil Attenuates Lipopolysaccharide (LPS)-Mediated Apoptosis and Inflammation in Pulmonary Microvascular Endothelial Cells via ROCK2/eNOS Signaling. Med. Sci. Monit. 2018, 24, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liao, M.; Zhao, W.; Zheng, X.; Xu, F.; Wang, H.; Huang, J. CXCL16/ROCK1 signaling pathway exacerbates acute kidney injury induced by ischemia-reperfusion. Biomed. Pharmacother. 2018, 98, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Diverse effects of pan-ROCK and ROCK2 inhibitors on 2 D and 3D cultured human trabecular meshwork (HTM) cells treated with TGFβ2. Sci. Rep. 2021, 11, 15286. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Hikage, F.; Ohguro, H. Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners. Molecules 2021, 26, 6382. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Higashide, M.; Furuhashi, M.; Ohguro, H. TGF-β-3 Induces Different Effects from TGF-β-1 and -2 on Cellular Metabolism and the Spatial Properties of the Human Trabecular Meshwork Cells. Int. J. Mol. Sci. 2023, 24, 4181. [Google Scholar] [CrossRef]
- Ichioka, H.; Hirohashi, Y.; Sato, T.; Furuhashi, M.; Watanabe, M.; Ida, Y.; Hikage, F.; Torigoe, T.; Ohguro, H. G-Protein-Coupled Receptors Mediate Modulations of Cell Viability and Drug Sensitivity by Aberrantly Expressed Recoverin 3 within A549 Cells. Int. J. Mol. Sci. 2023, 24, 771. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wang, K.; Zhang, S.; Yuan, J.; Liao, X.; Wu, C.; Zou, Y.; Ha, Y.; Shen, Z.; Guo, J.; et al. Ingenuity pathway analysis of differentially expressed genes involved in signaling pathways and molecular networks in RhoE gene-edited cardiomyocytes. Int. J. Mol. Med. 2020, 46, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Alimadadi, A.; Aryal, S.; Manandhar, I.; Joe, B.; Cheng, X. Identification of Upstream Transcriptional Regulators of Ischemic Cardiomyopathy Using Cardiac RNA-Seq Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3472. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Seto, M.; Noma, K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; Chen, X.Q.; Manser, E.; Lim, L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 1996, 16, 5313–5327. [Google Scholar] [CrossRef]
- Yoneda, A.; Multhaupt, H.A.; Couchman, J.R. The Rho kinases I and II regulate different aspects of myosin II activity. J. Cell Biol. 2005, 170, 443–453. [Google Scholar] [CrossRef]
- Yoneda, A.; Ushakov, D.; Multhaupt, H.A.; Couchman, J.R. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol. Biol. Cell 2007, 18, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.; Wei, L.; Shi, J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011, 7, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, P.; Eing, C.; Straessner, R.; Durst, S.; Frey, W.; Nick, P. Plant actin controls membrane permeability. Biochim. Biophys. Acta 2011, 1808, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, X.; Zack, D.J.; Zhu, H.; Qian, J. TiGER: A database for tissue-specific gene expression and regulation. BMC Bioinform. 2008, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Di Cunto, F.; Imarisio, S.; Hirsch, E.; Broccoli, V.; Bulfone, A.; Migheli, A.; Atzori, C.; Turco, E.; Triolo, R.; Dotto, G.P.; et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 2000, 28, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Roberts, W.; Wang, L.; Yamada, M.; Zhang, S.; Zhao, Z.; Rivkees, S.A.; Schwartz, R.J.; Imanaka-Yoshida, K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development 2001, 128, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Peng, D.; Shi, W.; Guo, J.; Huo, S.; Men, L.; Zhang, C.; Li, S.; Lv, J.; Lin, L. IL-6/STAT3 Signaling Promotes Cardiac Dysfunction by Upregulating FUNDC1-Dependent Mitochondria-Associated Endoplasmic Reticulum Membranes Formation in Sepsis Mice. Front. Cardiovasc. Med. 2021, 8, 790612. [Google Scholar] [CrossRef] [PubMed]
- Sadrkhanloo, M.; Paskeh, M.D.A.; Hashemi, M.; Raesi, R.; Motahhary, M.; Saghari, S.; Sharifi, L.; Bokaie, S.; Mirzaei, S.; Entezari, M.; et al. STAT3 signaling in prostate cancer progression and therapy resistance: An oncogenic pathway with diverse functions. Biomed. Pharmacother. 2023, 158, 114168. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ida, Y.; Hikage, F.; Umetsu, A.; Ichioka, H.; Watanabe, M.; Furuhashi, M. STAT3 Is the Master Regulator for the Forming of 3D Spheroids of 3T3-L1 Preadipocytes. Cells 2022, 11, 300. [Google Scholar] [CrossRef]
- Valle, M.L.; Dworshak, J.; Sharma, A.; Ibrahim, A.S.; Al-Shabrawey, M.; Sharma, S. Inhibition of interleukin-6 trans-signaling prevents inflammation and endothelial barrier disruption in retinal endothelial cells. Exp. Eye Res. 2019, 178, 27–36. [Google Scholar] [CrossRef]
- Brooks, G.D.; McLeod, L.; Alhayyani, S.; Miller, A.; Russell, P.A.; Ferlin, W.; Rose-John, S.; Ruwanpura, S.; Jenkins, B.J. IL6 Trans-signaling Promotes KRAS-Driven Lung Carcinogenesis. Cancer Res. 2016, 76, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, H.; Cao, W.; Wang, T.; Chen, W.; Yu, H.; Fu, Y.; Jiang, B.; Zhou, H.; Guo, H.; et al. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics 2019, 9, 3980–3991. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Mochita, M.; Inoue, T.; Kojima, S.; Futakuchi, A.; Fujimoto, T.; Sato-Ohira, S.; Tsutsumi, U.; Tanihara, H. Interleukin-6-mediated trans-signaling inhibits transforming growth factor-β signaling in trabecular meshwork cells. J. Biol. Chem. 2018, 293, 10975–10984. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Inoue, T.; Shobayashi, K.; Iwao, K.; Fukushima, M.; Tanihara, H. Simultaneous increase in multiple proinflammatory cytokines in the aqueous humor in neovascular glaucoma with and without intravitreal bevacizumab injection. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Rose-John, S. Dissecting Interleukin-6 Classic- and Trans-Signaling in Inflammation and Cancer. Methods Mol. Biol. 2018, 1725, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.B.; Luna, C.; Bodman, M.; Hong, A.; Epstein, D.L.; Gonzalez, P. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem. Biophys. Res. Commun. 2005, 337, 1229–1236. [Google Scholar] [CrossRef]
- Yemanyi, F.; Raghunathan, V. Lysophosphatidic Acid and IL-6 Trans-signaling Interact via YAP/TAZ and STAT3 Signaling Pathways in Human Trabecular Meshwork Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef]
- Ma, W.; Sze, K.M.; Chan, L.K.; Lee, J.M.; Wei, L.L.; Wong, C.M.; Lee, T.K.; Wong, C.C.; Ng, I.O. RhoE/ROCK2 regulates chemoresistance through NF-κB/IL-6/ STAT3 signaling in hepatocellular carcinoma. Oncotarget 2016, 7, 41445–41459. [Google Scholar] [CrossRef]
- Mettu, P.S.; Deng, P.F.; Misra, U.K.; Gawdi, G.; Epstein, D.L.; Rao, P.V. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Read, A.T.; Sumida, G.M.; Ethier, C.R. Sphingosine-1-phosphate effects on the inner wall of Schlemm’s canal and outflow facility in perfused human eyes. Exp. Eye Res. 2009, 89, 980–988. [Google Scholar] [CrossRef]
- Lee, M.J.; Van Brocklyn, J.R.; Thangada, S.; Liu, C.H.; Hand, A.R.; Menzeleev, R.; Spiegel, S.; Hla, T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998, 279, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Gonzalez-Cabrera, P.J.; Sanna, M.G.; Brown, S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 2009, 78, 743–768. [Google Scholar] [CrossRef] [PubMed]
- Sumida, G.M.; Stamer, W.D. S1P2 receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am. J. Physiol. Cell Physiol. 2011, 300, C1164–C1171. [Google Scholar] [CrossRef] [PubMed]
- Rivenbark, A.G.; Coleman, W.B. Epigenetic regulation of cystatins in cancer. Front. Biosci. 2009, 14, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.D.; AnithaChristy, S.A.; Buie, L.K.; Borrás, T. Cystatin a, a potential common link for mutant myocilin causative glaucoma. PLoS ONE 2012, 7, e36301. [Google Scholar] [CrossRef]
- Boerma, M.; Fu, Q.; Wang, J.; Loose, D.S.; Bartolozzi, A.; Ellis, J.L.; McGonigle, S.; Paradise, E.; Sweetnam, P.; Fink, L.M.; et al. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2008, 19, 709–718. [Google Scholar] [CrossRef]
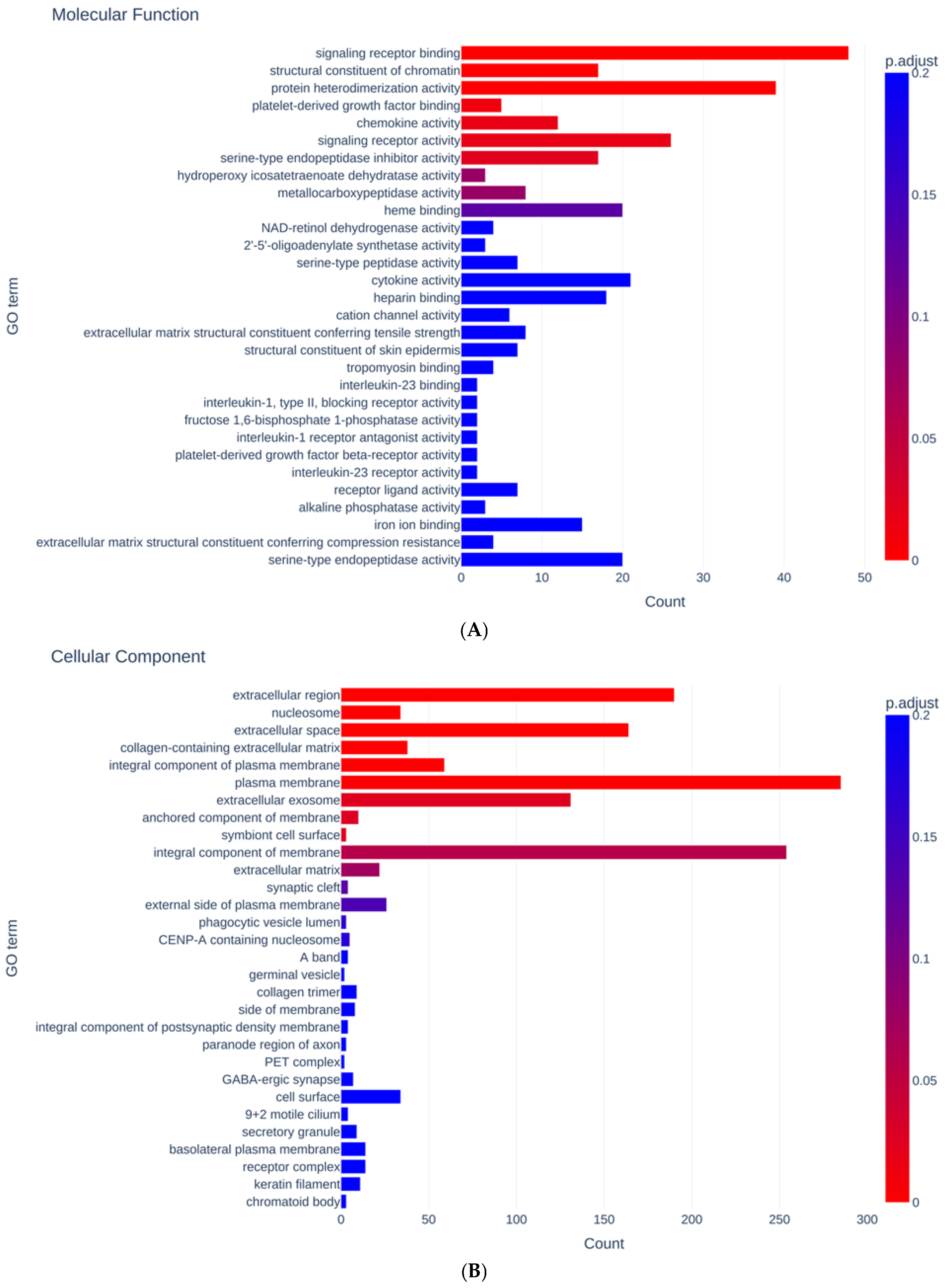
| Up Regulation | Down Regulation | ||
|---|---|---|---|
| Molecules | p-Value | Molecules | p-Value |
| KIF1A | 6.301 | CHRNA1 | −5.838 |
| CDHR1 | 6.156 | TNFSF18 | −5.603 |
| CSTA | 5.410 | IDO1 | −5.018 |
| LCN1 | 5.271 | LOC105377155 | −4.997 |
| HSPA6 | 4.990 | LOC102724434 | −4.661 |
| LINC01705 | 4.837 | ENSG00000289492 | −4.658 |
| PIP5K1B | 4.719 | CFI | −4.610 |
| LINC02568 | 4.679 | AKAP13-AS1 | −4.605 |
| ZBBX | 4.607 | MYO16 | −4.555 |
| AGA-DT | 4.593 | RP11_885L141 | −4.502 |
| Molecular and Cellular Functions | ||
|---|---|---|
| Name | p-Value of Range | Molecules |
| Cellular Movement | 1.58 × 10−0.6–1.98 × 10−21 | 278 |
| Cellular Development | 1.47 × 10−0.6–4.12 × 10−19 | 368 |
| Cellular Function and Maintenance | 5.00 × 10−0.7–4.12× 10−19 | 303 |
| Cellular Growth and Proliferation | 1.24 × 10−0.6–4.12 × 10−19 | 359 |
| Cell-To-Cell Signaling and Interaction | 1.58 × 10−0.6–6.61 × 10−16 | 227 |
| Top Networks | ||
|---|---|---|
| No. | Associated Network Functions | Score |
| 1 | Developmental Disorder, Hereditary Disorder, Organismal Injury and Abnormalities | 41 |
| 2 | Cardiac Dilation, Cardiac Enlargement, Cardiovascular Disease | 33 |
| 3 | Nervous System Development and Function, Neurological Disease, Organismal Injury and Abnormalities | 33 |
| 4 | Organ Morphology, Organismal Development, Renal and Urological System Development and Function | 33 |
| 5 | Cell Death and Survival, Organismal Injury and Abnormalities, Skeletal and Muscular Disorders | 33 |
| Upstream Regulator | Expression Log Ratio | Activation z-Score | p Value of Overlap |
|---|---|---|---|
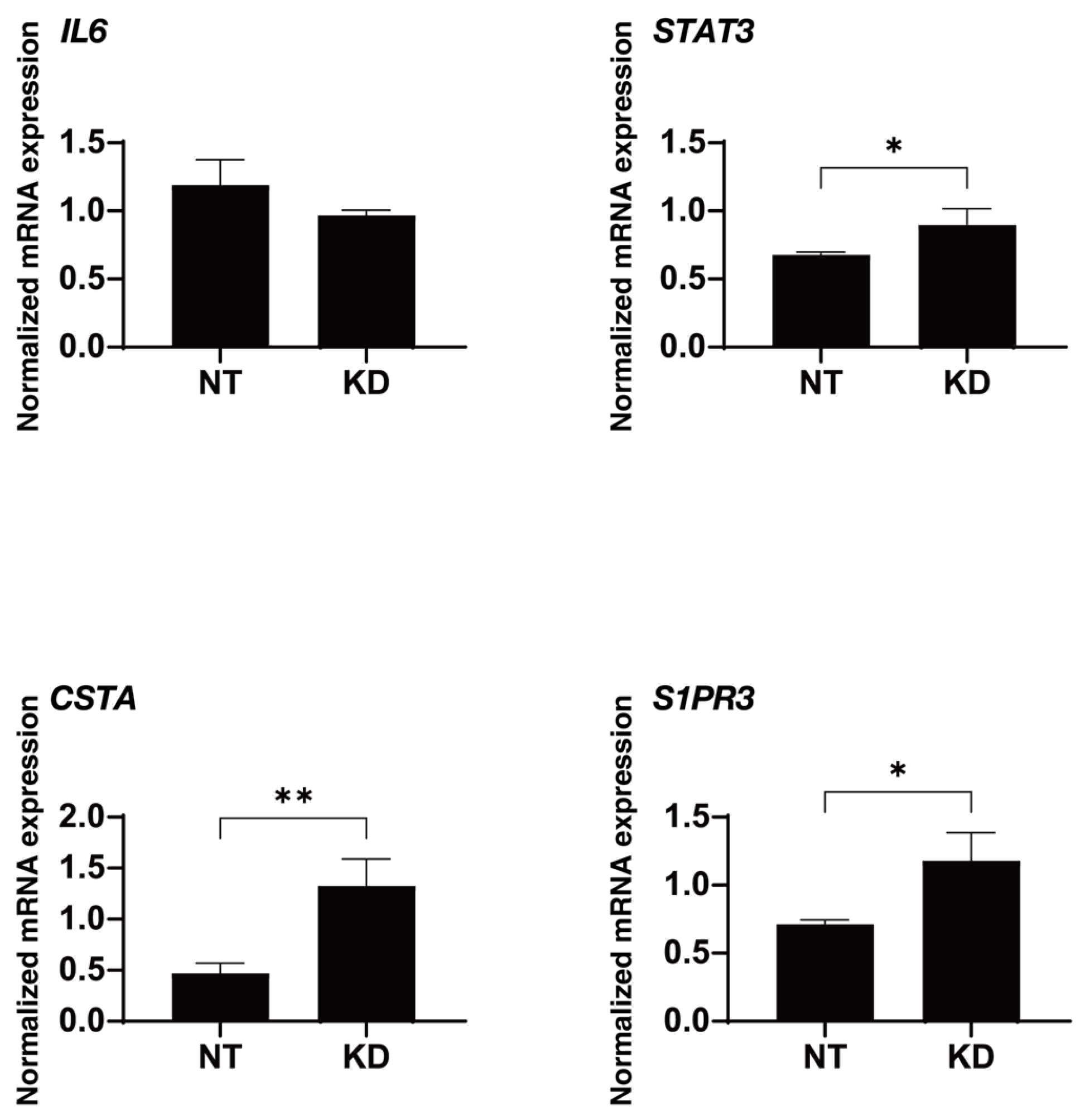
| IL6 | −1.688 | −2.474 | 0.00002 |
| STAT3 | 1.51 | 3.061 | 0.00008 |
| Causal Network Regulator | Expression Log Ratio | Activation z-Score | p Value of Overlap |
| CSTA | 5.41 | 3.312 | 0.001 |
| S1PR3 | 1.52 | 4.427 | 0.002 |
| D + Rip | D + KD | T + Rip | T + KD | |
|---|---|---|---|---|
| TEER | ↑ | ↑↑↑ | → | ↓↓↓ |
| Size | ↑↑↑↑ | ↓ | ↑↑↑↑ | ↑ |
| Stiffness | ↑↑↑ | ↓↓↓ | ↓↓↓ | ↑↑↑ |
| OCR | → | ↑ | ↓ | ↑↑ |
| ECRA | ↑ | ↓ | ↓ | ↓↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Sato, T.; Umetsu, A.; Ogawa, T.; Nishikiori, N.; Suzuki, M.; Furuhashi, M.; Ohguro, H. The Specific ROCK2 Inhibitor KD025 Alleviates Glycolysis through Modulating STAT3-, CSTA- and S1PR3-Linked Signaling in Human Trabecular Meshwork Cells. Biomedicines 2024, 12, 1165. https://doi.org/10.3390/biomedicines12061165
Watanabe M, Sato T, Umetsu A, Ogawa T, Nishikiori N, Suzuki M, Furuhashi M, Ohguro H. The Specific ROCK2 Inhibitor KD025 Alleviates Glycolysis through Modulating STAT3-, CSTA- and S1PR3-Linked Signaling in Human Trabecular Meshwork Cells. Biomedicines. 2024; 12(6):1165. https://doi.org/10.3390/biomedicines12061165
Chicago/Turabian StyleWatanabe, Megumi, Tatsuya Sato, Araya Umetsu, Toshifumi Ogawa, Nami Nishikiori, Megumi Suzuki, Masato Furuhashi, and Hiroshi Ohguro. 2024. "The Specific ROCK2 Inhibitor KD025 Alleviates Glycolysis through Modulating STAT3-, CSTA- and S1PR3-Linked Signaling in Human Trabecular Meshwork Cells" Biomedicines 12, no. 6: 1165. https://doi.org/10.3390/biomedicines12061165
APA StyleWatanabe, M., Sato, T., Umetsu, A., Ogawa, T., Nishikiori, N., Suzuki, M., Furuhashi, M., & Ohguro, H. (2024). The Specific ROCK2 Inhibitor KD025 Alleviates Glycolysis through Modulating STAT3-, CSTA- and S1PR3-Linked Signaling in Human Trabecular Meshwork Cells. Biomedicines, 12(6), 1165. https://doi.org/10.3390/biomedicines12061165








