A New Approach to Postoperative Rehabilitation following Mosaicplasty and Bone Marrow Aspiration Concentrate (BMAC) Augmentation
Abstract
1. Introduction
1.1. Mosaicplasty
1.2. BMAC Augmentation
1.3. Rehabilitation
2. Materials and Methods
2.1. Study Design
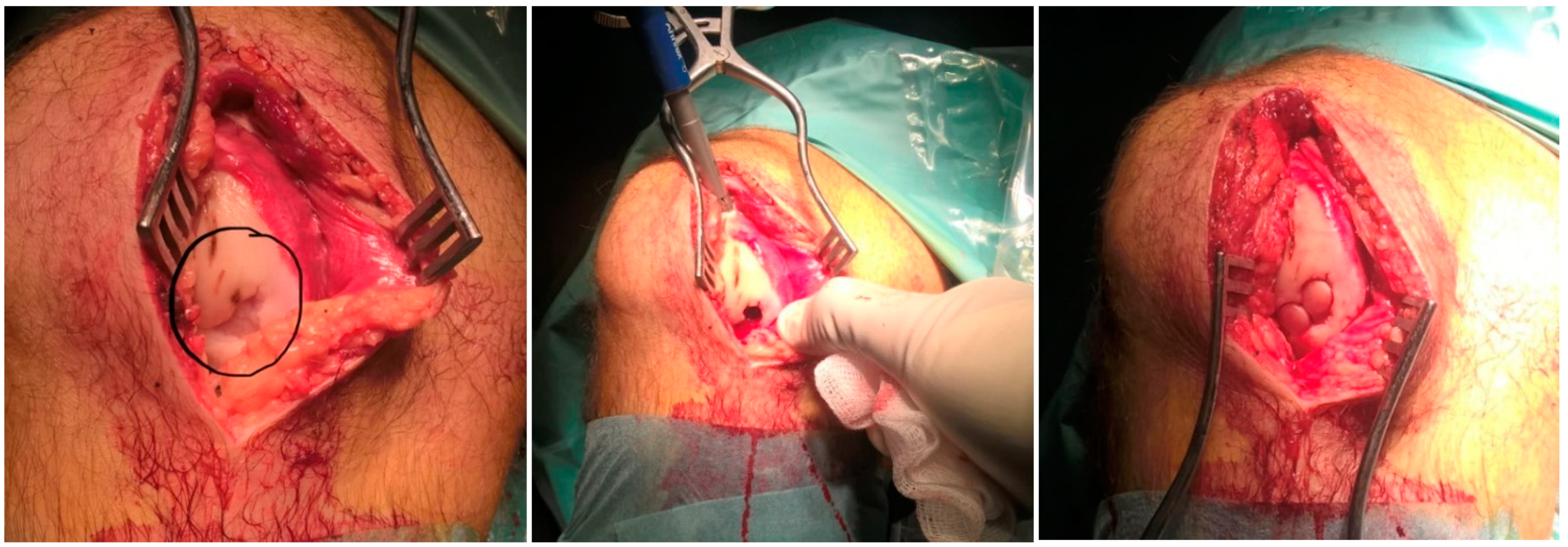
2.2. Surgical Treatment and BMAC Augmentation
2.3. Subjects Evaluation
2.4. Physiotherapy Treatment (PT)
3. Results
3.1. Statistical Analysis
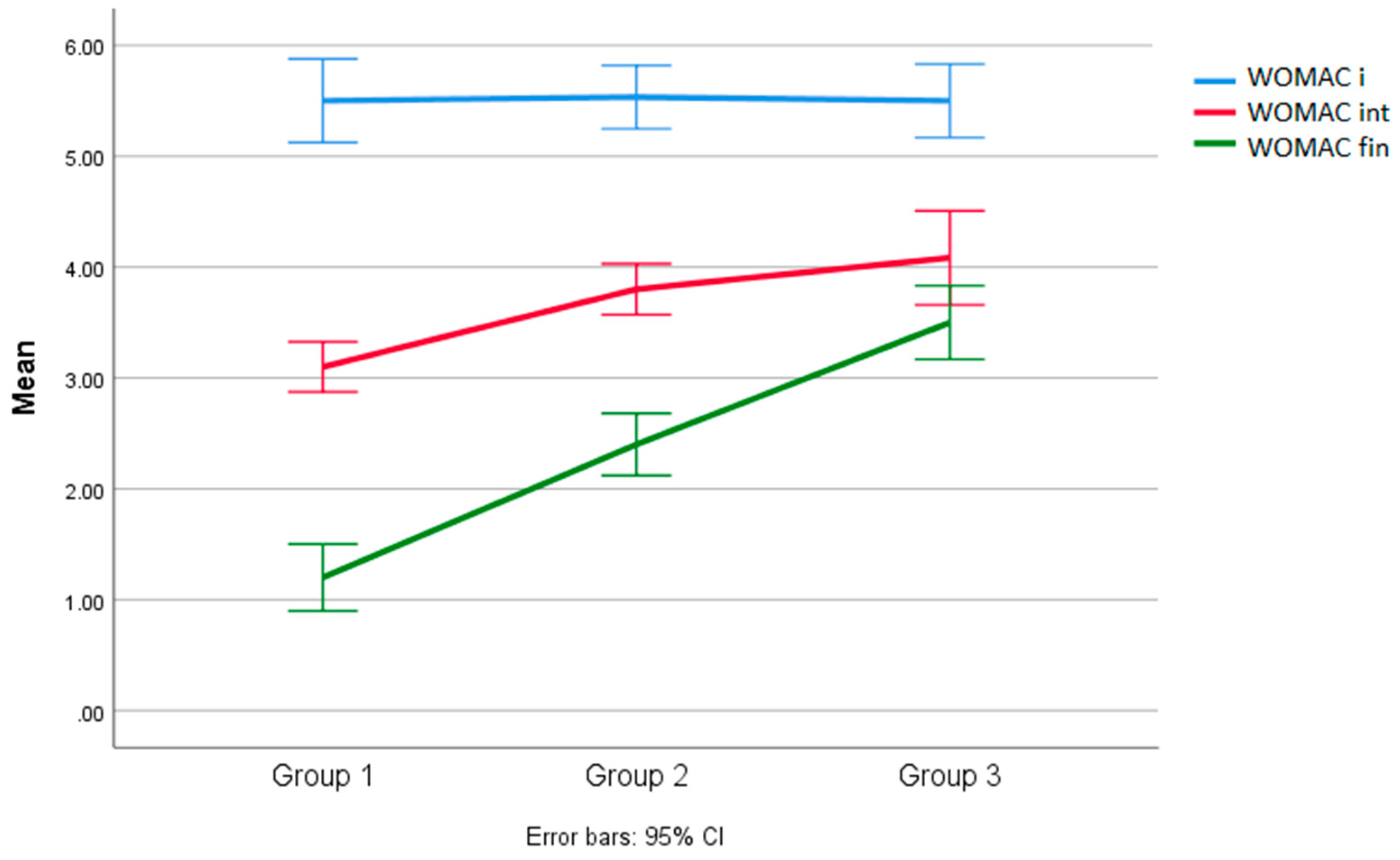
3.2. Analysis of Results between Groups for the WOMAC Scale
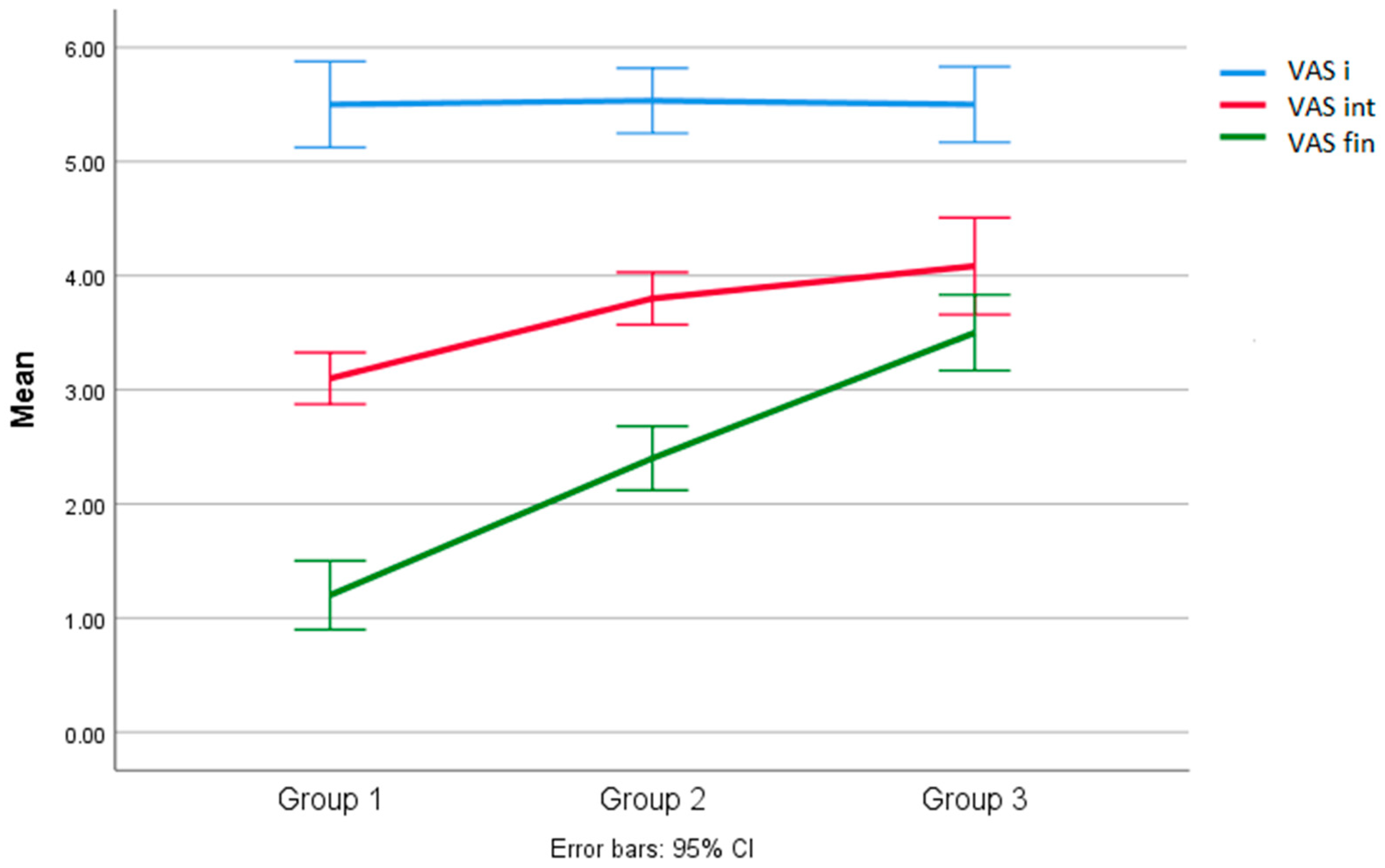
3.3. Analysis of between-Group Results for the VAS Scale
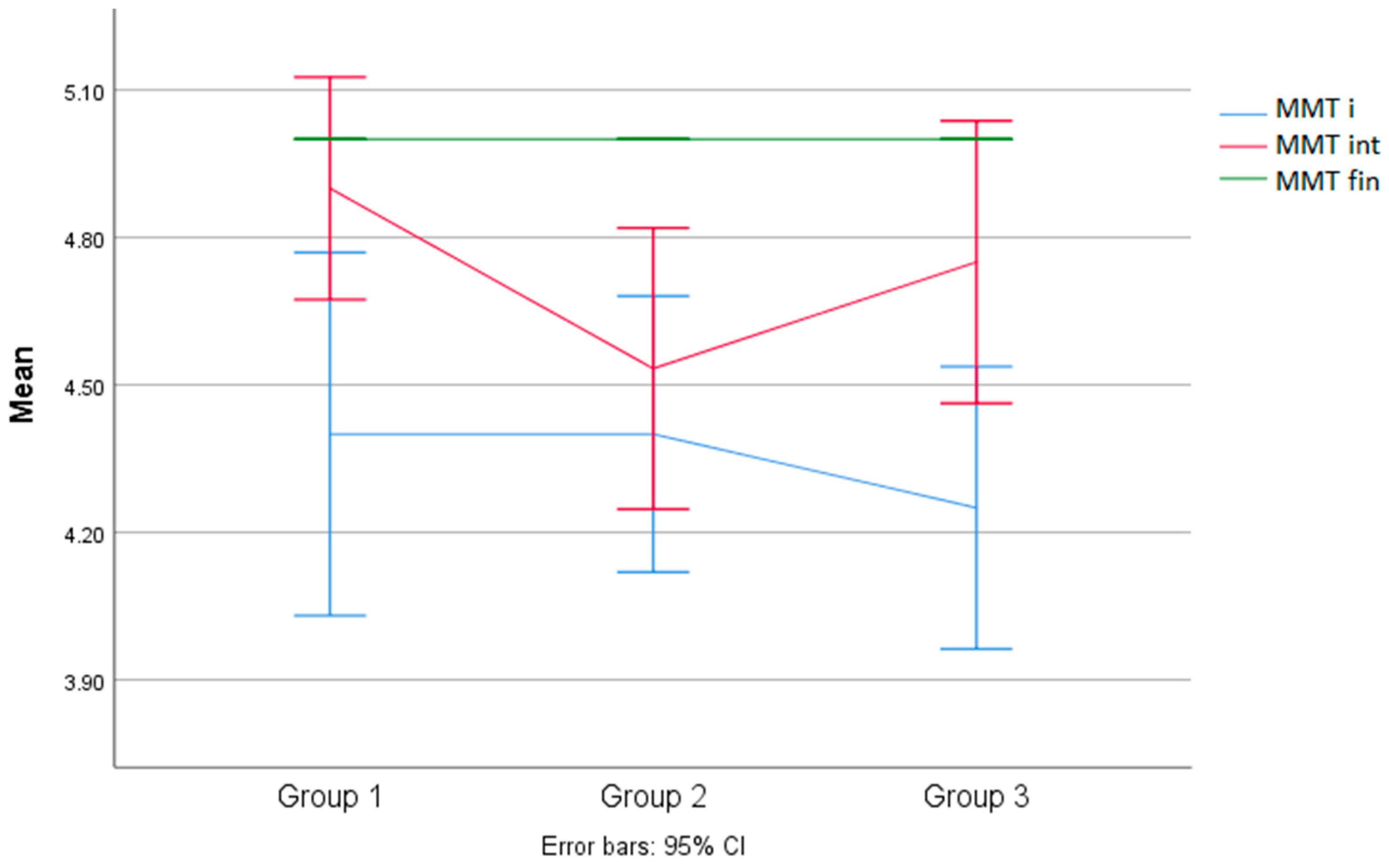
3.4. Analysis of Results between Groups for the MMT of the Quadriceps
3.5. Analysis of the between-Group Results of the Knee ROM Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curl, W.W.; Krome, J.; Gordon, E.S.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage Injuries: A Review of 31,516 Knee Arthroscopies. Arthroscopy 1997, 13, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Onu, I.; Matei, D.; Sardaru, D.-P.; Cascaval, D.; Onu, A.; Gherghel, R.; Serban, I.L.; Mocanu, G.D.; Iordan, D.A.; Murariu, G.; et al. Rehabilitation of Patients with Moderate Knee Osteoarthritis Using Hyaluronic Acid Viscosupplementation and Physiotherapy. Appl. Sci. 2022, 12, 3165. [Google Scholar] [CrossRef]
- Gherghel, R.; Macovei, L.A.; Burlui, M.-A.; Cardoneanu, A.; Rezus, I.-I.; Mihai, I.R.; Rezus, E. Osteoarthritis—The Role of Mesenchymal Stem Cells in Cartilage Regeneration. Appl. Sci. 2023, 13, 10617. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, A.; Ozdemir, M.R.; Ozkan, Y. Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int. Orthop. 2006, 30, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Mosaicplasty|Patient Education. Available online: https://cartilage.org/patient/about-cartilage/cartilage-repair/mosaicplasty/ (accessed on 9 March 2024).
- Andrade, R.; Vasta, S.; Pereira, R.; Pereira, H.; Papalia, R.; Karahan, M.; Oliveira, J.M.; Reis, R.L.; Espregueira-Mendes, J. Knee donor-site morbidity after mosaicplasty—A systematic review. J. Exp. Orthop. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.J.; Rueda, T.D.; Del Sol, S.M.R.; Bryant, S.A.; Jenkins, S.; Gardner, B.; McGahan, P.J.; Chen, J.L. Arthroscopic Osteochondral Autograft Transfer System Procedure of the Lateral Femoral Condyle with Donor-Site Backfill Using Osteochondral Allograft Plug. Arthrosc. Tech. 2021, 10, e2683–e2689. [Google Scholar] [CrossRef] [PubMed]
- Autograft OATS® Technique. Available online: https://www.arthrex.com/knee/oats-technique (accessed on 12 March 2024).
- Markus, N.; Jeyakumar, V.; Muellner, T.; Nehrer, S. Bone-marrow-aspirate-concentrate for chondral defects: Surgical techniques, clinical applications and basic science. Ann. Jt. 2018, 3, 107. [Google Scholar] [CrossRef]
- Holton, J.; Imam, M.A.; Snow, M. Bone Marrow Aspirate in the Treatment of Chondral Injuries. Front. Surg. 2016, 3, 33. [Google Scholar] [CrossRef]
- Gobbi, A.; Karnatzikos, G.; Scotti, C.; Mahajan, V.; Mazzucco, L.; Grigolo, B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: Results at 2-year follow-up. Cartilage 2011, 2, 286–299. [Google Scholar] [CrossRef]
- Giannini, S.; Buda, R.; Cavallo, M.; Ruffilli, A.; Cenacchi, A.; Cavallo, C.; Vannini, F. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: From open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury 2010, 41, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, M.F.; Horng, A.; Glaser, C.; Albrecht, D.; Bruns, J.; Scheffler, S.; Marlovits, S.; Angele, P.; Aurich, M.; Bosch, U.; et al. Nachbehandlung bei der autologen Chondrozytentransplantation: Eine Bestandsaufnahme und Empfehlung der AG Klinische Geweberegeneration der DGU/DGOOC [Post-treatment rehabilitation after autologous chondrocyte implantation: State of the art and recommendations of the Clinical Tissue Regeneration Study Group of the German Society for Accident Surgery and the German Society for Orthopedics and Orthopedic Surgery]. Der Unfallchirurg 2014, 117, 235–241. (In German) [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.K.; Ackland, T.; Ebert, J.R. Clinical rehabilitation guidelines for matrix-induced autologous chondrocyte implantation on the tibiofemoral joint. J. Orthop. Sports Phys. Ther. 2014, 44, 102–119. [Google Scholar] [CrossRef]
- BioCUE® Blood and Bone Marrow Aspiration (bBMA) Concentration System. Available online: https://www.zimmerbiomet.com/en/products-and-solutions/specialties/biologics/biocue-bbma-concentration-system.html (accessed on 18 April 2024).
- Kim, G.B.; Kim, J.-D.; Choi, Y.; Choi, C.H.; Lee, G.W. Intra-Articular Bone Marrow Aspirate Concentrate Injection in Patients with Knee Osteoarthritis. Appl. Sci. 2020, 10, 5945. [Google Scholar] [CrossRef]
- Keeling, L.E.; Belk, J.W.; Kraeutler, M.J.; Kallner, A.C.; Lindsay, A.; McCarty, E.C.; Postma, W.F. Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis: A Systematic Review. Am. J. Sports Med. 2022, 50, 2315–2323. [Google Scholar] [CrossRef]
- Skowroński, J.; Skowroński, R.; Rutka, M. Large cartilage lesions of the knee treated with bone marrow concentrate and collagen membrane–results. Ortop. Traumatol. Rehabil. 2013, 15, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Krych, A.J.; Nawabim, D.H.; Farshad-Amacker, N.A.; Jones, K.J.; Maak, T.G.; Potter, H.G.; Williams, R.J., III. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: A comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am. J. Sports Med. 2016, 44, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, K.M.; Burge, A.J.; Balazs, G.C.; Williams, R.J., 3rd. Bone Marrow Aspirate Concentrate Does Not Improve Osseous Integration of Osteochondral Allografts for the Treatment of Chondral Defects in the Knee at 6 and 12 Months: A Comparative Magnetic Resonance Imaging Analysis. Am. J. Sports Med. 2019, 47, 339–346. [Google Scholar] [CrossRef]
- Solheim, E.; Hegna, J.; Inderhaug, E. Clinical outcome after mosaicplasty of knee articular cartilage defects of patellofemoral joint versus tibiofemoral joint. J. Orthop. 2019, 18, 36–40. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xiang, D.; Shao, J.; Fu, Q.; Han, Y.; Zhu, J.; Chen, Y.; Qian, Q. The clinical efficacy of arthroscopic therapy with knee infrapatellar fat pad cell concentrates in treating knee cartilage lesion: A prospective, randomized, and controlled study. J. Orthop. Surg. Res. 2021, 16, 87. [Google Scholar] [CrossRef]
- Johnson, M. Transcutaneous Electrical Nerve Stimulation: Mechanisms, Clinical Application and Evidence. Rev. Pain 2007, 1, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, J.; Meng, X.; Wang, F. Effects of Electrical Stimulation on Articular Cartilage Regeneration with a Focus on Piezoelectric Biomaterials for Articular Cartilage Tissue Repair and Engineering. Int. J. Mol. Sci. 2023, 24, 1836. [Google Scholar] [CrossRef] [PubMed]
- do Carmo Almeida, T.C.; Dos Santos Figueiredo, F.W.; Barbosa Filho, V.C.; de Abreu, L.C.; Fonseca, F.L.A.; Adami, F. Effects of Transcutaneous Electrical Nerve Stimulation on Proinflammatory Cytokines: Systematic Review and Meta-Analysis. Mediat. Inflamm. 2018, 2018, 1094352. [Google Scholar] [CrossRef] [PubMed]
- Cherian, J.J.; Harrison, P.E.; Benjamin, S.A.; Bhave, A.; Harwin, S.F.; Mont, M.A. Do the Effects of Transcutaneous Electrical Nerve Stimulation on Knee Osteoarthritis Pain and Function Last? J. Knee Surg. 2016, 29, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Deep Oscillation Can Help Fast and Effectively. Pain, Swelling, Edema or Wound Healing Problems? Available online: https://physiomed.de/en/tiefenoszillation/ (accessed on 19 March 2024).
- Hausmann, M.; Ober, J.; Lepley, A.S. The Effectiveness of Deep Oscillation Therapy on Reducing Swelling and Pain in Athletes with Acute Lateral Ankle Sprains. J. Sport Rehabil. 2019, 28, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Vladeva, E.; Mihaylova, M.; Panayotovam, L. Deep Oscillations- Reducing Edema and Improving Kinesiology in the Early Stages after Knee Joint Arthroplasty. J. IMAB 2021, 2, 3577–3581. [Google Scholar] [CrossRef]
- Watson, T. Ultrasound in contemporary physiotherapy practice. Ultrasonics 2008, 48, 321–329. [Google Scholar] [CrossRef]
- Watson, T. Electrotherapy and Tissue Repair. Sportex Med. 2006, 29, 7–13. [Google Scholar]
- Schumann, D.; Kujat, R.; Zellner, J.; Angele, M.K.; Nerlich, M.; Mayr, E.; Angele, P. Treatment of Human Mesenchymal Stem Cells with Pulsed Low Intensity Ultrasound Enhances the Chondrogenic Phenotype In Vitro. Biorheology 2006, 43, 431–443. [Google Scholar]
- de Oliveira Perrucini, P.D.; Poli-Frederico, R.C.; de Almeida Pires-Oliveira, D.A.; Dragonetti Bertin, L.; Beltrão Pires, F.; Shimoya-Bittencourt, W.; Santos, V.M.; Coelho, J.M.; de Oliveira, R.F. Anti-Inflammatory and Healing Effects of Pulsed Ultrasound Therapy on Fibroblasts. Am. J. Phys. Med. Rehabil. 2020, 99, 9–25. [Google Scholar] [CrossRef]
- Wyatt, P.B.; Nelson, C.T.; Cyrus, J.W.; Goldman, A.H.; Patel, N.K. The Role of Cryotherapy After Total Knee Arthroplasty: A Systematic Review. J. Arthroplast. 2023, 38, 950–956. [Google Scholar] [CrossRef]
- van Ooij, B.; Wiegerinck, J.I.; Wegener, J.T.; Van Dijk, C.N.; Schafroth, M. UCryotherapy after Total Knee Arthroplasty provides faster recovery and better ranges of motion in short term follow up. Results of a prospective comparative study. Acta Orthop. Belg. 2020, 86, 463–469. [Google Scholar] [PubMed]
- Matei, D.; Luca, C.; Onu, I.; Matei, P.; Iordan, D.A.; Buculei, I. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350. [Google Scholar] [CrossRef]
- Onu, I.; Iordan, D.A.; Codreanu, C.M.; Matei, D.; Galaction, A.I. Anti-inflammatory effects of exercise training. A systematic review. Balneo Res. J. 2021, 12, 418–425. [Google Scholar] [CrossRef]
- MOSAICPLASTY and OATS SURGERY. Here Are Guidelines that Will Help you in Preparing for Cartilage Transfer Surgery. Available online: https://www.massgeneral.org/assets/mgh/pdf/orthopaedics/sports-medicine/physical-therapy/rehabilitation-protocol-for-mosaicplasty-and-oats-knee-surgery.pdf (accessed on 21 March 2024).
- Inderhaug, E.; Solheim, E. Osteochondral Autograft Transplant (Mosaicplasty) for Knee Articular Cartilage Defects. JBJS Essent. Surg. Tech. 2019, 9, e34. [Google Scholar] [CrossRef]
- Nischal, N.; Iyengar, K.P.; Herlekar, D.; Botchu, R. Imaging of Cartilage and Chondral Defects: An Overview. Life 2023, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Sofu, H.; Oner, A.; Camurcu, Y.; Gursu, S.; Ucpunar, H.; Sahin, V. Predictors of the Clinical Outcome After Arthroscopic Partial Meniscectomy for Acute Trauma–Related Symptomatic Medial Meniscal Tear in Patients More Than 60 Years of Age. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 1125–1132. [Google Scholar] [CrossRef]
- Kemp, J.L.; Makdissi, M.; Schache, A.G.; Pritchard, M.G.; Pollard, T.C.B.; Crossley, K.M. Hip chondropathy at arthroscopy: Prevalence and relationship to labral pathology, femoroacetabular impingement and patient-reported outcomes. Br. J. Sports Med. 2014, 48, 1102–1107. [Google Scholar] [CrossRef]
- Slattery, C.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 476, 2101–2104. [Google Scholar] [CrossRef]


| Initial Assessment Results | ||||||||
| Group | M | SD | DF | F | Sig. | Turkey t | ||
| 1 | 64.1 | 2.92 | InterG | IntraG | 2.06 | <0.142 | Mean Difference | Sig. |
| 2 | 64.86 | 4.24 | 2 | 34 | - | - | ||
| 3 | 66.91 | 2.53 | ||||||
| Interim evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 30.10 | 6.80 | InterG | IntraG | 5.24 | <0.010 | G1/G2 = 1.23 | <0.813 |
| 2 | 31.33 | 4.67 | 2 | 34 | G1/G3 = 6.23 | <0.015 | ||
| 3 | 36.33 | 3.02 | G3/G2 = 5.00 | <0.034 | ||||
| Final evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 15.10 | 3.07 | InterG | IntraG | 111 | <0.000 | G1/G2 = 3.90 | <0.010 |
| 2 | 19.00 | 2.53 | 2 | 34 | G1/G3 = 17.98 | <0.000 | ||
| 3 | 33.08 | 3.60 | G3/G2 = 14.08 | <0.000 | ||||
| Initial Assessment Results | ||||||||
| Group | M | SD | DF | F | Sig. | Turkey t | ||
| 1 | 5.50 | 0.52 | InterG | IntraG | 0.18 | <0.982 | Mean Difference | Sig. |
| 2 | 5.53 | 0.51 | 2 | 34 | - | - | ||
| 3 | 5.50 | 0.52 | ||||||
| Interim evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 3.10 | 0.31 | InterG | IntraG | 11.40 | <0.000 | G1/G2 = 0.70 | <0.004 |
| 2 | 3.80 | 0.41 | 2 | 34 | G1/G3 = 0.98 | <0.000 | ||
| 3 | 4.08 | 0.66 | G3/G2 = 0.28 | <0.309 | ||||
| Final evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 1.20 | 0.42 | InterG | IntraG | 59.87 | <0.000 | G1/G2 = 1.20 | <0.000 |
| 2 | 2.40 | 0.50 | 2 | 34 | G1/G3 = 2.30 | <0.000 | ||
| 3 | 3.50 | 0.52 | G3/G2 = 1.10 | <0.000 | ||||
| Initial Assessment Results | ||||||||
| Group | M | SD | DF | F | Sig. | Turkey t | ||
| 1 | 4.40 | 0.51 | InterG | IntraG | 0.376 | <0.689 | Mean Difference | Sig. |
| 2 | 4.40 | 0.50 | 2 | 34 | - | - | ||
| 3 | 4.25 | 0.45 | ||||||
| Interim evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 4.90 | 0.31 | InterG | IntraG | 2.090 | <0.139 | - | - |
| 2 | 4.53 | 0.51 | 2 | 34 | ||||
| 3 | 4.75 | 0.45 | ||||||
| Final evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 5.0000 | 0.00 | InterG | IntraG | - | - | - | - |
| 2 | 5.0000 | 0.00 | 2 | 34 | ||||
| 3 | 5.0000 | 0.00 | ||||||
| Initial Assessment Results | ||||||||
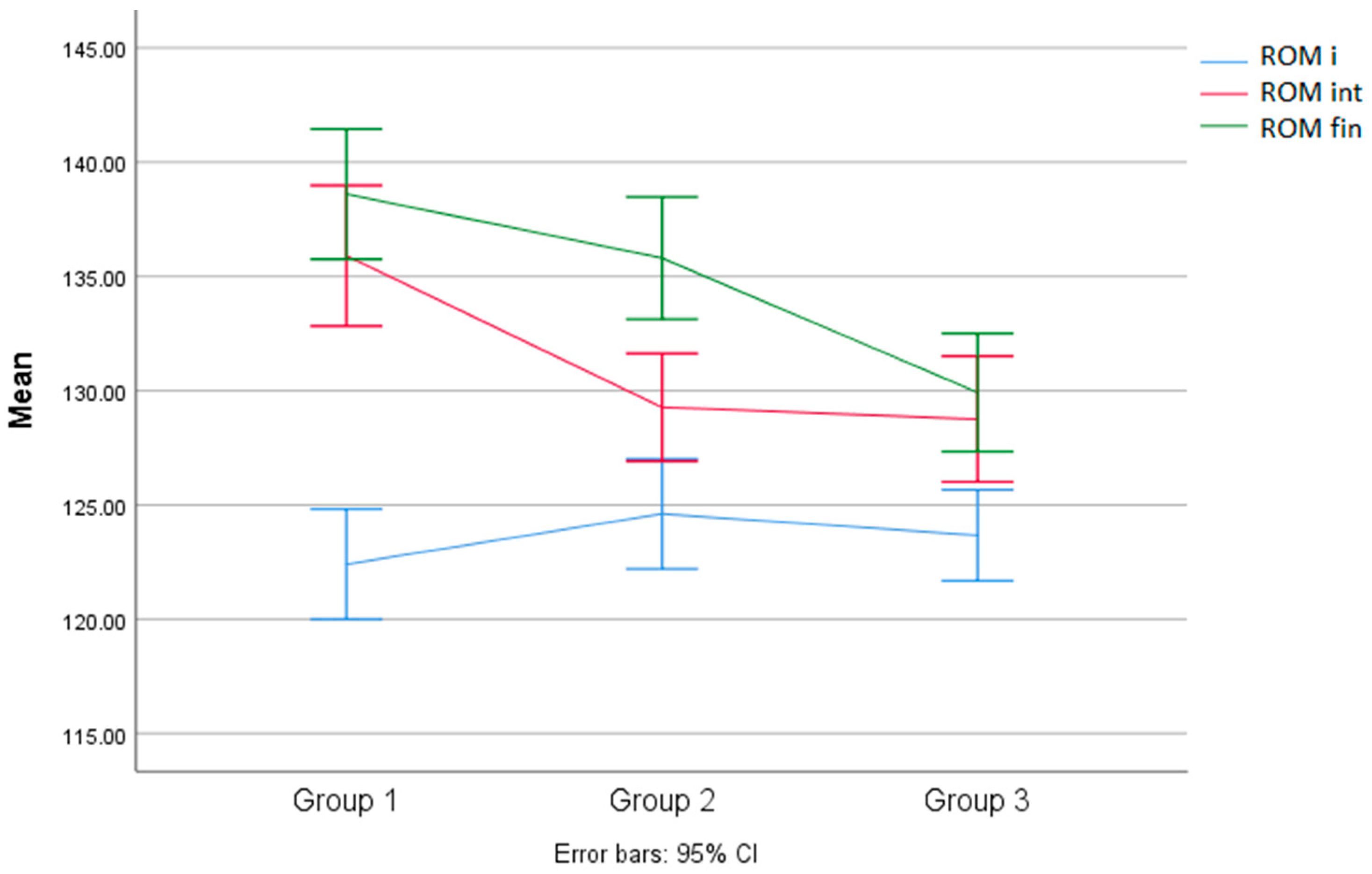
| Group | M | SD | DF | F | Sig. | Turkey t | ||
| 1 | 122.40 | 3.37 | InterG | IntraG | 1.037 | <0.366 | Mean Difference | Sig. |
| 2 | 124.60 | 4.35 | 2 | 34 | - | - | ||
| 3 | 123.66 | 3.14 | ||||||
| Interim evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 135.90 | 4.30 | InterG | IntraG | 9.38 | <0.001 | G1/G2 = 6.63 | <0.002 |
| 2 | 129.26 | 4.25 | 2 | 34 | G1/G3 = 7.15 | <0.001 | ||
| 3 | 128.75 | 4.33 | G3/G2 = 1.66 | <0.984 | ||||
| Final evaluation results | Turkey t | |||||||
| Group | M | SD | DF | F | Sig. | Mean Difference | Sig. | |
| 1 | 138.60 | 3.97 | InterG | IntraG | 11.60 | <0.000 | G1/G2 = 2.80 | <0.274 |
| 2 | 135.80 | 4.82 | 2 | 34 | G1/G3 = 8.68 | <0.000 | ||
| 3 | 129.91 | 4.07 | G3/G2 = 5.83 | <0.004 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherghel, R.; Onu, I.; Iordan, D.A.; Antohe, B.A.; Rezus, I.-I.; Alexa, O.; Macovei, L.A.; Rezus, E. A New Approach to Postoperative Rehabilitation following Mosaicplasty and Bone Marrow Aspiration Concentrate (BMAC) Augmentation. Biomedicines 2024, 12, 1164. https://doi.org/10.3390/biomedicines12061164
Gherghel R, Onu I, Iordan DA, Antohe BA, Rezus I-I, Alexa O, Macovei LA, Rezus E. A New Approach to Postoperative Rehabilitation following Mosaicplasty and Bone Marrow Aspiration Concentrate (BMAC) Augmentation. Biomedicines. 2024; 12(6):1164. https://doi.org/10.3390/biomedicines12061164
Chicago/Turabian StyleGherghel, Robert, Ilie Onu, Daniel Andrei Iordan, Bogdan Alexandru Antohe, Ioana-Irina Rezus, Ovidiu Alexa, Luana Andreea Macovei, and Elena Rezus. 2024. "A New Approach to Postoperative Rehabilitation following Mosaicplasty and Bone Marrow Aspiration Concentrate (BMAC) Augmentation" Biomedicines 12, no. 6: 1164. https://doi.org/10.3390/biomedicines12061164
APA StyleGherghel, R., Onu, I., Iordan, D. A., Antohe, B. A., Rezus, I.-I., Alexa, O., Macovei, L. A., & Rezus, E. (2024). A New Approach to Postoperative Rehabilitation following Mosaicplasty and Bone Marrow Aspiration Concentrate (BMAC) Augmentation. Biomedicines, 12(6), 1164. https://doi.org/10.3390/biomedicines12061164









