Non-Invasive Spinal Cord Stimulation for Motor Rehabilitation of Patients with Spinal Muscular Atrophy Treated with Orphan Drugs
Abstract
1. Introduction
1.1. Spinal Muscular Atrophy
1.2. SMA Orphan Drugs
1.3. Transcutaneous Spinal Cord Stimulation
1.4. Purpose and Hypothesis of This Study
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Treatment Goals
2.2.2. Physical Therapy
2.2.3. Transcutaneous Spinal Cord Stimulation
2.3. Outcome Measures
2.3.1. Joint Goniometry
2.3.2. Revised Upper Limb Module
2.3.3. Hammersmith Function Motor Scale Expanded
2.3.4. Forced Vital Capacity
2.4. Statistics
3. Results
3.1. SMA Type 2 and Type 3 Participants
3.2. Transcutaneous Spinal Cord Stimulation
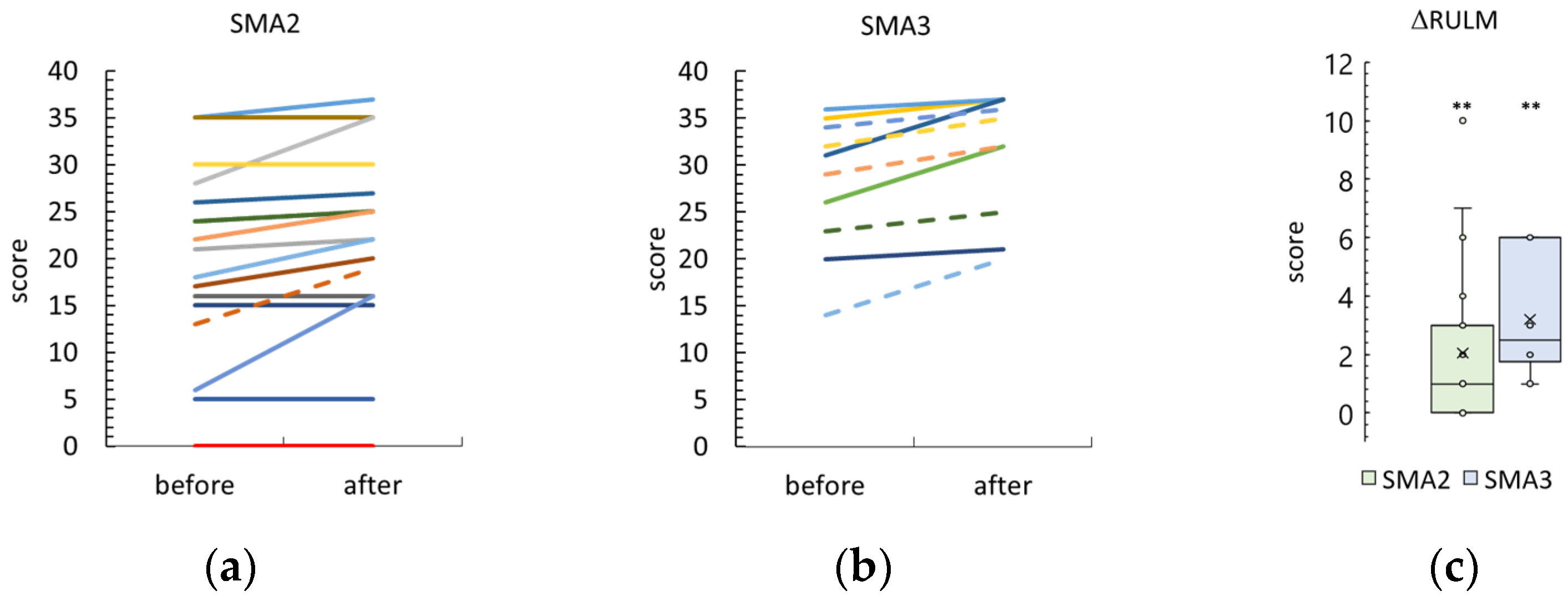
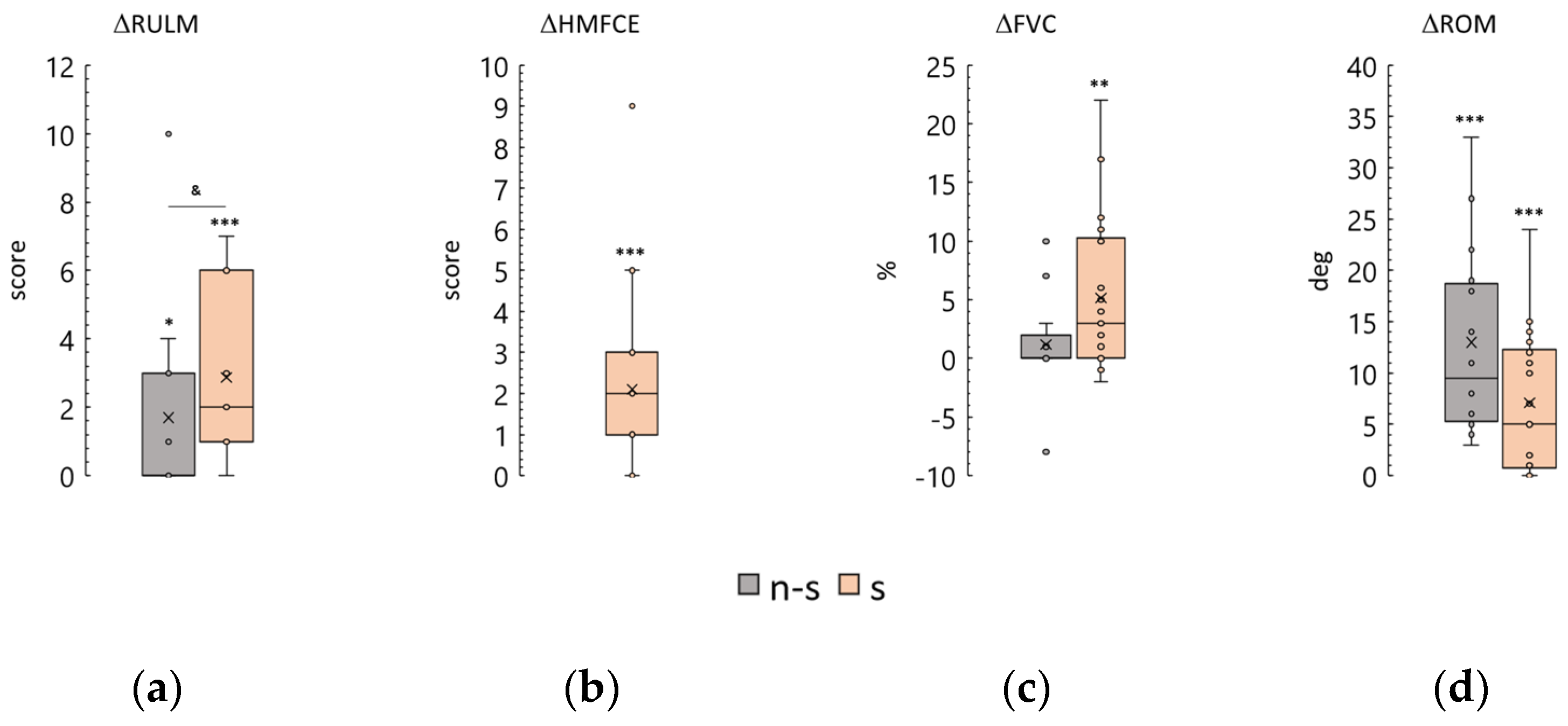
3.3. Revised Upper Limb Module
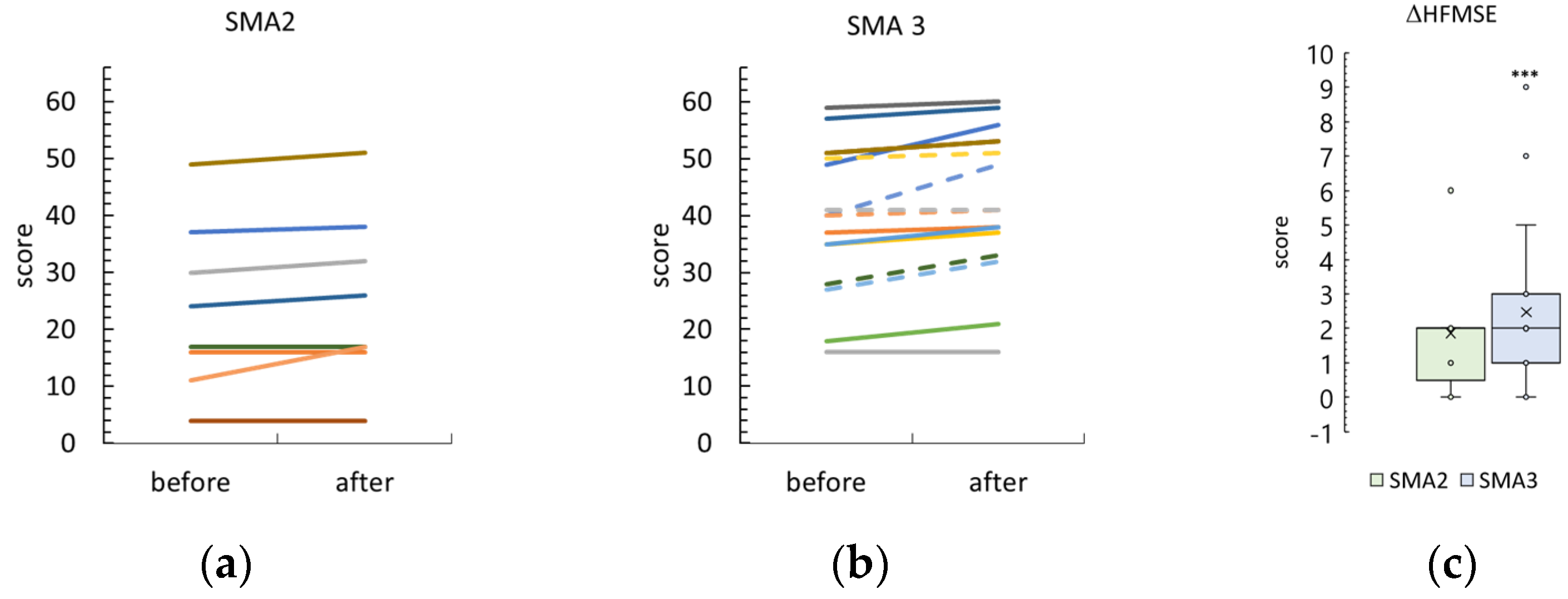
3.4. Hammersmith Function Motor Scale Expanded
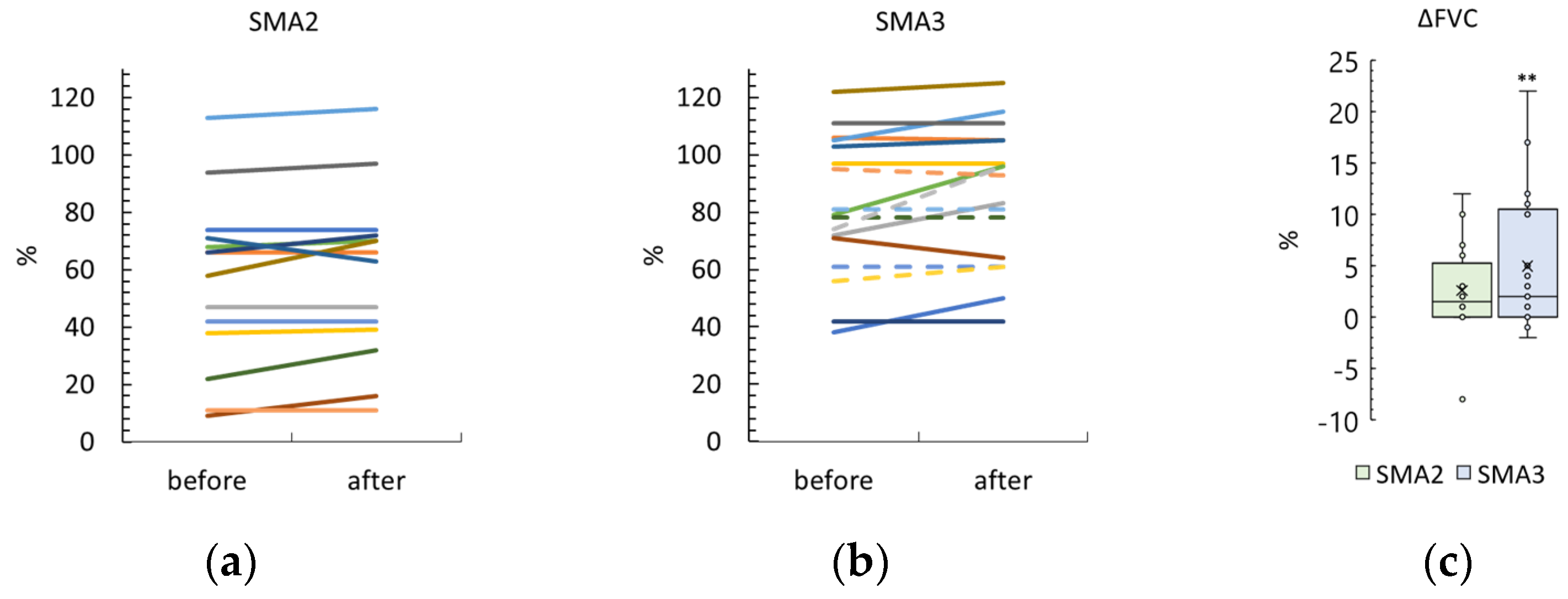
3.5. Forced Vital Capacity
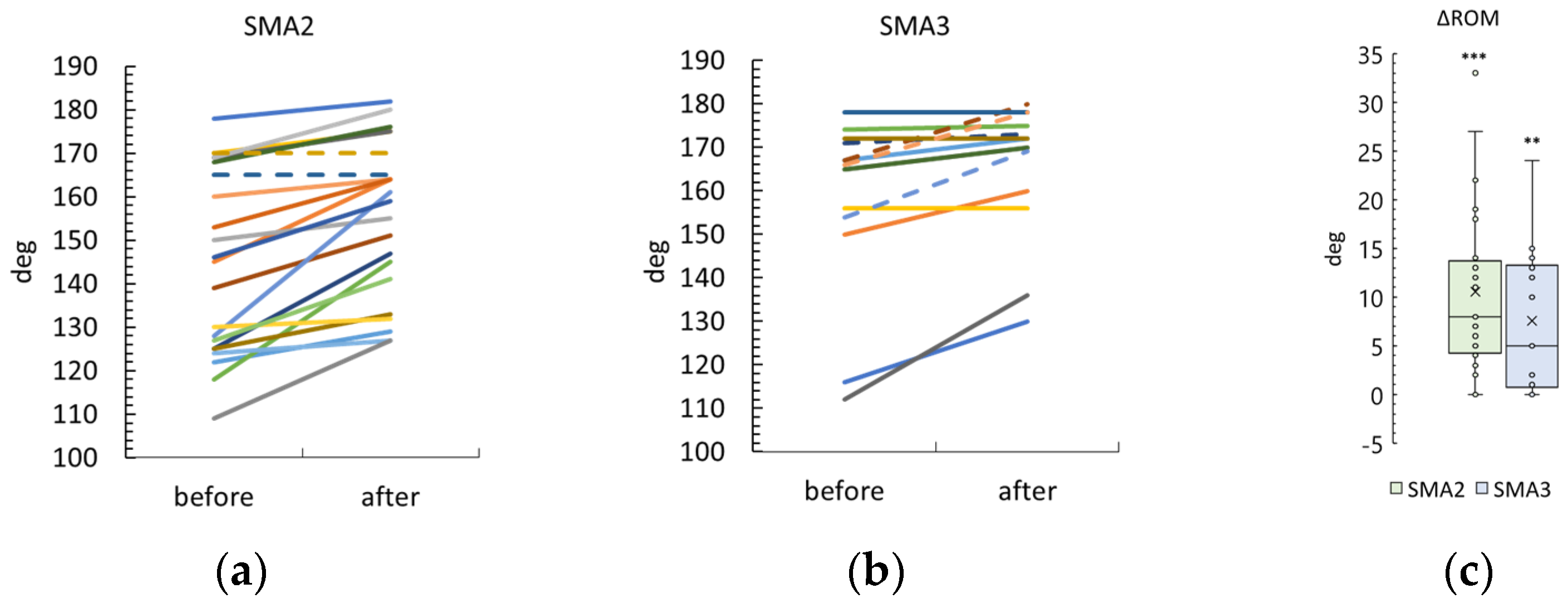
3.6. Knee Range of Motion
3.7. Motor Skills
3.8. Functional Status
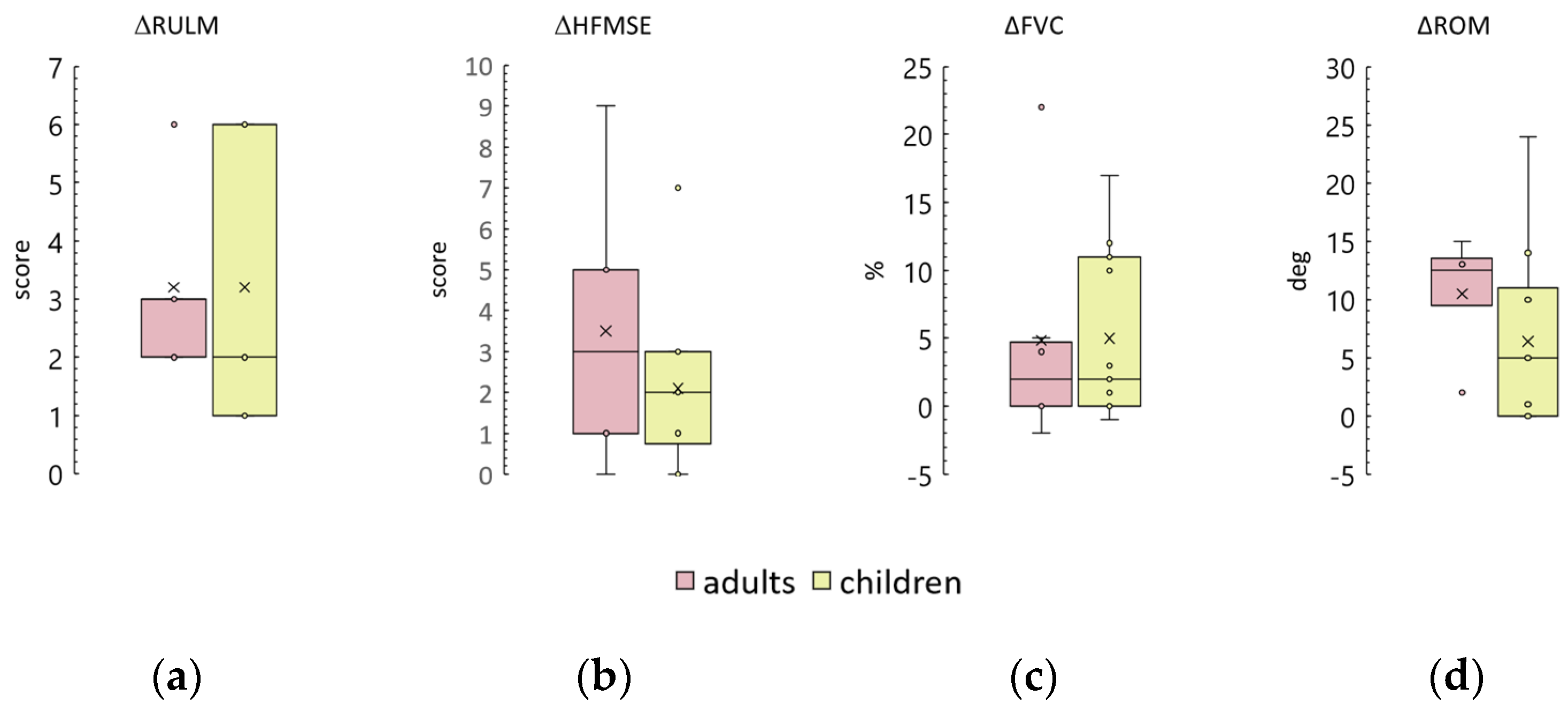
3.9. Age and Adults
3.10. Disease Duration
3.11. Duration of Drug Therapy
4. Discussion
4.1. Minimal Clinically Important Difference and Stimulation Results
4.1.1. RULM and HMFSE Scales
4.1.2. ROM Results
4.1.3. FVC Results
4.1.4. Motor Skill Results
4.2. Effects of SMA Severity
4.3. Effects of Age, Disease Duration, and Medication Duration
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms and Abbreviations
| FVC | Forced vital capacity |
| HFMSE | Hammersmith Function Motor Scale Expanded scale |
| N-S | Non-sitters |
| OA | Onasemnogene abeparvovec |
| ROM | Range of Movements of joints with contracture |
| RULM | Revised Upper Limb Module scale |
| S | Sitters |
| tSCS | transcutaneous Spinal Cord Stimulation |
| W | Walkers |
References
- Novikov, A.; Maldova, M.; Shandybina, N.; Shalmiev, I.; Shoshina, E.; Epoyan, N.; Moshonkina, T. First Use of Non-Invasive Spinal Cord Stimulation in Motor Rehabilitation of Children with Spinal Muscular Atrophy. Life 2023, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, Incidence and Carrier Frequency of 5q–Linked Spinal Muscular Atrophy—A Literature Review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Sumner, C.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal Muscular Atrophy. Nat. Rev. 2022, 371, 2120–2133. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell 1995, 1, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and Management of Spinal Muscular Atrophy: Part 1: Recommendations for Diagnosis, Rehabilitation, Orthopedic and Nutritional Care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Nishio, H.; Niba, E.T.E.; Saito, T.; Okamoto, K.; Takeshima, Y.; Awano, H. Spinal Muscular Atrophy: The Past, Present, and Future of Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11939. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, C.A.; Ivan, R.; Samaha, F.J.; Ionita-Radu, F.; Jianu, D.C.; Vasiliu, O.; Constantin, C.; Tută, S. Orphan Drugs in Neurology—A Narrative Review. J. Pers. Med. 2023, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Botty, C.; Beaudart, C.; Servais, L.; Hiligsmann, M. Systematic Literature Review of the Economic Burden of Spinal Muscular Atrophy and Economic Evaluations of Treatments. Orphanet J. Rare Dis. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, N.L.; Kuntz, N.L.; Kirschner, C.A.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Dangouloff, T.; Servais, L. Clinical Evidence Supporting Early Treatment Of Patients with Spinal Muscular Atrophy: Current Perspectives. TCRM 2019, 15, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.; Lowes, L.P.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.F.; Kwon, J.M. Spinal Muscular Atrophy: Past, Present, and Future. NeoReviews 2019, 20, e437–e451. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Reggie Edgerton, V. Transcutaneous Electrical Spinal-Cord Stimulation in Humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gorodnichev, R.M.; Pivovarova, E.A.; Puhov, A.; Moiseev, S.A.; Gerasimenko, Y.P.; Savochin, A.A.; Moshonkina, T.R.; Chsherbakova, N.A.; Kilimnik, V.A.; Selionov, V.A.; et al. Transcutaneous Electrical Stimulation of the Spinal Cord: A Noninvasive Tool for the Activation of Stepping Pattern Generators in Humans. Hum. Physiol. 2012, 38, 158–167. [Google Scholar] [CrossRef]
- Moshonkina, T.; Grishin, A.; Bogacheva, I.; Gorodnichev, R.; Ovechkin, A.; Siu, R.; Edgerton, V.R.; Gerasimenko, Y. Novel Non-Invasive Strategy for Spinal Neuromodulation to Control Human Locomotion. Front. Hum. Neurosci. 2021, 14, 622533. [Google Scholar] [CrossRef] [PubMed]
- Shamantseva, N.; Timofeeva, O.; Gvozdeva, A.; Andreeva, I.; Moshonkina, T. Posture of Healthy Subjects Modulated by Transcutaneous Spinal Cord Stimulation. Life 2023, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Megía García, A.; Serrano-Muñoz, D.; Taylor, J.; Avendaño-Coy, J.; Gómez-Soriano, J. Transcutaneous Spinal Cord Stimulation and Motor Rehabilitation in Spinal Cord Injury: A Systematic Review. Neurorehabilit. Neural Repair 2020, 34, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.-P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J. Clin. Med. 2022, 11, 1550. [Google Scholar] [CrossRef]
- Singh, G.; Lucas, K.; Keller, A.; Martin, R.; Behrman, A.; Vissarionov, S.; Gerasimenko, Y. Transcutaneous Spinal Stimulation from Adults to Children: A Review. Top. Spinal Cord Inj. Rehabil. 2023, 29, 16–23. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; McKinney, Z.; Sayenko, D.G.; Gad, P.; Gorodnichev, R.M.; Grundfest, W.; Edgerton, V.R.; Kozlovskaya, I.B. Spinal and Sensory Neuromodulation of Spinal Neuronal Networks in Humans. Hum. Physiol. 2017, 43, 492–500. [Google Scholar] [CrossRef]
- Moshonkina, T.R.; Zharova, E.N.; Ananev, S.S.; Shandybina, N.D.; Vershinina, E.A.; Lyakhovetskii, V.A.; Grishin, A.A.; Shlyakhto, E.V.; Gerasimenko, Y.P. A New Technology for Recovery of Locomotion in Patients after a Stroke. Dokl. Biochem. Biophys. 2022, 507, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, G. Development and Reliability of a System to Classify Gross Motor Function in Children with Cerebral Palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Solopova, I.A.; Sukhotina, I.A.; Zhvansky, D.S.; Ikoeva, G.A.; Vissarionov, S.V.; Baindurashvili, A.G.; Edgerton, V.R.; Gerasimenko, Y.P.; Moshonkina, T.R. Effects of Spinal Cord Stimulation on Motor Functions in Children with Cerebral Palsy. Neurosci. Lett. 2017, 639, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Hastings, H.; Zhong, H.; Seth, G.; Kandhari, S.; Edgerton, V.R. Transcutaneous Spinal Neuromodulation Reorganizes Neural Networks in Patients with Cerebral Palsy. Neurotherapeutics 2021, 18, 1953–1962. [Google Scholar] [CrossRef]
- Kariyawasam, D.; D’Silva, A.; Howells, J.; Herbert, K.; Geelan-Small, P.; Lin, C.S.-Y.; Farrar, M.A. Motor Unit Changes in Children with Symptomatic Spinal Muscular Atrophy Treated with Nusinersen. J. Neurol. Neurosurg. Psychiatry 2021, 92, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Sam, G.; Wick, W.; Weiler, M. Evaluation of Risdiplam Efficacy in 5q Spinal Muscular Atrophy: A Systematic Comparison of Electrophysiologic with Clinical Outcome Measures. Eur. J. Neurol. 2024, 31, e16099. [Google Scholar] [CrossRef] [PubMed]
- Bovend’Eerdt, T.J.; Botell, R.E.; Wade, D.T. Writing SMART Rehabilitation Goals and Achieving Goal Attainment Scaling: A Practical Guide. Clin. Rehabil. 2009, 23, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Solopova, I.A.; Selionov, V.A.; Zhvansky, D.S.; Gurfinkel, V.S.; Ivanenko, Y.P. Human Cervical Spinal Cord Circuitry Activated by Tonic Input Can Generate Rhythmic Arm Movements. J. Neurophysiol. 2016, 115, 1018–1030. [Google Scholar] [CrossRef]
- Baindurashvili, A.; Vissarionov, S.; Belianchikov, S.; Kartavenko, K.; Solokhina, I.; Kozyrev, A.; Pukhov, A.; Moshonkina, T.; Gerasimenko, Y. Comprehensive Treatment of a Patient with Complicated Thoracic Spine Injury Using Percutaneous Electrical Spinal Cord Stimulation (Case Report). Genij Ortop. 2020, 26, 79–88. [Google Scholar] [CrossRef]
- Huang, R.; Nikooyan, A.A.; Moore, L.D.; Zdunowski, S.; Morikawa, E.; Sierro, T.; Sayenko, D.; Gad, P.; Homsey, T.; Le, T.; et al. Minimal Handgrip Force Is Needed for Transcutaneous Electrical Stimulation to Improve Hand Functions of Patients with Severe Spinal Cord Injury. Sci. Rep. 2022, 14, 7733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Momeni, K.; Ramanujam, A.; Ravi, M.; Carnahan, J.; Kirshblum, S.; Forrest, G.F. Cervical Spinal Cord Transcutaneous Stimulation Improves Upper Extremity and Hand Function in People with Complete Tetraplegia: A Case Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, S.T. Outcome Measures for Pediatric Spinal Muscular Atrophy. Arch. Neurol. 2002, 59, 1445. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Pepler, L.; Maturi, B.; Afonso, A.C.F.; Sarmiento, J.; Haldenby, R. Systematic Review of Motor Function Scales and Patient-Reported Outcomes in Spinal Muscular Atrophy. Am. J. Phys. Med. Rehabil. 2022, 101, 590–608. [Google Scholar] [CrossRef]
- Salazar, R.; Montes, J.; Dunaway Young, S.; McDermott, M.P.; Martens, W.; Pasternak, A.; Quigley, J.; Mirek, E.; Glanzman, A.M.; Civitello, M.; et al. Quantitative Evaluation of Lower Extremity Joint Contractures in Spinal Muscular Atrophy: Implications for Motor Function. Pediatr. Phys. Ther. 2018, 30, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Young, S.D.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised Upper Limb Module for Spinal Muscular Atrophy: Development of a New Module. Muscle Nerve 2017, 55, 869–874. [Google Scholar] [CrossRef] [PubMed]
- O’Hagen, J.M.; Glanzman, A.M.; McDermott, M.P.; Ryan, P.A.; Flickinger, J.; Quigley, J.; Riley, S.; Sanborn, E.; Irvine, C.; Martens, W.B.; et al. An Expanded Version of the Hammersmith Functional Motor Scale for SMA II and III Patients. Neuromuscul. Disord. 2007, 17, 693–697. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Norkin, C.C.; White, D.J. Measurement of Joint Motion: A Guide to Goniometry, 3rd ed.; F.A. Davis Company: Philadelphia, PA, USA, 2004; ISBN 978-0-8036-4566-0. [Google Scholar]
- Iannaccone, S.T.; Hynan, L.S.; Morton, A.; Buchanan, R.; Limbers, C.A.; Varni, J.W. The PedsQLTM in Pediatric Patients with Spinal Muscular Atrophy: Feasibility, Reliability, and Validity of the Pediatric Quality of Life InventoryTM Generic Core Scales and Neuromuscular Module. Neuromuscul. Disord. 2009, 19, 805–812. [Google Scholar] [CrossRef]
- Wijngaarde, C.A.; Veldhoen, E.S.; van Eijk, R.P.A.; Stam, M.; Otto, L.A.M.; Asselman, F.-L.; Wösten-van Asperen, R.M.; Hulzebos, E.H.J.; Verweij-van den Oudenrijn, L.P.; Bartels, B.; et al. Natural History of Lung Function in Spinal Muscular Atrophy. Orphanet J. Rare Dis. 2020, 15, 88. [Google Scholar] [CrossRef]
- Samaha, F.J.; Buncher, C.R.; Russman, B.S.; White, M.L.; Iannaccone, S.T.; Barker, L.; Burhans, K.; Smith, C.; Perkins, B.; Zimmerman, L. Pulmonary Function in Spinal Muscular Atrophy. J. Child Neurol. 1994, 9, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; Garber, C.E.; Kramer, S.S.; Montgomery, M.J.; Dunaway, S.; Kamil-Rosenberg, S.; Carr, B.; Cruz, R.; Strauss, N.E.; Sproule, D.; et al. Single-Blind, Randomized, Controlled Clinical Trial of Exercise in Ambulatory Spinal Muscular Atrophy: Why Are the Results Negative? JND 2015, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Mirea, A.; Leanca, M.C.; Onose, G.; Sporea, C.; Padure, L.; Shelby, E.-S.; Dima, V.; Daia, C. Physical Therapy and Nusinersen Impact on Spinal Muscular Atrophy Rehabilitative Outcome. FBL 2022, 27, 179. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Beaton, D.; Shea, B.; Boers, M.; Simon, L.; Strand, V.; Brooks, P.; Tugwell, P. Minimal Clinically Important Differences: Review of Methods. J. Rheumatol. 2001, 28, 406–412. [Google Scholar] [PubMed]
- Stolte, B.; Bois, J.-M.; Bolz, S.; Kizina, K.; Totzeck, A.; Schlag, M.; Kleinschnitz, C.; Hagenacker, T. Minimal Clinically Important Differences in Functional Motor Scores in Adults with Spinal Muscular Atrophy. Eur. J. Neurol. 2020, 27, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- Pera, M.C.; Coratti, G.; Forcina, N.; Mazzone, E.S.; Scoto, M.; Montes, J.; Pasternak, A.; Mayhew, A.; Messina, S.; Sframeli, M.; et al. Content Validity and Clinical Meaningfulness of the HFMSE in Spinal Muscular Atrophy. BMC Neurol. 2017, 17, 39. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Mazzone, E.S.; Montes, J.; Scoto, M.; De Sanctis, R.; Main, M.; Mayhew, A.; Muni Lofra, R.; Dunaway Young, S.; et al. Revised Upper Limb Module for Spinal Muscular Atrophy: 12 Month Changes. Muscle Nerve 2019, 59, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and Management of Spinal Muscular Atrophy: Part 2: Pulmonary and Acute Care; Medications, Supplements and Immunizations; Other Organ Systems; and Ethics. Neuromuscul. Disord. 2018, 28, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Chabanon, A.; Seferian, A.M.; Daron, A.; Péréon, Y.; Cances, C.; Vuillerot, C.; De Waele, L.; Cuisset, J.-M.; Laugel, V.; Schara, U.; et al. Prospective and Longitudinal Natural History Study of Patients with Type 2 and 3 Spinal Muscular Atrophy: Baseline Data NatHis-SMA Study. PLoS ONE 2018, 13, e0201004. [Google Scholar] [CrossRef]
- Elsheikh, B.; Severyn, S.; Zhao, S.; Kline, D.; Linsenmayer, M.; Kelly, K.; Tellez, M.; Bartlett, A.; Heintzman, S.; Reynolds, J.; et al. Safety, Tolerability, and Effect of Nusinersen in Non-Ambulatory Adults with Spinal Muscular Atrophy. Front. Neurol. 2021, 12, 650532. [Google Scholar] [CrossRef]
- Landfeldt, E.; Abner, S.; Pechmann, A.; Sejersen, T.; McMillan, H.J.; Lochmüller, H.; Kirschner, J. Caregiver Burden of Spinal Muscular Atrophy: A Systematic Review. PharmacoEconomics 2023, 41, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, C.A. Pharmacotherapy for Spinal Muscular Atrophy in Babies and Children: A Review of Approved and Experimental Therapies. Pediatr. Drugs 2022, 24, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Mentis, G.Z.; Blivis, D.; Liu, W.; Drobac, E.; Crowder, M.E.; Kong, L.; Alvarez, F.J.; Sumner, C.J.; O’Donovan, M.J. Early Functional Impairment of Sensory-Motor Connectivity in a Mouse Model of Spinal Muscular Atrophy. Neuron 2011, 69, 453–467. [Google Scholar] [CrossRef] [PubMed]

| Group | N | Sex (m/f) | Age (years) | Orphan Drug (n) | ||
|---|---|---|---|---|---|---|
| Nusinersen | Risdiplam | OA | ||||
| SMA2 | 20 | 11/9 | 8 [6; 13] 1 | 13 | 6 | 1 |
| SMA3 | 17 | 10/7 | 12 [10; 24] | 17 | - | - |
| Group | Funct. Status (n) | RULM | HFMSE | FVC | ||
|---|---|---|---|---|---|---|
| N-S | S | W | (Score) | (%) | ||
| SMA2 | 12 | 8 | - | 20 ± 10 1 (20) 2 | 24 [11; 37] 3 (7) | 56 ± 30 (14) |
| SMA3 | 1 | 14 | 2 | 35 [28; 37] (17) | 40 ± 13 (16) | 82 ± 24 (17) |
| Funct. Status | n | Age | RULM | HFMSE | FVC |
|---|---|---|---|---|---|
| (Years) | (Score) | (%) | |||
| N-S | 13 | 9.5 ± 4.1 1 | 16 ± 8 | 4; 11 (2) 3 | 47 ± 27 (11) |
| S | 22 | 11.5 [8; 24] 2 | 35 [26; 37] | 36 ± 13 (19) | 84 ± 21 (18) |
| W | 2 | 7; 14 | 31; 37 | 49; 57 | 38; 103 |
| Group | ΔRULM | ΔHFMSE | ΔFVC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | n | r | p | n | r | p | n | |
| SMA2 | 0.26 | 0.27 | 9 | 0.32 | 0.48 | 7 | 0.11 | 0.71 | 14 |
| SMA3 | 0.49 | 0.16 | 10 | 0.09 | 0.75 | 16 | −0.09 | 0.74 | 17 |
| Group | ΔRULM | ΔHFMSE | ΔFVC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | n | r | p | n | r | p | n | |
| SMA2 | 0.41 | 0.11 | 16 | 0.15 | 0.83 | 5 | 0.16 | 0.59 | 13 |
| SMA3 | 0.37 | 0.41 | 7 | 0.26 | 0.40 | 12 | 0.39 | 0.19 | 13 |
| Group | ΔRULM | ΔHFMSE | ΔFVC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | n | r | p | n | r | p | n | |
| SMA2 | −0.44 | 0.08 | 17 | 0.62 | 0.24 | 6 | −0.47 | 0.09 | 14 |
| SMA3 | −0.15 | 0.72 | 8 | 0.06 | 0.84 | 14 | −0.01 | 0.96 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikov, A.; Maldova, M.; Shamantseva, N.; Shalmiev, I.; Shoshina, E.; Epoyan, N.; Krutikova, N.; Moshonkina, T. Non-Invasive Spinal Cord Stimulation for Motor Rehabilitation of Patients with Spinal Muscular Atrophy Treated with Orphan Drugs. Biomedicines 2024, 12, 1162. https://doi.org/10.3390/biomedicines12061162
Novikov A, Maldova M, Shamantseva N, Shalmiev I, Shoshina E, Epoyan N, Krutikova N, Moshonkina T. Non-Invasive Spinal Cord Stimulation for Motor Rehabilitation of Patients with Spinal Muscular Atrophy Treated with Orphan Drugs. Biomedicines. 2024; 12(6):1162. https://doi.org/10.3390/biomedicines12061162
Chicago/Turabian StyleNovikov, Anton, Maria Maldova, Natalia Shamantseva, Ivan Shalmiev, Elena Shoshina, Natalia Epoyan, Natalia Krutikova, and Tatiana Moshonkina. 2024. "Non-Invasive Spinal Cord Stimulation for Motor Rehabilitation of Patients with Spinal Muscular Atrophy Treated with Orphan Drugs" Biomedicines 12, no. 6: 1162. https://doi.org/10.3390/biomedicines12061162
APA StyleNovikov, A., Maldova, M., Shamantseva, N., Shalmiev, I., Shoshina, E., Epoyan, N., Krutikova, N., & Moshonkina, T. (2024). Non-Invasive Spinal Cord Stimulation for Motor Rehabilitation of Patients with Spinal Muscular Atrophy Treated with Orphan Drugs. Biomedicines, 12(6), 1162. https://doi.org/10.3390/biomedicines12061162








