The Polyamine Analogue Ivospemin Increases Chemotherapeutic Efficacy in Murine Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Culture Conditions, and Reagents
2.2. Cell Viability Assays
2.3. Intracellular Polyamine Pool Determination
2.4. Syngeneic Mouse Model
2.5. Statistical Analysis
3. Results
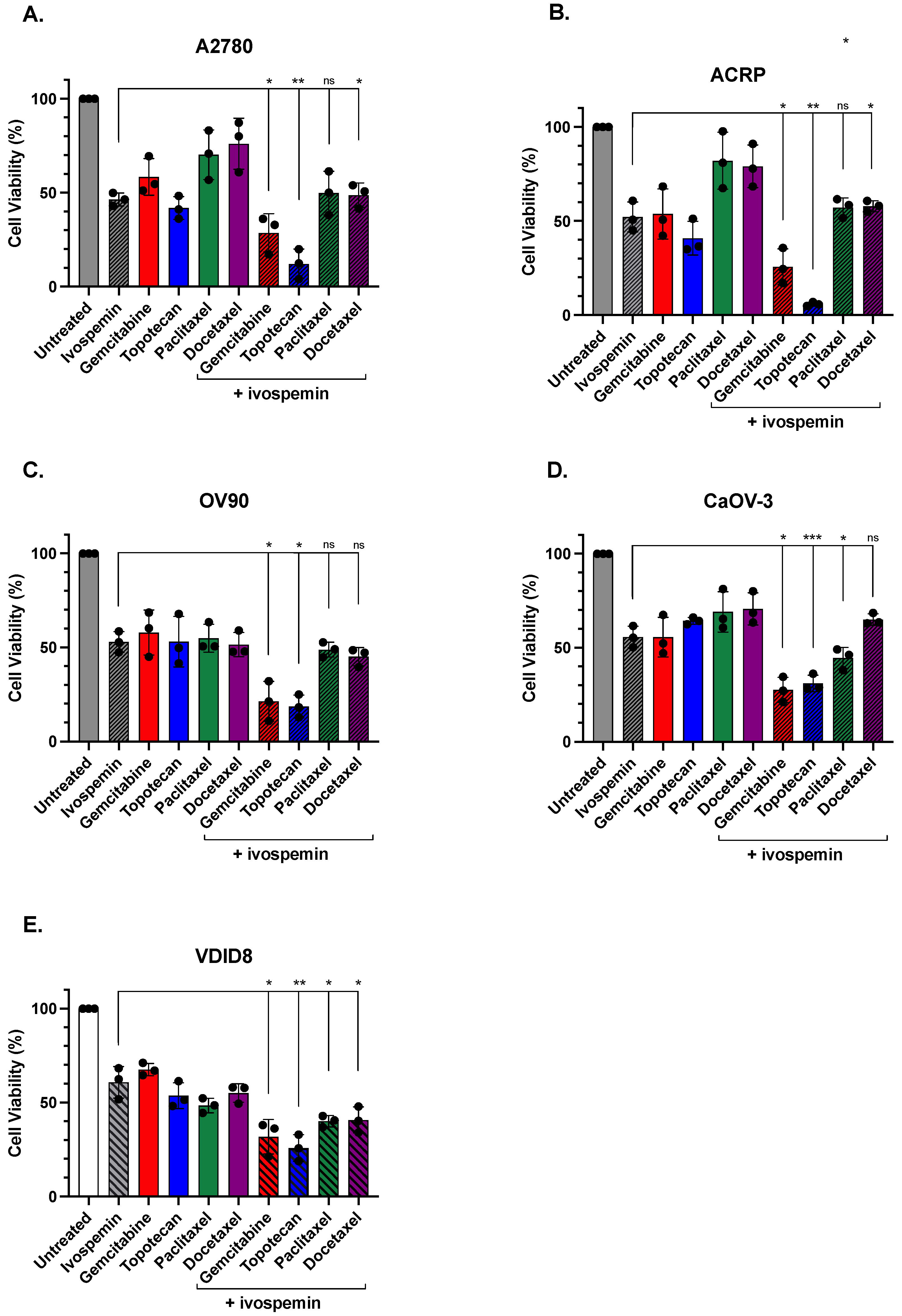
3.1. Ivospemin Co-Treatment Increases Chemotherapy Toxicity in Ovarian Adenocarcinoma Cell Lines Regardless of Cisplatin Sensitivity
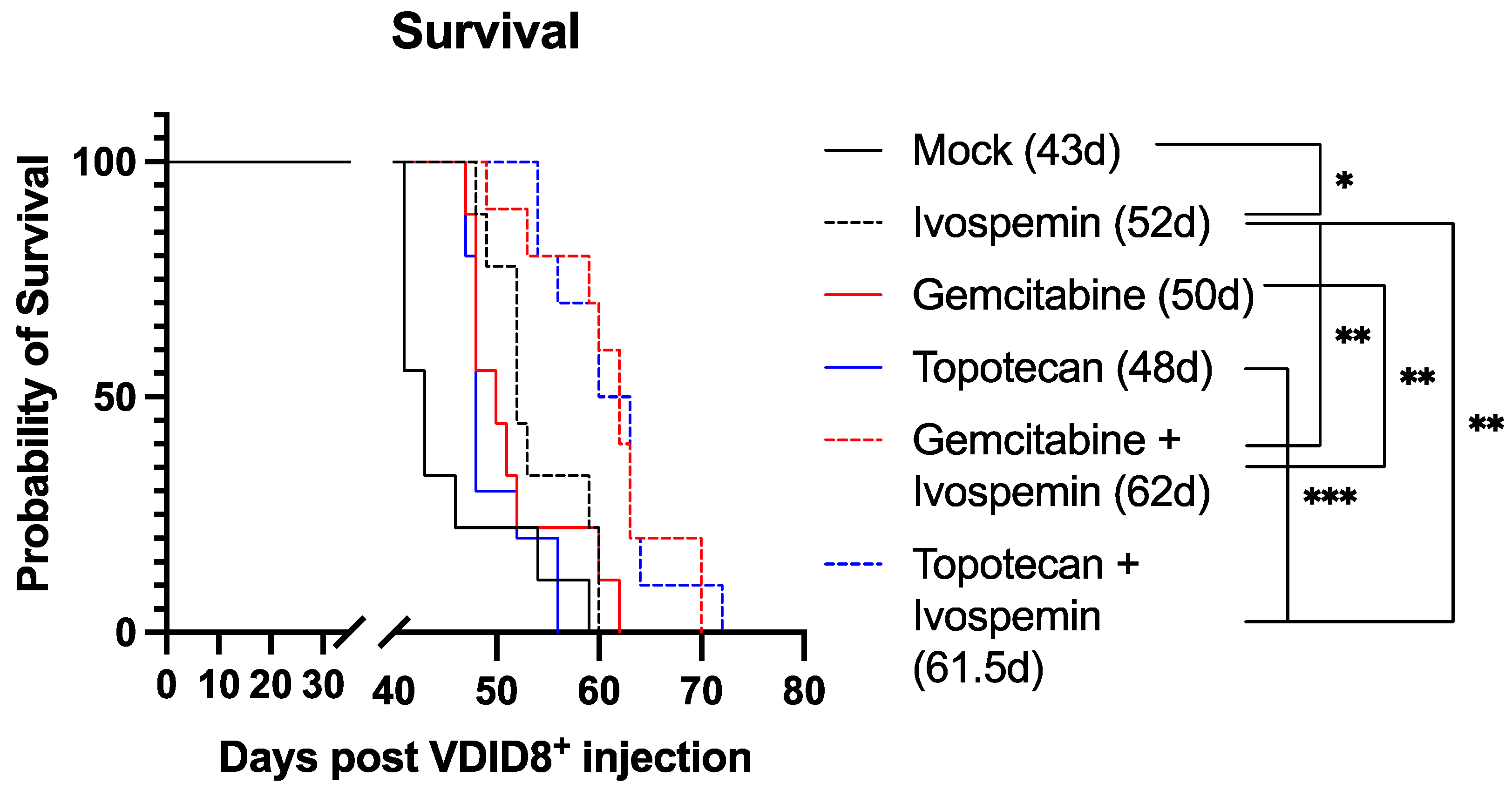
3.2. Ivospemin Treatment in Combination with Chemotherapy Increases Survival in a Murine Ovarian Adenocarcinoma Model Compared to Chemotherapy Alone
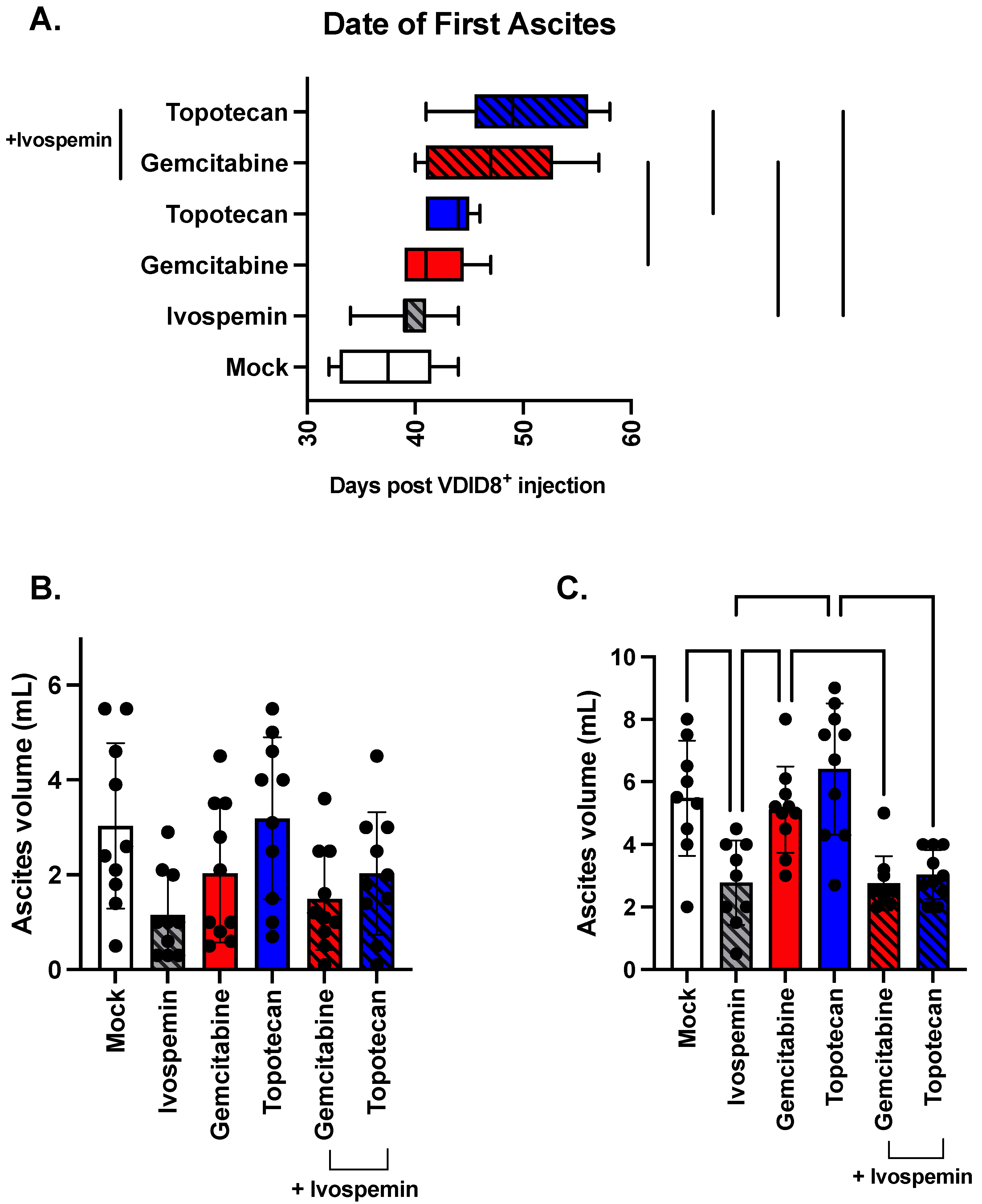
3.3. Ivospemin Increases Chemotherapeutic Efficacy by Delaying Disease Onset and Decreasing Overall Tumor Burden in a Murine Ovarian Adenocarcinoma Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Meyskens, F.L. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Stewart, T.M.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. USA 1993, 90, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Thomas, T.J. Polyamine metabolism and cancer. J. Cell Mol. Med. 2003, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, R.J.; Müller, R.; Bussenius, J.; McManis, J.S.; Merriman, R.L.; Smith, R.E.; Yao, H.; Weimar, W.R. Synthesis and evaluation of hydroxylated polyamine analogues as antiproliferatives. J. Med. Chem. 2000, 43, 224–235. [Google Scholar] [CrossRef]
- Bergeron, R.J.; Neims, A.H.; McManis, J.S.; Hawthorne, T.R.; Vinson, J.R.; Bortell, R.; Ingeno, M.J. Synthetic polyamine analogues as antineoplastics. J. Med. Chem. 1988, 31, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Woster, P.M. Terminally alkylated polyamine analogues as chemotherapeutic agents. J. Med. Chem. 2001, 44, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Fogel-Petrovic, M.; Vujcic, S.; Miller, J.; Porter, C.W. Differential post-transcriptional control of ornithine decarboxylase and spermidine-spermine N1-acetyltransferase by polyamines. FEBS Lett. 1996, 391, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Fogel-Petrovic, M.; Vujcic, S.; Brown, P.J.; Haddox, M.K.; Porter, C.W. Effects of polyamines, polyamine analogs, and inhibitors of protein synthesis on spermidine-spermine N1-acetyltransferase gene expression. Biochemistry 1996, 35, 14436–14444. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Hu, R.H. Effect of polyamine analogues and inhibition of polyamine oxidase on spermidine/spermine N1-acetyltransferase activity and cell proliferation. Cancer Lett. 1995, 95, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hahm, H.A.; Ettinger, D.S.; Bowling, K.; Hoker, B.; Chen, T.L.; Zabelina, Y.; Casero, R.A., Jr. Phase I study of N(1),N(11)-diethylnorspermine in patients with non-small cell lung cancer. Clin. Cancer Res. 2002, 8, 684–690. [Google Scholar] [PubMed]
- Creaven, P.J.; Perez, R.; Pendyala, L.; Meropol, N.J.; Loewen, G.; Levine, E.; Berghorn, E.; Raghavan, D. Unusual central nervous system toxicity in a phase I study of N1N11 diethylnorspermine in patients with advanced malignancy. Investig. New Drugs 1997, 15, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wilding, G.; King, D.; Tutsch, K.; Pomplun, M.; Feierabend, C.; Alberti, D.; Arzoomanian, R. Phase I trial of the polyamine analog N1,N14-diethylhomospermine (DEHSPM) in patients with advanced solid tumors. Investig. New Drugs 2004, 22, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.M.; Desai, A.A.; Fitzgerald, M.L.; Marton, L.J.; Casero, R.A. A phase I dose-escalation study of the polyamine analog PG-11047 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2020, 85, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.M.; Von Hoff, D.; Fitzgerald, M.; Marton, L.J.; Becerra, C.H.R.; Boyd, T.E.; Conkling, P.R.; Garbo, L.E.; Jotte, R.M.; Richards, D.A.; et al. A Phase Ib mulitcenter, dose-escalation study of the polyamine analogue PG-11047 in combination with gemcitabine, docetaxel, bevacizumab, erlotinib, cisplatin, 5-fluorouracil, or sunitinib in patients with advanced solid tumors or lymphoma. Cancer Chemother. Pharmacol. 2020, 87, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Foley, J.R.; Stewart, T.M.; Casero, R.A. Expanded Potential of the Polyamine Analogue SBP-101 (Diethyl Dihydroxyhomospermine) as a Modulator of Polyamine Metabolism and Cancer Therapeutic. Int. J. Mol. Sci. 2022, 23, 6798. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Cullen, M.T.; Baker, C.H. Abstract 3128: Efficacy of diethyldihydroxyhomospermine against human pancreatic adenocarcinoma using orthotopic implantation of human pancreatic L3.6pl cells into the pancreas of nude mice. Cancer Res. 2014, 74, 3128. [Google Scholar] [CrossRef]
- Singhal, N.; Sigal, D.; Tebbutt, N.C.; Hezel, A.F.; Nagrial, A.; Lumba, S.; George, T.J.; Smith, S.L.; Gagnon, S.; Walker, M.; et al. SBP-101, a polyamine metabolic inhibitor, administered in combination with gemcitabine and nab-paclitaxel, shows signals of efficacy as first-line treatment for subjects with metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2021, 39 (Suppl. S15), 4127. [Google Scholar] [CrossRef]
- Therapeutics. Phase 1A/1B Dose Escalation and Expansion Study of SBP-101 in Combination with Nab-Paclitaxel and Gemcitabine in Subjects with Previously Untreated Metastatic Pancreatic Ductal Adenocarcinoma. NCT03412799. Updated 25 May 2022. Available online: https://clinicaltrials.gov/study/NCT03412799 (accessed on 12 May 2023).
- Therapeutics, P. A Randomized, Double-Blind, Placebo-Controlled Study of Nab-Paclitaxel and Gemcitabine with or without SBP-101 in Subjects Previously Untreated for Metastatic Pancreatic Ductal Adenocarcinoma. NCT05254171. Updated 4 April 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05254171 (accessed on 23 August 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Galván, S.; Carnero, A. Targeting Cancer Stem Cells to Overcome Therapy Resistance in Ovarian Cancer. Cells 2020, 9, 1402. [Google Scholar] [CrossRef]
- da Costa, A.A.B.A.; Baiocchi, G. Genomic profiling of platinum-resistant ovarian cancer: The road into druggable targets. Semin. Cancer Biol. 2021, 77, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Almousa, S.; Warren, B.; Abdulfattah, A.Y.; Shu, J.; Abouelfadl, H.; Gonzalez, D.; Livingston, C.; Said, N. Exploring the clinical value of tumor microenvironment in platinum-resistant ovarian cancer. Semin. Cancer Biol. 2021, 77, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Sherman-Baust, C.A.; Weeraratna, A.T.; Rangel, L.B.; Pizer, E.S.; Cho, K.R.; Schwartz, D.R.; Shock, T.; Morin, P.J. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 2003, 3, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kabra, P.M.; Lee, H.K.; Lubich, W.P.; Marton, L.J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: Improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J. Chromatogr. 1986, 380, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Small molecule inhibitor of c-Met (PHA665752) suppresses the growth of ovarian cancer cells and reverses cisplatin resistance. Tumour Biol. 2016, 37, 7843–7852. [Google Scholar]
- Hayakawa, J.; Ohmichi, M.; Kurachi, H.; Ikegami, H.; Kimura, A.; Matsuoka, T.; Jikihara, H.; Mercola, D.; Murata, Y. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J. Biol. Chem. 1999, 274, 31648–31654. [Google Scholar] [CrossRef] [PubMed]
- Katano, K.; Kondo, A.; Safaei, R.; Holzer, A.; Samimi, G.; Mishima, M.; Kuo, Y.-M.; Rochdi, M.; Howell, S.B. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002, 62, 6559–6565. [Google Scholar] [PubMed]
- Varma, R.; Hector, S.; Greco, W.R.; Clark, K.; Hawthorn, L.; Porter, C.; Pendyala, L. Platinum drug effects on the expression of genes in the polyamine pathway: Time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells. Cancer Chemother. Pharmacol. 2007, 59, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Diegelman, P.; Hector, S.; Kramer, D.L.; Clark, K.; Zagst, P.; Fetterly, G.; Porter, C.W.; Pendyala, L. Combination effects of platinum drugs and N1, N11 diethylnorspermine on spermidine/spermine N1-acetyltransferase, polyamines and growth inhibition in A2780 human ovarian carcinoma cells and their oxaliplatin and cisplatin-resistant variants. Cancer Chemother. Pharmacol. 2011, 67, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Hector, S.; Porter, C.W.; Kramer, D.L.; Clark, K.; Prey, J.; Kisiel, N.; Diegelman, P.; Chen, Y.; Pendyala, L. Polyamine catabolism in platinum drug action: Interactions between oxaliplatin and the polyamine analogue N1,N11-diethylnorspermine at the level of spermidine/spermine N1-acetyltransferase. Mol. Cancer Ther. 2004, 3, 813–822. [Google Scholar] [CrossRef] [PubMed]

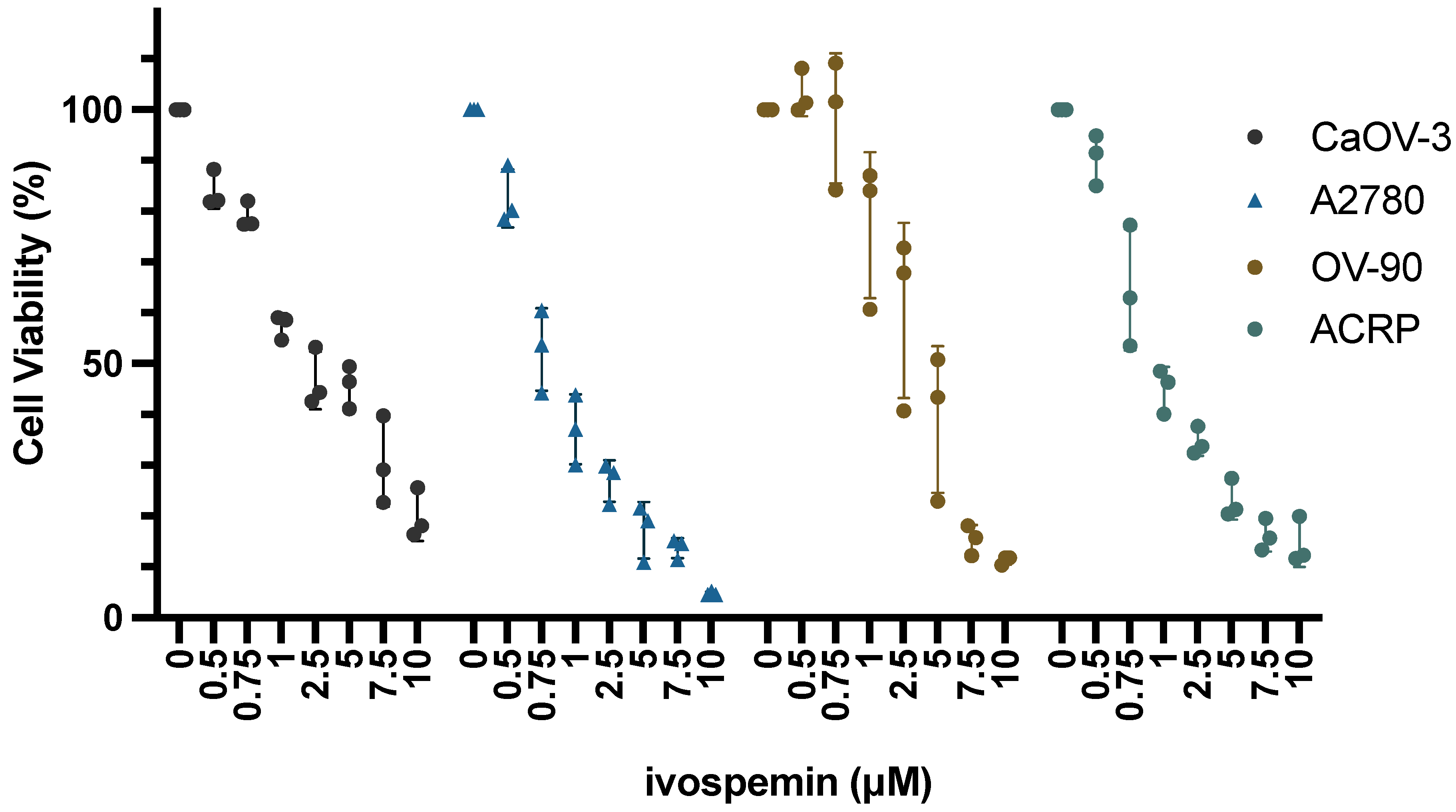
| Cell Line | Cisplatin 48 h IC50 | Ivospemin 96 h IC50 |
|---|---|---|
| CaOV-3 | 4.24 μM | 2.56 μM |
| A2780 | 5.21 μM | 0.95 μM |
| OV90 | 14.13 μM | 3.24 μM |
| ACRP | 28.42 μM | 1.41 μM |
| Comparison | p-Value | Significance |
|---|---|---|
| Gemcitabine (A2780) | 0.021 | * |
| Gemcitabine (ACRP) | 0.041 | * |
| Gemcitabine (CaOV-3) | 0.018 | * |
| Gemcitabine (OV90) | 0.016 | * |
| Gemcitabine (VDID8) | 0.016 | * |
| Topotecan (A2780) | 0.007 | ** |
| Topotecan (ACRP) | 0.003 | ** |
| Topotecan (CaOV-3) | 0.0003 | *** |
| Topotecan (OV90) | 0.016 | * |
| Topotecan (VDID8) | 0.005 | ** |
| Paclitaxel (A2780) | 0.114 | ns |
| Paclitaxel (ACRP) | 0.054 | ns |
| Paclitaxel (CaOV-3) | 0.025 | * |
| Paclitaxel (OV90) | 0.281 | ns |
| Paclitaxel (VDID8) | 0.016 | * |
| Docetaxel (A2780) | 0.034 | * |
| Docetaxel (ACRP) | 0.035 | * |
| Docetaxel (CaOV-3) | 0.338 | ns |
| Docetaxel (OV90) | 0.243 | ns |
| Docetaxel (VDID8) | 0.033 | * |
| Comparison | p-Value | Significance |
|---|---|---|
| Mock vs. Ivo | 0.026 | * |
| Mock vs. Gem | 0.053 | ns |
| Mock vs. Topo | 0.170 | ns |
| Ivo vs. Gem | 0.559 | ns |
| Ivo vs. Topo | 0.037 | * |
| Ivo vs. Gem + Ivo | 0.004 | ** |
| Ivo vs. Topo + Ivo | 0.003 | ** |
| Gem vs. Gem + Ivo | 0.004 | ** |
| Topo vs. Topo + Ivo | 0.0002 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holbert, C.E.; Foley, J.R.; Casero, R.A., Jr.; Stewart, T.M. The Polyamine Analogue Ivospemin Increases Chemotherapeutic Efficacy in Murine Ovarian Cancer. Biomedicines 2024, 12, 1157. https://doi.org/10.3390/biomedicines12061157
Holbert CE, Foley JR, Casero RA Jr., Stewart TM. The Polyamine Analogue Ivospemin Increases Chemotherapeutic Efficacy in Murine Ovarian Cancer. Biomedicines. 2024; 12(6):1157. https://doi.org/10.3390/biomedicines12061157
Chicago/Turabian StyleHolbert, Cassandra E., Jackson R. Foley, Robert A. Casero, Jr., and Tracy Murray Stewart. 2024. "The Polyamine Analogue Ivospemin Increases Chemotherapeutic Efficacy in Murine Ovarian Cancer" Biomedicines 12, no. 6: 1157. https://doi.org/10.3390/biomedicines12061157
APA StyleHolbert, C. E., Foley, J. R., Casero, R. A., Jr., & Stewart, T. M. (2024). The Polyamine Analogue Ivospemin Increases Chemotherapeutic Efficacy in Murine Ovarian Cancer. Biomedicines, 12(6), 1157. https://doi.org/10.3390/biomedicines12061157







