Gut Microbiome-Mediated Mechanisms in Alleviating Opioid Addiction with Aqueous Extract of Anacyclus pyrethrum
Abstract
1. Introduction
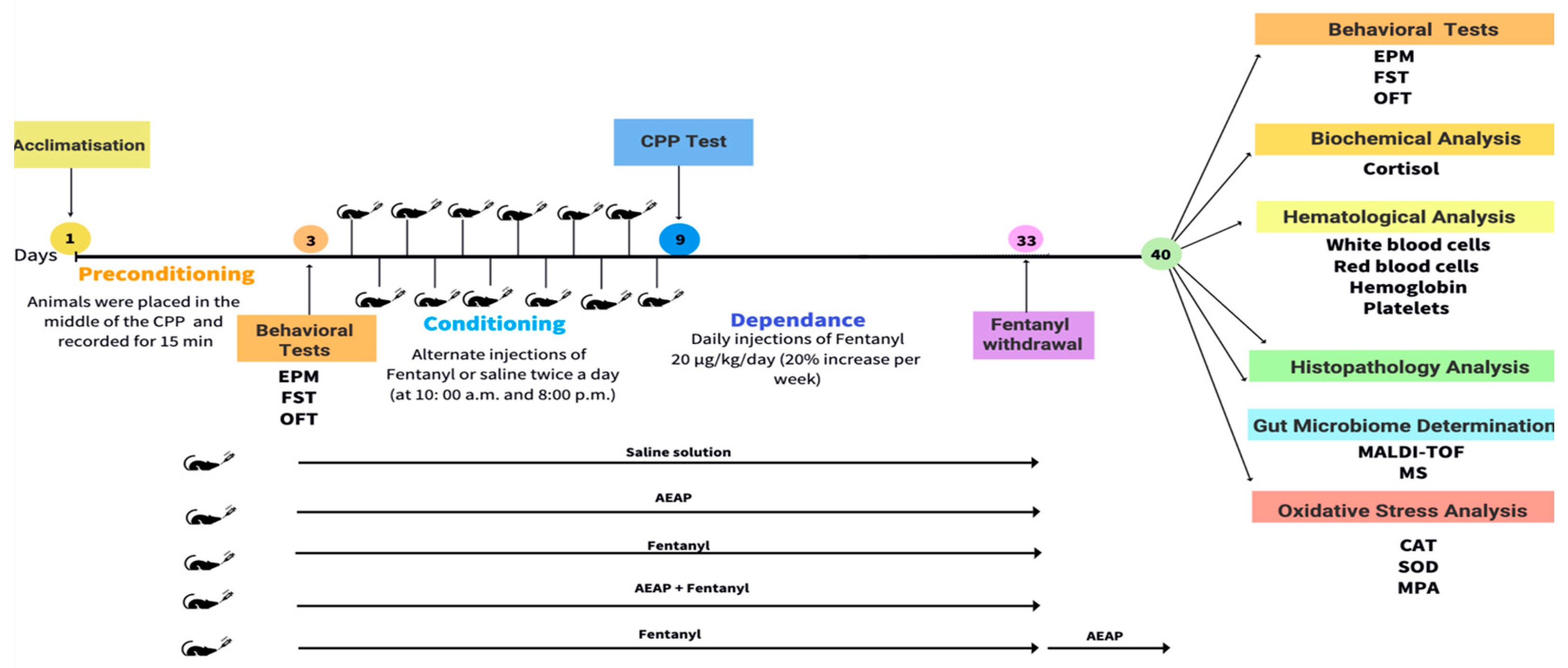
2. Materials and Methods
2.1. Plant Material and Preparation of the Aqueous Extract
2.2. AEAP Metabolite Profiling
2.3. Animals
2.4. Drug Administration
2.5. Behavioral Assessment
2.5.1. Conditioned Place Preference (CPP)
2.5.2. Open Field Test (OFT)
2.5.3. Porsolt’s Forced Swim Test (FST)
2.5.4. Elevated Plus Maze (EPM)
2.6. Biochemical and Hematological Analyses
2.7. Oxidative Stress
2.7.1. LPO Assay
2.7.2. CAT Activity
2.7.3. SOD Activity
2.8. Gut Microbiota Determination
2.8.1. MALDI-TOF MS
2.8.2. MALDI-TOF MS Analysis
2.9. Histological Study
2.10. Statistical Analyses
3. Results
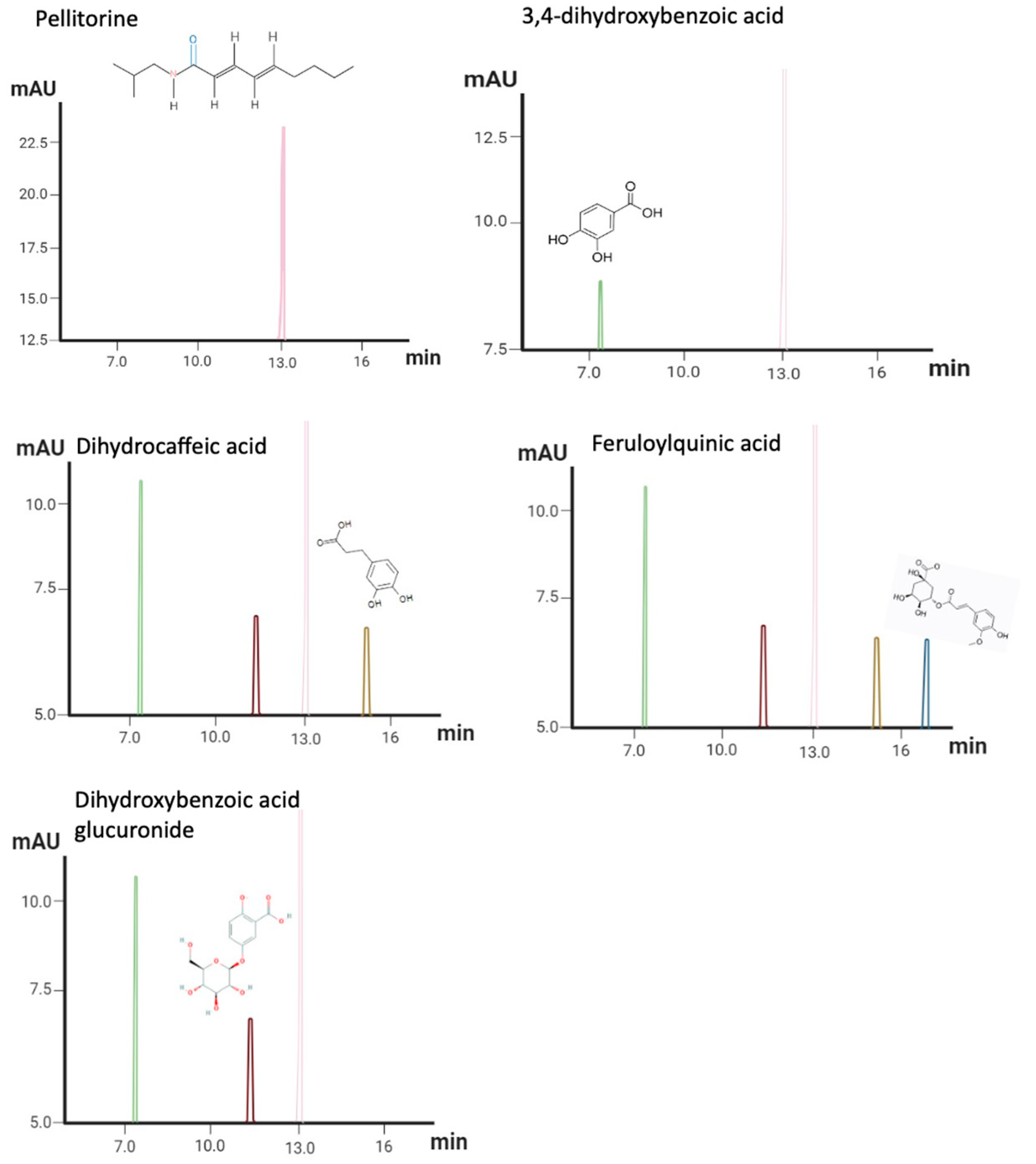
3.1. HPLC–PDA–MS Aqueous Extract of Anacyclus Pyrethrum Profiles
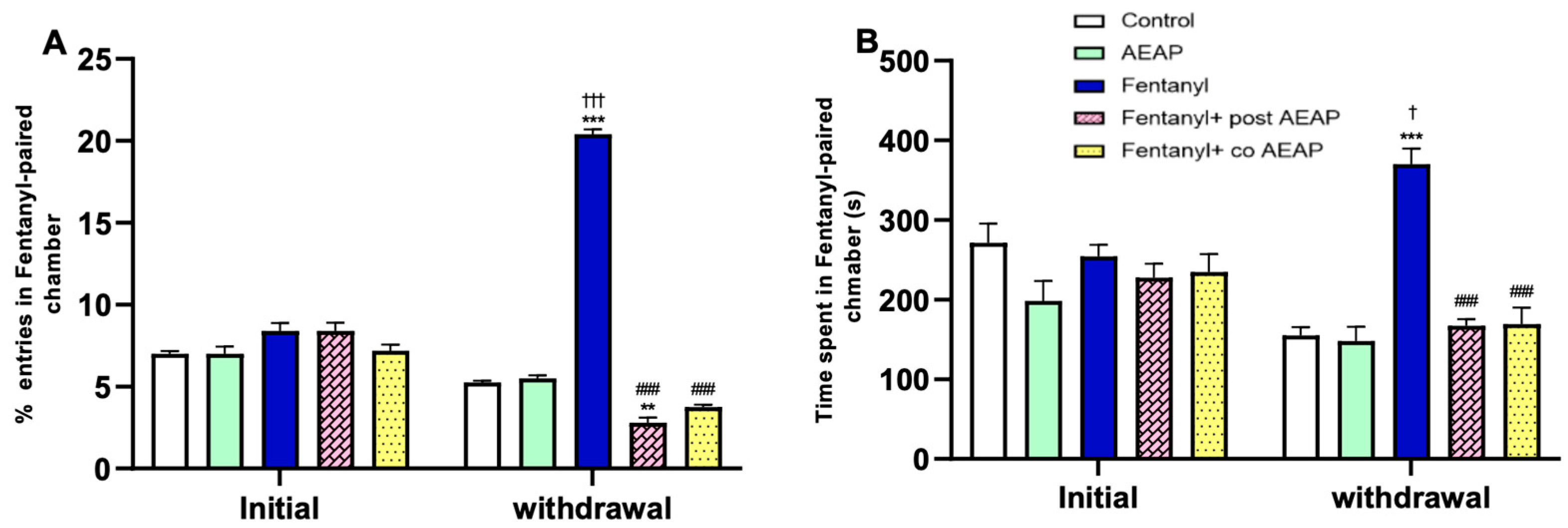
3.2. Fentanyl-Induced Conditioned Place Preference (CPP)
3.3. Fentanyl Withdrawal-Induced Anxiety and Depression and the Potential of AEAP to Alleviate Adverse Outcomes
3.4. Fentanyl Withdrawal-Induced Cortisol Level Elevation
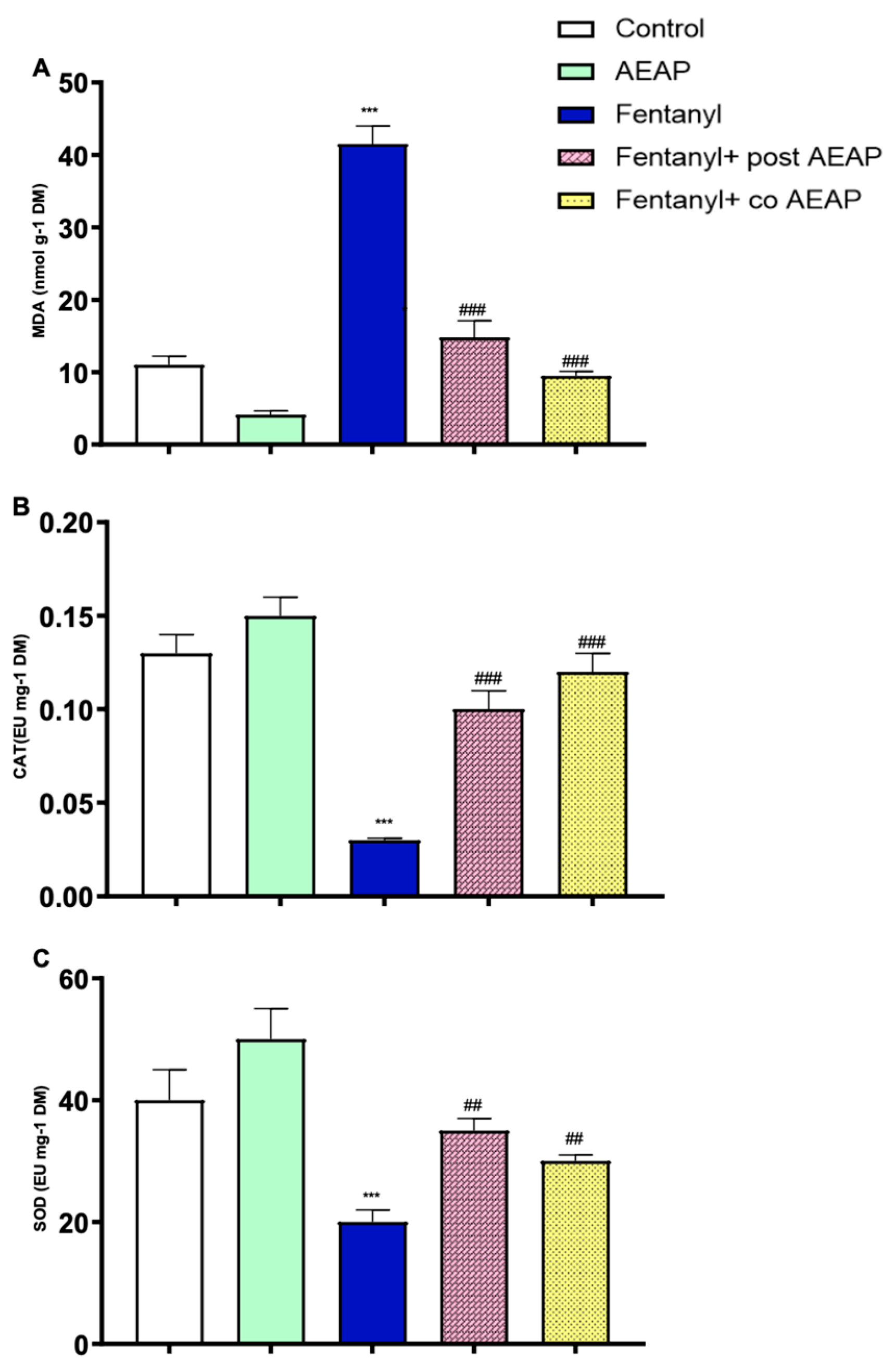
3.5. Fentanyl Withdrawal-Induced Oxidative Stress and the Antioxidant Capacity of AEAP
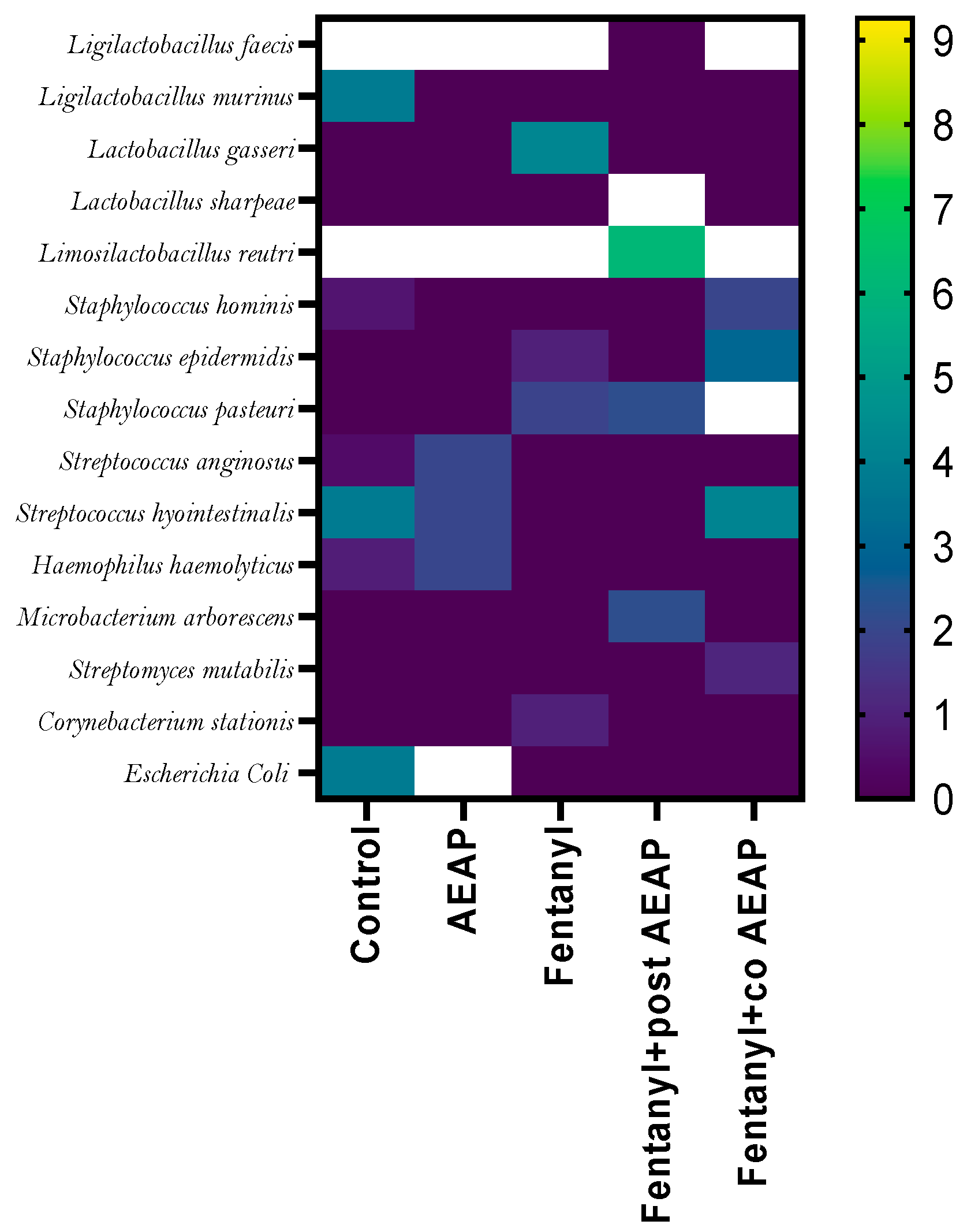
3.6. Alterations in Microbiota Density and Composition during Fentanyl Withdrawal and the Ameliorative Effect of AEAP
3.7. Alterations in Intestinal Tissue during Fentanyl Withdrawal and the Ameliorative Effect of AEAP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baslam, A.; Azraida, H.; Aboufatima, R.; Ait-El-Mokhtar, M.; Dilagui, I.; Boussaa, S.; Chait, A.; Baslam, M. Trihexyphenidyl Alters Its Host’s Metabolism, Neurobehavioral Patterns, and Gut Microbiome Feedback Loop—The Modulating Role of Anacyclus pyrethrum. Antioxidants 2024, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P.; et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926. [Google Scholar] [CrossRef] [PubMed]
- Baslam, A.; Hamid kabdy Ait Baba, A.; Aboufatima, R.; Boussaa, S.; Abderrahman, C. Substance use among university students and affecting factors in Central Morocco: A cross-sectional Study. Res. Sq. 2023, 38, 146–153. [Google Scholar]
- Towers, E.B.; Setaro, B.; Lynch, W.J. Sex- and Dose-Dependent Differences in the Development of an Addiction-Like Phenotype Following Extended-Access Fentanyl Self-Administration. Front. Pharmacol. 2022, 13, 841873. [Google Scholar] [CrossRef]
- Le Merrer, J.; Becker, J.A.J.; Befort, K.; Kieffer, B.L. Reward Processing by the Opioid System in the Brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Lüscher, C. The Molecular Basis of Drug Addiction: Linking Epigenetic to Synaptic and Circuit Mechanisms. Neuron 2019, 102, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Cárcamo, P.; Quezada, M.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y.; Ezquer, F. Oxidative Stress and Neuroinflammation as a Pivot in Drug Abuse. A Focus on the Therapeutic Potential of Antioxidant and Anti-Inflammatory Agents and Biomolecules. Antioxidants 2020, 9, 830. [Google Scholar] [CrossRef]
- Caspani, G.; Swann, J. Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr. Opin. Pharmacol. 2019, 48, 99–106. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Lin, C.H.; Lane, H.Y. d-glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2676. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, M.P.; Fox, B.W.; Chao, P.-H.; Schroeder, F.C.; Sengupta, P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; Nega, M.; Nguyen, M.T.; Ebner, P.; Götz, F. SadA-Expressing Staphylococci in the Human Gut Show Increased Cell Adherence and Internalization. Cell Rep. 2018, 22, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, S.E.; Larochelle, M.R.; Ameli, O.; Chaisson, C.E.; McPheeters, J.T.; Crown, W.H.; Azocar, F.; Sanghavi, D.M. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw. Open 2020, 3, e1920622. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Barnes, J. Prevalence of Use of Herbal and Traditional Medicines. In Pharmacovigilance for Herbal and Traditional Medicines: Advances, Challenges and International Perspectives; Barnes, J., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 15–25. [Google Scholar] [CrossRef]
- Bezza, K.; Laadraoui, J.; El Gabbas, Z.; Laaradia, M.A.; Oufquir, S.; Aboufatima, R.; Gharrassi, I.; Chait, A. Effects of Anacyclus pyrethrum on affective behaviors and memory during withdrawal from cigarette smoke exposure in rats. Pharmacogn. Mag. 2020, 16, 123–132. [Google Scholar] [CrossRef]
- Baslam, A.; Aitbaba, A.; Aboufatima, R.; Agouram, F.; Boussaa, S.; Chait, A.; Baslam, M. Phytochemistry, Antioxidant Potential, and Antibacterial Activities of Anacyclus pyrethrum: Promising Bioactive Compounds. Horticulturae 2023, 9, 1196. [Google Scholar] [CrossRef]
- Baslam, A.; Aitbaba, A.; Lamrani Hanchi, A.; Tazart, Z.; Aboufatima, R.; Soraa, N.; Ait-El-Mokhtar, M.; Boussaa, S.; Baslam, M.; Chait, A. Modulation of Gut Microbiome in Ecstasy/MDMA-Induced Behavioral and Biochemical Impairment in Rats and Potential of Post-Treatment with Anacyclus pyrethrum L. Aqueous Extract to Mitigate Adverse Effects. Int. J. Mol. Sci. 2023, 24, 9086. [Google Scholar] [CrossRef] [PubMed]
- Seaman, R.W., Jr.; Collins, G.T. Impact of Morphine Dependence and Withdrawal on the Reinforcing Effectiveness of Fentanyl, Cocaine, and Methamphetamine in Rats. Front. Pharmacol. 2021, 12, 691700. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.L.; Vendruscolo, L.F.; E Schlosburg, J.; O Hernandez, D.; Koob, G.F. Compulsive-Like Responding for Opioid Analgesics in Rats with Extended Access. Neuropsychopharmacology 2015, 40, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bakhti-Suroosh, A.; Towers, E.B.; Lynch, W.J. A buprenorphine-validated rat model of opioid use disorder optimized to study sex differences in vulnerability to relapse. Psychopharmacology 2021, 238, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Banerjee, S. Effect of Valeriana wallichii on alcohol addiction in mice. Pharmacogn. Mag. 2018, 14, S613–S618. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test: Characterization Using Behavioral Tests. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.A.; Leergaard, T.B.; Calabrese, E.; Johnson, G.A.; Bjaalie, J.G. Waxholm Space atlas of the Sprague Dawley rat brain. NeuroImage 2014, 97, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. 1993, 57, 779S–786S. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. Available online: https://www.sciencedirect.com/science/article/pii/S0076687984050163 (accessed on 20 April 2023).
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Tsuchida, S.; Umemura, H.; Nakayama, T. Current Status of Matrix-Assisted Laser Desorption/Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS) in Clinical Diagnostic Microbiology. Molecules 2020, 25, 4775. [Google Scholar] [CrossRef]
- Malatesta, M. Histological and Histochemical Methods—Theory and practice. Eur. J. Histochem. 2016, 60, 2639. [Google Scholar] [CrossRef]
- McKendrick, G.; Graziane, N.M. Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Front. Behav. Neurosci. 2020, 14, 582147. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, D.M.; Sellers, E.M. Addiction and the brain: The role of neurotransmitters in the cause and treatment of drug dependence. CMAJ Can. Med. Assoc. J. 2001, 164, 817–821. [Google Scholar]
- Boumosleh, J.M.; Jaalouk, D. Depression, anxiety, and smartphone addiction in university students—A cross sectional study. PLoS ONE 2017, 12, e0182239. [Google Scholar] [CrossRef]
- Neumann, I.D.; Landgraf, R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012, 35, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Burkett, J.P.; Young, L.J. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology 2012, 224, 1–26. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, e92–e123. [Google Scholar] [CrossRef]
- Koob, G.F. Negative reinforcement in drug addiction: The darkness within. Curr. Opin. Neurobiol. 2013, 23, 559–563. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001, 24, 97–129. [Google Scholar] [CrossRef]
- King, C.E.; Gano, A.; Becker, H.C. The Role of Oxytocin in Alcohol and Drug Abuse. Brain Res. 2020, 1736, 146761. [Google Scholar] [CrossRef]
- Koob, G.F. A role for brain stress systems in addiction. Neuron 2008, 59, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Xu, G.; Zeng, X. The Biology of Morphine and Oxidative Stress. In Handbook of Substance Misuse and Addictions: From Biology to Public Health; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1955–1975. [Google Scholar] [CrossRef]
- Zeng, X.-S.; Geng, W.-S.; Wang, Z.-Q.; Jia, J.-J. Morphine Addiction and Oxidative Stress: The Potential Effects of Thioredoxin-1. Front. Pharmacol. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Salarian, A.; Kadkhodaee, M.; Zahmatkesh, M.; Seifi, B.; Bakhshi, E.; Akhondzadeh, S.; Adeli, S.; Askari, H.; Arbabi, M. Opioid Use Disorder Induces Oxidative Stress and Inflammation: The Attenuating Effect of Methadone Maintenance Treatment. Iran J. Psychiatry 2018, 13, 46–54. [Google Scholar]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Bravo, J.A.; Julio-Pieper, M.; Forsythe, P.; Kunze, W.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Communication between gastrointestinal bacteria and the nervous system. Curr. Opin. Pharmacol. 2012, 12, 667–672. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, N.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Habtemariam, S.; Adejare, A. Chemistry and Pharmacology of Alkylamides from Natural Origin. Rev. Bras. Farmacogn. Orgao. Soc. Bras. Farmacogn. 2020, 30, 622–640. [Google Scholar] [CrossRef]
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bras. Farm. 2018, 28, 339–343. [Google Scholar] [CrossRef]
- Manouze, H.; Bouchatta, O.; Gadhi, A.C.; Bennis, M.; Sokar, Z.; Ba-M’hamed, S. Anti-inflammatory, Antinociceptive, and Antioxidant Activities of Methanol and Aqueous Extracts of Anacyclus pyrethrum Roots. Front. Pharmacol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. Anacyclus pyrethrum (L): Chemical Composition, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef] [PubMed]
- Baslam, A.; Kabdy, H.; Aitlaaradia, M.; Laadraroui, J.; Oufquir, S.; Aboufatima, R.; Boussaa, S.; Chait, A. Therapeutic potential of Anacyclus pyrethrum aqueous extract in managing Clonazepam withdrawal in rats. J. Appl. Pharm. Sci. 2024, 14, 81–91. [Google Scholar] [CrossRef]
- Hosseinzadeh Sahafi, O.; Sardari, M.; Alijanpour, S.; Rezayof, A. Shared Mechanisms of GABAergic and Opioidergic Transmission Regulate Corticolimbic Reward Systems and Cognitive Aspects of Motivational Behaviors. Brain Sci. 2023, 13, 815. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.F.; Cheng, Y.F.; You, S.T.; Kuo, W.C.; Huang, C.W.; Chiou, J.J.; Hsu, C.C.; Hsieh-Li, H.M.; Wang, S.; Tsai, Y.C. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson’s disease. Brain Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-F.; Yan, X.-F.; Ruan, Z.-R.; Peng, F.-Y.; Cai, D.; Yuan, H.; Sun, L.; Ding, D.-Y.; Xu, S.-S. Heroin abuse and nitric oxide, oxidation, peroxidation, lipoperoxidation. Biomed. Environ. Sci. 2000, 13, 139. [Google Scholar]
- Usmani, A.; Khushtar, M.; Arif, M.; Siddiqui, M.A.; Sing, S.P. Mujahid Pharmacognostic and phytopharmacology study of Anacyclus pyrethrum: An insight. J. Appl. Pharm. Sci. 2016, 6, 144–150. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Jin, X. Mechanism of ethanolic extract of root of Anacyclus pyrethrum on two models of inflammation. Chin. J. Clin. Pharmacol. Ther. 2018, 23, 47. [Google Scholar]
- Bezza, K.; El Gabbas, Z.; Laadraoui, J.; Laaradia, M.A.; Oufquir, S.; Chait, A. Ameliorative potential of Anacyclus pyrethrum extract in generalized seizures in rat: Possible cholinergic mediated mechanism. Bangladesh J. Pharmacol. 2019, 14, 188–195. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baslam, A.; Kabdy, H.; Chait, Y.; Azraida, H.; El Yazouli, L.; Aboufatima, R.; Chait, A.; Baslam, M. Gut Microbiome-Mediated Mechanisms in Alleviating Opioid Addiction with Aqueous Extract of Anacyclus pyrethrum. Biomedicines 2024, 12, 1152. https://doi.org/10.3390/biomedicines12061152
Baslam A, Kabdy H, Chait Y, Azraida H, El Yazouli L, Aboufatima R, Chait A, Baslam M. Gut Microbiome-Mediated Mechanisms in Alleviating Opioid Addiction with Aqueous Extract of Anacyclus pyrethrum. Biomedicines. 2024; 12(6):1152. https://doi.org/10.3390/biomedicines12061152
Chicago/Turabian StyleBaslam, Abdelmounaim, Hamid Kabdy, Yassine Chait, Hajar Azraida, Loubna El Yazouli, Rachida Aboufatima, Abderrahman Chait, and Marouane Baslam. 2024. "Gut Microbiome-Mediated Mechanisms in Alleviating Opioid Addiction with Aqueous Extract of Anacyclus pyrethrum" Biomedicines 12, no. 6: 1152. https://doi.org/10.3390/biomedicines12061152
APA StyleBaslam, A., Kabdy, H., Chait, Y., Azraida, H., El Yazouli, L., Aboufatima, R., Chait, A., & Baslam, M. (2024). Gut Microbiome-Mediated Mechanisms in Alleviating Opioid Addiction with Aqueous Extract of Anacyclus pyrethrum. Biomedicines, 12(6), 1152. https://doi.org/10.3390/biomedicines12061152







