Polydopamine Coating of Graphitic Carbon Nitride, g-C3N4, Improves Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
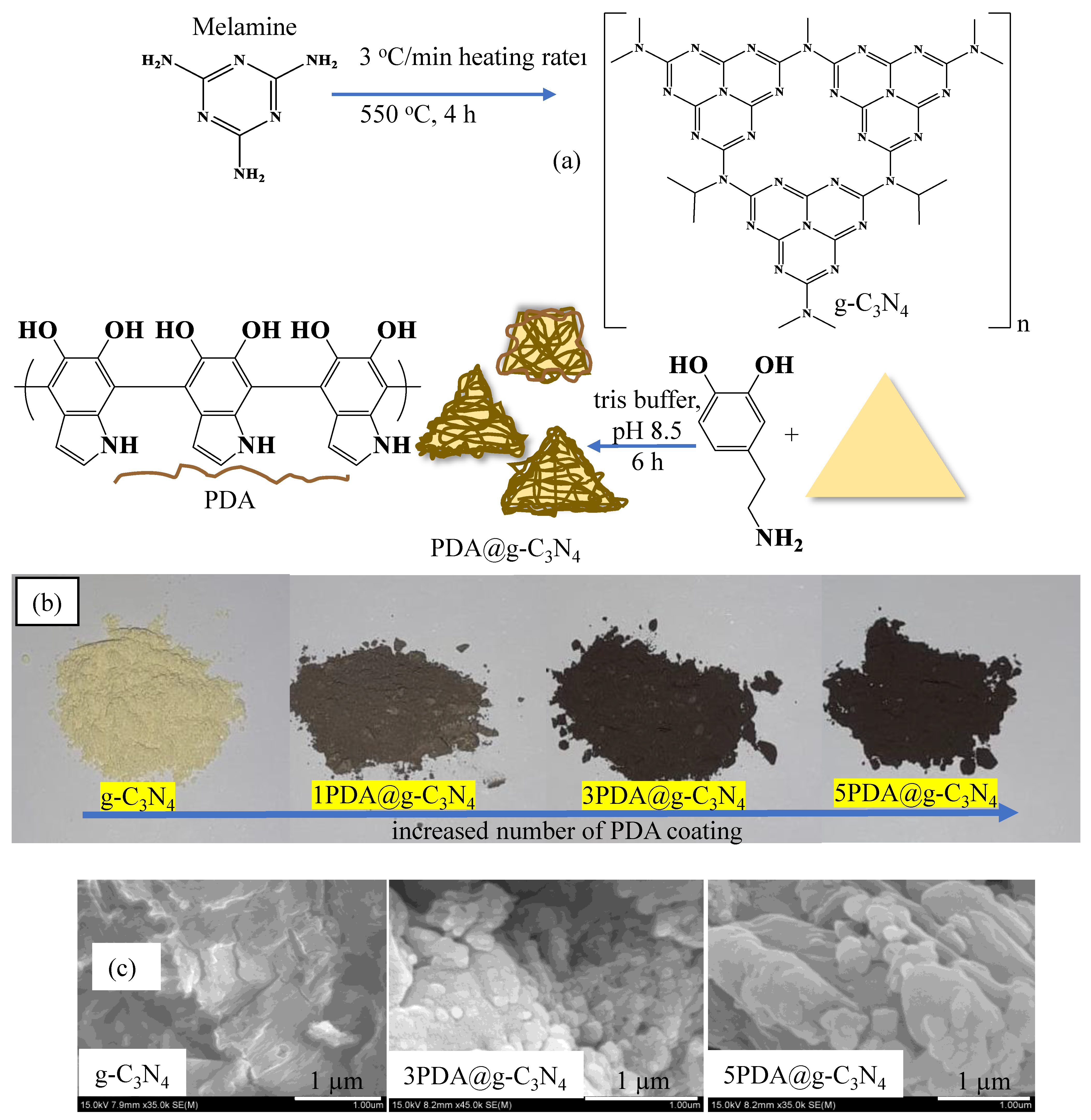
2.2. Synthesis of g-C3N4 and Its PDA Coating
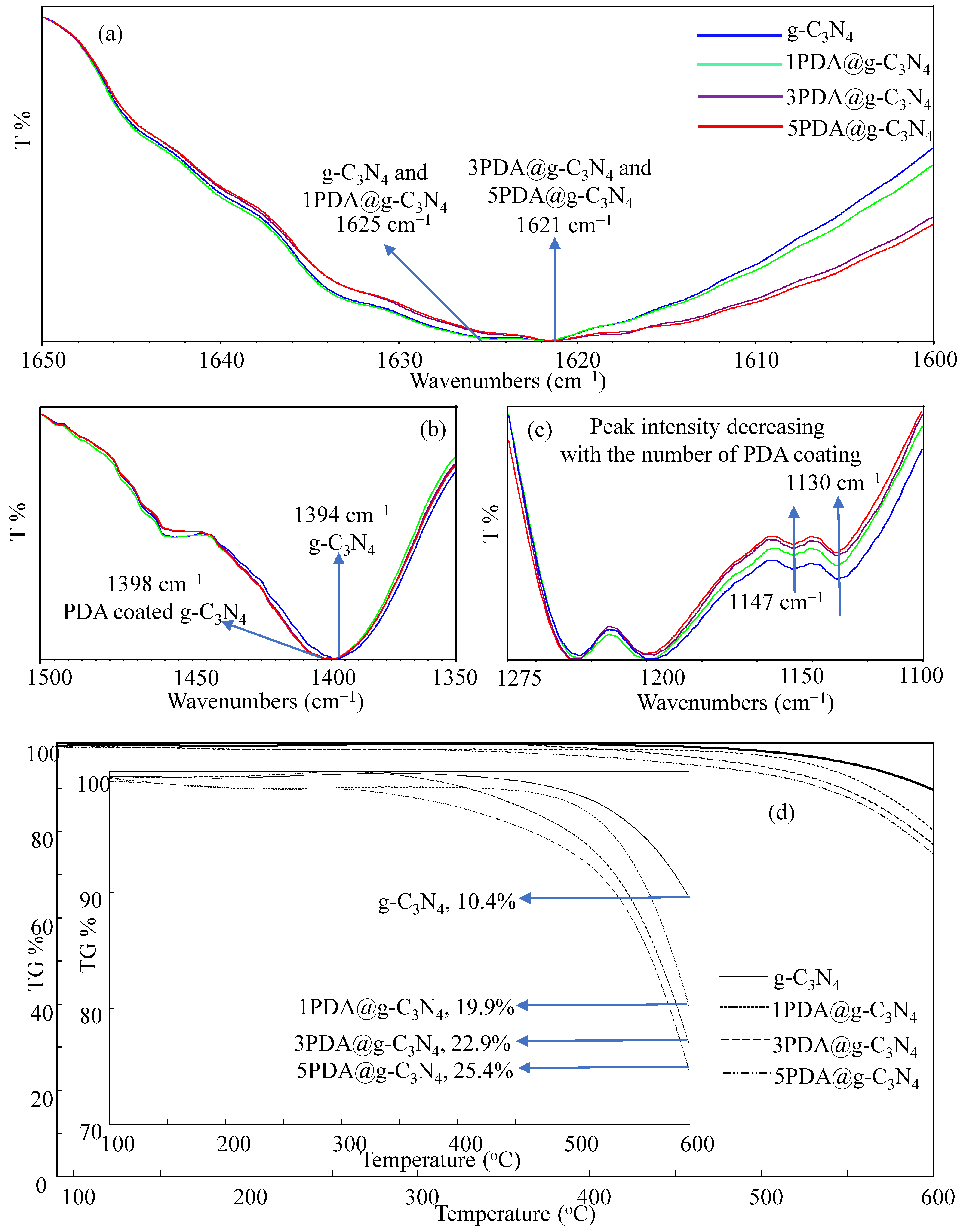
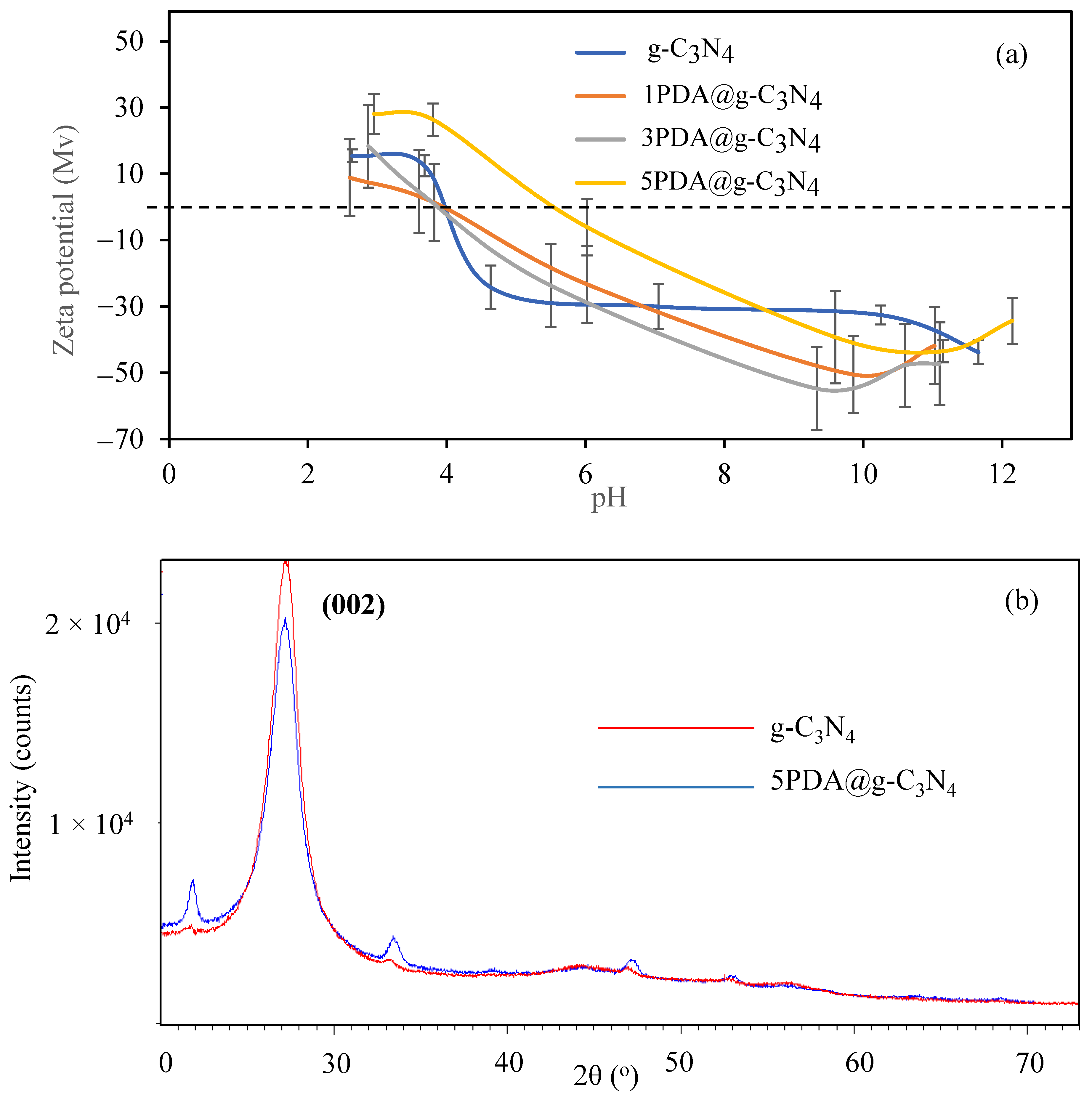
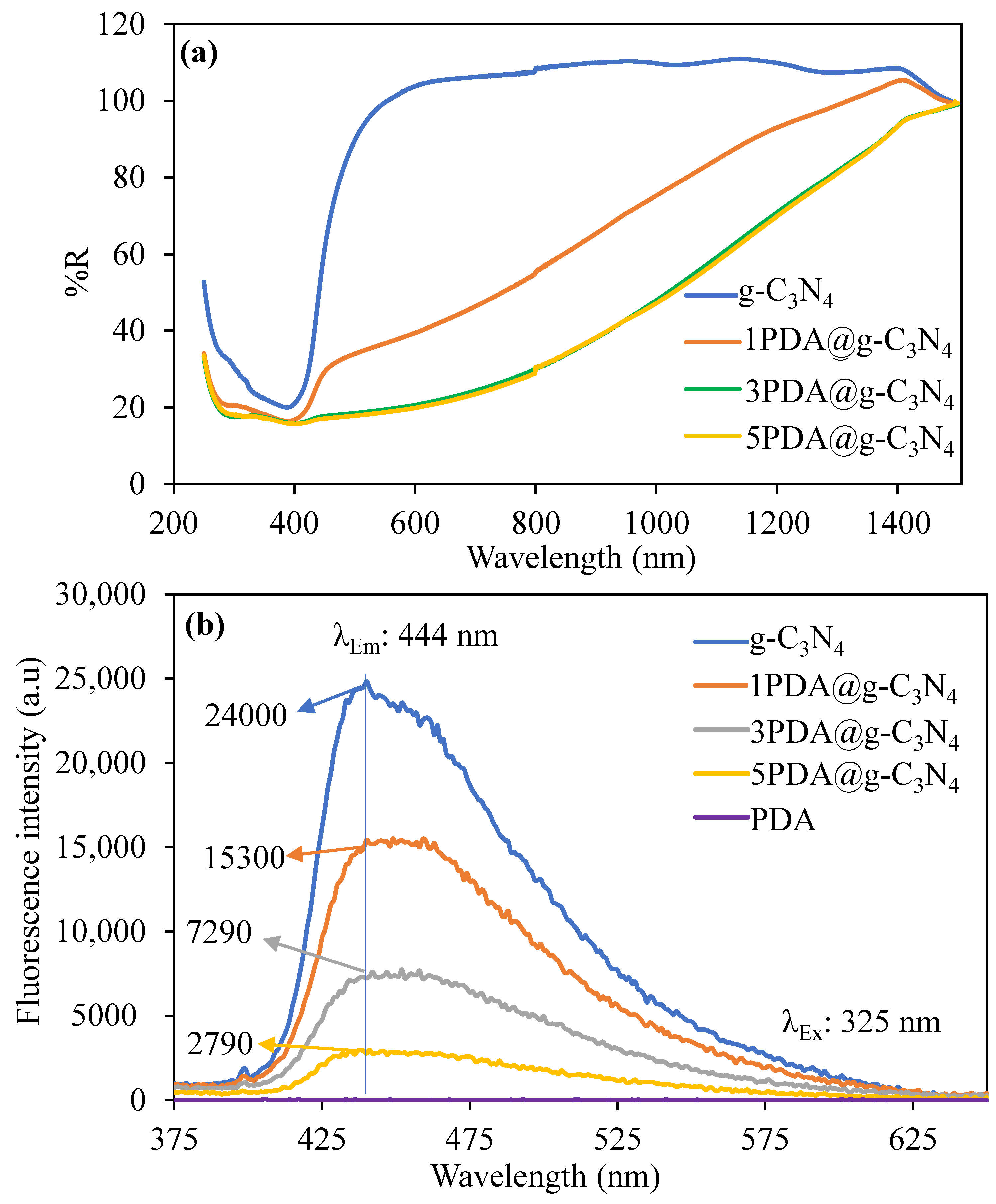
2.3. Characterization of PDA@g-C3N4
2.4. Biomedical Properties of PDA Coated g-C3N4
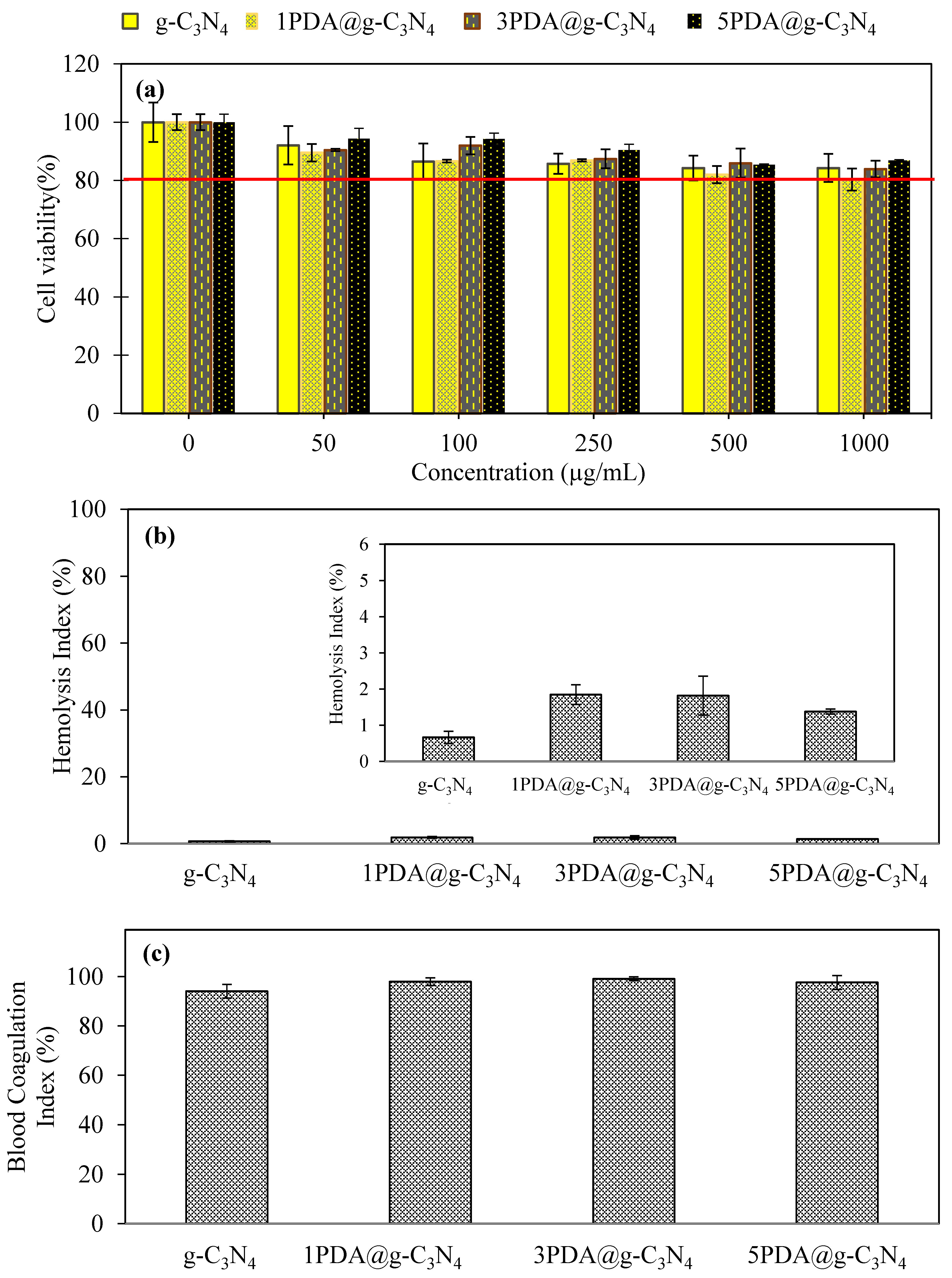
2.4.1. Cytotoxicity and Blood Compatibility of PDA-Coated g-C3N4
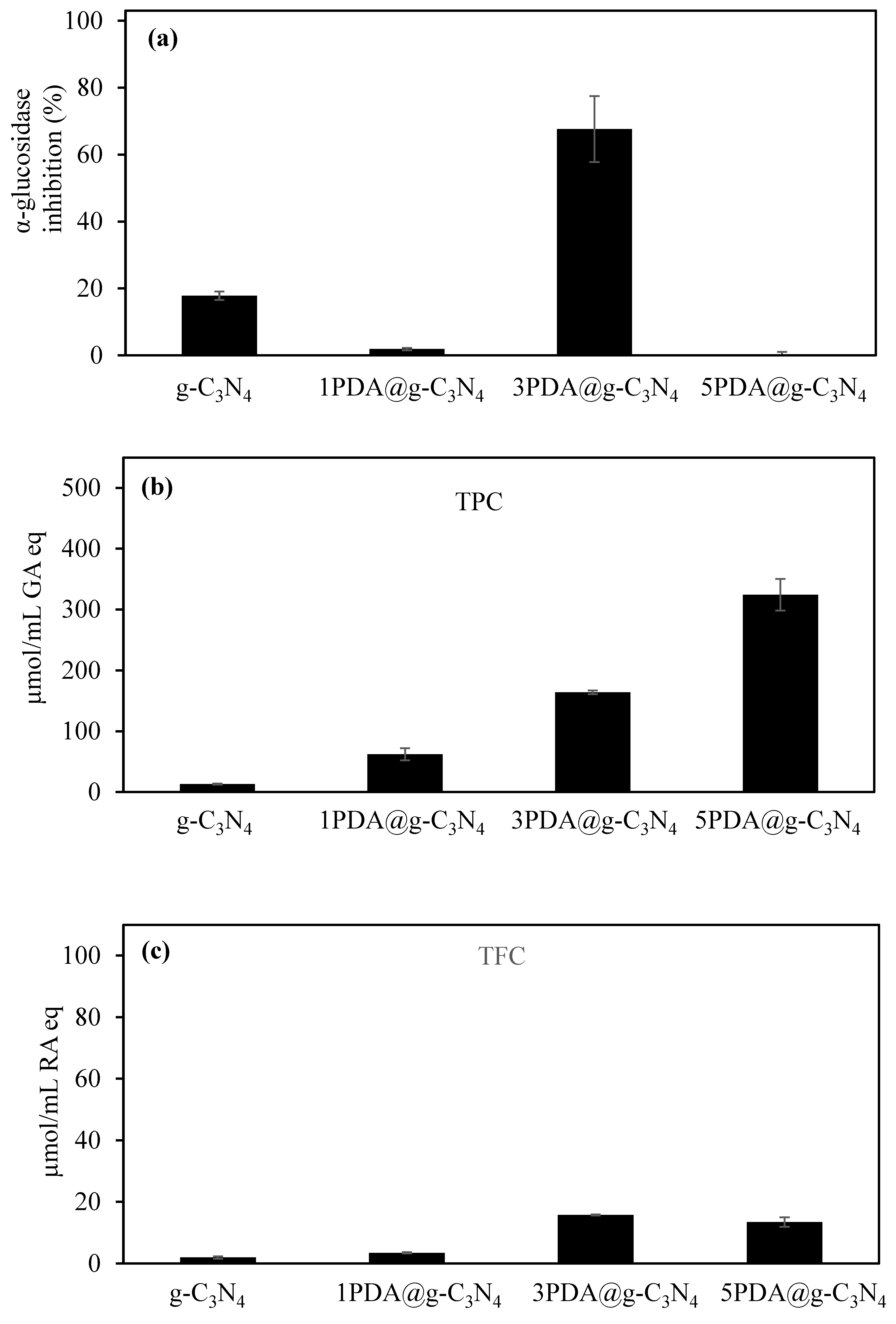
2.4.2. Antioxidant Activity Assays for PDA-Coated g-C3N4
2.4.3. Antibacterial Activity Assay for PDA Coated g-C3N4
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging Graphitic Carbon Nitride-Based Materials for Biomedical Applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.; Nie, R. Synthesis and Biomedical Applications of Graphitic Carbon Nitride Quantum Dots. J. Mater. Chem. B 2019, 7, 5432–5448. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yan, Y.; Zheng, S.; Xue, H.; Pang, H. Graphitic Carbon Nitride Based Materials for Electrochemical Energy Storage. J. Mater. Chem. A 2019, 7, 901–924. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon Nitride Based Nanocomposites: A Review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Asif, K.; Perveen, M.; Khera, R.A.; Nazir, S.; Raza Ayub, A.; Asif, T.; Shabbir, M.; Iqbal, J. Computational and Theoretical Study of Graphitic Carbon Nitride (g-C3N4) as a Drug Delivery Carrier for Lonidamine Drug to Treat Cancer. Comput. Theor. Chem. 2021, 1206, 113459. [Google Scholar] [CrossRef]
- Shamim, M.; Perveen, M.; Nazir, S.; Hussnain, M.; Mehmood, R.; Khan, M.I.; Iqbal, J. DFT Study of Therapeutic Potential of Graphitic Carbon Nitride (g-C3N4) as a New Drug Delivery System for Carboplatin to Treat Cancer. J. Mol. Liq. 2021, 331, 115607. [Google Scholar] [CrossRef]
- Qusain Afzal, Q.; Rafique, J.; Jaffar, K.; Perveen, M.; Iqbal, J.; Al-Buriahi, M.S.; Alomairy, S.; Alrowaili, Z.A.; Somaily, H.H. DFT Study of 2D Graphitic Carbon Nitride Based Preferential Targeted Delivery of Levosimendan, a Cardiovascular Drug. Comput. Theor. Chem. 2022, 1209, 113584. [Google Scholar] [CrossRef]
- Rajabzadeh-Khosroshahi, M.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Rasekh, B. Chitosan/Agarose/Graphitic Carbon Nitride Nanocomposite as an Efficient PH-Sensitive Drug Delivery System for Anticancer Curcumin Releasing. J. Drug Deliv. Sci. Technol. 2022, 74, 103443. [Google Scholar] [CrossRef]
- Ilyas, M.; Ayu, A.R.; Shehzad, R.A.; Khan, M.A.; Perveen, M.; Amin, S.; Muhammad, S.; Iqbal, J. A DFT Approach for Finding Therapeutic Potential of Two Dimensional (2D) Graphitic Carbon Nitride (GCN) as a Drug Delivery Carrier for Curcumin to Treat Cardiovascular Diseases. J. Mol. Struct. 2022, 1257, 132547. [Google Scholar] [CrossRef]
- Lin, L.-S.; Cong, Z.-X.; Li, J.; Ke, K.-M.; Guo, S.-S.; Yang, H.-H.; Chen, G.-N. Graphitic-Phase C3N4 Nanosheets as Efficient Photosensitizers and PH-Responsive Drug Nanocarriers for Cancer Imaging and Therapy. J. Mater. Chem. B 2014, 2, 1031. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.-Y.; Liu, Y.-X. Effective Route to Graphitic Carbon Nitride from Ball-Milled Amorphous Carbon in NH3 Atmosphere Under Annealing. Chin. Phys. Lett. 2003, 20, 1554–1557. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Zhang, Q.; Wang, X.; Dai, W.-L. Superior Sponge-like Carbon Self-Doping Graphitic Carbon Nitride Nanosheets Derived from Supramolecular Pre-Assembly of a Melamine–Cyanuric Acid Complex for Photocatalytic H2 Evolution. Nanotechnology 2021, 32, 155604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, J.; Ding, Y.; Liu, B.; Zhao, L.; Zhang, S. Research Progress on g–C3N4–Based Photocatalysts for Organic Pollutants Degradation in Wastewater: From Exciton and Carrier Perspectives. Ceram. Int. 2021, 47, 31005–31030. [Google Scholar] [CrossRef]
- Demirci, S.; Sahiner, M.; Suner, S.S.; Sahiner, N. Improved Biomedical Properties of Polydopamine-Coated Carbon Nanotubes. Micromachines 2021, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Guo, R.; Lan, J.; Jiang, S.; Lin, S.; Cheng, C.; Zhao, L. Bismuth Tungstate Coating on Polyester Fabric Modified with Dopamine for Photocatalytic Property under Visible Light Irradiation. Surf. Coat. Technol. 2017, 319, 219–229. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Blake, D.A.; Reed, W.F. Polydopamine Particles as Nontoxic, Blood Compatible, Antioxidant and Drug Delivery Materials. Colloids Surf. B Biointerfaces 2018, 172, 618–626. [Google Scholar] [CrossRef]
- Moura, C.; Correia, A.S.; Vale, N. Exploring the Interaction of Indole-3-Acetonitrile with Neuroblastoma Cells: Understanding the Connection with the Serotonin and Dopamine Pathways. Biomedicines 2023, 11, 3325. [Google Scholar] [CrossRef]
- Abramova, O.; Zorkina, Y.; Ushakova, V.; Zubkov, E.; Morozova, A.; Chekhonin, V. The Role of Oxytocin and Vasopressin Dysfunction in Cognitive Impairment and Mental Disorders. Neuropeptides 2020, 83, 102079. [Google Scholar] [CrossRef]
- Maraming, P.; Aye, N.N.S.; Boonsiri, P.; Daduang, S.; Buhome, O.; Daduang, J. Polydopamine Nanoparticles Functionalized Electrochemical DNA Aptasensor for Serum Glycated Albumin Detection. Int. J. Mol. Sci. 2022, 23, 13699. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dong, X.; Wang, Y.; Wu, X.; Dai, H. Dopamine-Modified Chitosan Hydrogel for Spinal Cord Injury. Carbohydr. Polym. 2022, 298, 120047. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Wu, M.; Yan, J.-M.; Zhang, X.; Zhao, M. Synthesis of g-C3N4 with Heating Acetic Acid Treated Melamine and Its Photocatalytic Activity for Hydrogen Evolution. Appl. Surf. Sci. 2015, 354, 196–200. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, X.; Wang, X.; Cai, H.; He, P.; Ma, J.; Li, L.; Ding, Y.; Fan, X. Large-Scale Preparation of g-C3N4 Porous Nanotubes with Enhanced Photocatalytic Activity by Using Salicylic Acid and Melamine. Ind. Eng. Chem. Res. 2020, 59, 1065–1072. [Google Scholar] [CrossRef]
- Sahiner, M.; Demirci, S.; Sahiner, N. Enhanced Bioactive Properties of Halloysite Nanotubes via Polydopamine Coating. Polymers 2022, 14, 4346. [Google Scholar] [CrossRef] [PubMed]
- Rāutiu, R.; White, D.A.; Adeleye, S.A.; Adkins, L. The Use of Zeta Potential Measurements in Inorganic Ion Exchange Studies. Hydrometallurgy 1994, 35, 361–374. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.C.; Zhu, M.; Mao, L.; Spanopoulos, I.; Liu, J.; Kontsevoi, O.Y.; Chen, M.; Sarma, D.; Zhang, Y.; et al. Enhanced Photovoltaic Performance and Stability with a New Type of Hollow 3D Perovskite {en}FASnI3. Sci. Adv. 2017, 3, e1701293. [Google Scholar] [CrossRef]
- Gao, F.; Ma, S.; Li, J.; Dai, K.; Xiao, X.; Zhao, D.; Gong, W. Rational Design of High Quality Citric Acid-Derived Carbon Dots by Selecting Efficient Chemical Structure Motifs. Carbon 2017, 112, 131–141. [Google Scholar] [CrossRef]
- Demirci, S.; Suner, S.S.; Neli, O.U.; Koca, A.; Sahiner, N. B, P, and S Heteroatom Doped, Bio- and Hemo-Compatible 2D Graphitic-Carbon Nitride (g-C3N4) with Antioxidant, Light-Induced Antibacterial, and Bioimaging Endeavors. Nanotechnology 2023, 35, 025101. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Blake, D.A.; Fullerton, M.L.; Suner, S.S.; Sunol, A.K.; Sahiner, N. Enhancement of Biocompatibility and Carbohydrate Absorption Control Potential of Rosmarinic Acid through Crosslinking into Microparticles. Int. J. Biol. Macromol. 2019, 137, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Sasson, Y. Functionalized Graphitic Carbon Nitride Decorated with Palladium: An Efficient Heterogeneous Catalyst for Hydrogenation Reactions Using KHCO2 as a Mild and Noncorrosive Source of Hydrogen. ACS Omega 2020, 5, 12302–12312. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, N.; Shizuno, M.; Kanazawa, T.; Kato, K.; Yamakata, A.; Nozawa, S.; Ito, T.; Terashima, K.; Maeda, K.; Tamaki, Y.; et al. Surface-Specific Modification of Graphitic Carbon Nitride by Plasma for Enhanced Durability and Selectivity of Photocatalytic CO2 Reduction with a Supramolecular Photocatalyst. ACS Appl. Mater. Interfaces 2023, 15, 13205–13218. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A Review of the Current Status of Graphitic Carbon Nitride. Crit. Rev. Solid State Mater. Sci. 2021, 46, 189–217. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-Free Carbon-Based Catalyst: An Overview and Directions for Future Research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- Clancy, A.J.; Bayazit, M.K.; Hodge, S.A.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S.P. Charged Carbon Nanomaterials: Redox Chemistries of Fullerenes, Carbon Nanotubes, and Graphenes. Chem. Rev. 2018, 118, 7363–7408. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.A.; Bayazit, M.K.; Coleman, K.S.; Shaffer, M.S.P. Unweaving the Rainbow: A Review of the Relationship between Single-Walled Carbon Nanotube Molecular Structures and Their Chemical Reactivity. Chem. Soc. Rev. 2012, 41, 4409. [Google Scholar] [CrossRef] [PubMed]
- Fidan, T.; Torabfam, M.; Saleem, Q.; Wang, C.; Kurt, H.; Yüce, M.; Tang, J.; Bayazit, M.K. Functionalized Graphitic Carbon Nitrides for Environmental and Sensing Applications. Adv. Energy Sustain. Res. 2021, 2, 2000073. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, M.B.; Teixeira, J.F.L.; Dehli, J.; Karlsson, C.; Kraft, D.; Souza, P.P.C.; Foss, M. Post-Treatments of Polydopamine Coatings Influence Cellular Response. Colloids Surf. B Biointerfaces 2021, 207, 111972. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Liu, M.; Cheng, B.; Yu, J.; Zhang, L. Dopamine Modified g-C3N4 and Its Enhanced Visible-Light Photocatalytic H2-Production Activity. ACS Sustain. Chem. Eng. 2018, 6, 8945–8953. [Google Scholar] [CrossRef]
- Fan, K.W.; Roberts, J.J.; Martens, P.J.; Stenzel, M.H.; Granville, A.M. Copolymerization of an Indazole Ligand into the Self-Polymerization of Dopamine for Enhanced Binding with Metal Ions. J. Mater. Chem. B 2015, 3, 7457–7465. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Saka, C. Metal-Free Catalysts with Phosphorus and Oxygen Doped on Carbon-Based on Chlorella Vulgaris Microalgae for Hydrogen Generation via Sodium Borohydride Methanolysis Reaction. Int. J. Hydrogen Energy 2021, 46, 5150–5157. [Google Scholar] [CrossRef]
- Saka, C. Facile Fabrication of P-Doped g-C3N4 Particles with Nitrogen Vacancies for Efficient Dehydrogenation of Sodium Borohydride Methanolysis. Fuel 2022, 313, 122688. [Google Scholar] [CrossRef]
- Li, G.; Yang, N.; Wang, W.; Zhang, W.F. Synthesis, Photophysical and Photocatalytic Properties of N-Doped Sodium Niobate Sensitized by Carbon Nitride. J. Phys. Chem. C 2009, 113, 14829–14833. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M.; Aravindh, S.A.; Dabare, R.; Gao, X.; Jiang, Z.-T.; Wong, K.W. Repurposing N-Doped Grape Marc for the Fabrication of Supercapacitors with Theoretical and Machine Learning Models. Nanomaterials 2022, 12, 1847. [Google Scholar] [CrossRef]
- Tan, L.; Xu, J.; Zhang, X.; Hang, Z.; Jia, Y.; Wang, S. Synthesis of g-C3N4/CeO2 Nanocomposites with Improved Catalytic Activity on the Thermal Decomposition of Ammonium Perchlorate. Appl. Surf. Sci. 2015, 356, 447–453. [Google Scholar] [CrossRef]
- Yu, Z.; Li, F.; Yang, Q.; Shi, H.; Chen, Q.; Xu, M. Nature-Mimic Method to Fabricate Polydopamine/Graphitic Carbon Nitride for Enhancing Photocatalytic Degradation Performance. ACS Sustain. Chem. Eng. 2017, 5, 7840–7850. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, G.; Wang, M.; Li, B.; Dang, M.; Ren, H.; Xia, A. The Modulation of g-C3N4 Energy Band Structure by Excitons Capture and Dissociation. Mater. Res. Bull. 2020, 122, 110685. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Nitrogen Doped g-C3N4 with the Extremely Narrow Band Gap for Excellent Photocatalytic Activities under Visible Light. Appl. Catal. B Environ. 2021, 281, 119474. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, S.-P. Band Gap of C3N4 in the GW Approximation. Int. J. Hydrogen Energy 2012, 37, 11072–11080. [Google Scholar] [CrossRef]
- del Valle, J.C.; Catalán, J. Kasha’s Rule: A Reappraisal. Phys. Chem. Chem. Phys. 2019, 21, 10061–10069. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-C.; Hsin, S.-W.; Lin, H.-C.; Yeh, C.-H.; Wu, R.; Cherng, W.-J. Oxidized Dopamine as the Interlayer between Heparin/Collagen Polyelectrolyte Multilayers and Titanium Substrate: An Investigation of the Coating’s Adhesion and Hemocompatibility. Surf. Coat. Technol. 2016, 303, 277–282. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Liu, J. Recent Development of Polydopamine Anti-Bacterial Nanomaterials. Int. J. Mol. Sci. 2022, 23, 7278. [Google Scholar] [CrossRef]
| Materials | Elements (wt%) | ||
|---|---|---|---|
| C | N | O | |
| g-C3N4 | 22.1 | 71.8 | 5.4 |
| 1PDA@g-C3N4 | 23.2 | 67.0 | 9.8 |
| 3PDA@g-C3N4 | 26.0 | 60.9 | 13.1 |
| 5PDA@g-C3N4 | 32.6 | 48.3 | 19.1 |
| Samples | g-C3N4 | 1PDA@g-C3N4 | 3PDA@g-C3N4 | 5PDA@g-C3N4 |
|---|---|---|---|---|
| QY% | 19.8 ± 1.2 | 13.7 ± 1.1 | 9.1 ± 0.7 | 4.8 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahiner, M.; Demirci, S.; Sahiner, N. Polydopamine Coating of Graphitic Carbon Nitride, g-C3N4, Improves Biomedical Application. Biomedicines 2024, 12, 1151. https://doi.org/10.3390/biomedicines12061151
Sahiner M, Demirci S, Sahiner N. Polydopamine Coating of Graphitic Carbon Nitride, g-C3N4, Improves Biomedical Application. Biomedicines. 2024; 12(6):1151. https://doi.org/10.3390/biomedicines12061151
Chicago/Turabian StyleSahiner, Mehtap, Sahin Demirci, and Nurettin Sahiner. 2024. "Polydopamine Coating of Graphitic Carbon Nitride, g-C3N4, Improves Biomedical Application" Biomedicines 12, no. 6: 1151. https://doi.org/10.3390/biomedicines12061151
APA StyleSahiner, M., Demirci, S., & Sahiner, N. (2024). Polydopamine Coating of Graphitic Carbon Nitride, g-C3N4, Improves Biomedical Application. Biomedicines, 12(6), 1151. https://doi.org/10.3390/biomedicines12061151









