Simple and Fast Prediction of Gestational Diabetes Mellitus Based on Machine Learning and Near-Infrared Spectra of Serum: A Proof of Concept Study at Different Stages of Pregnancy
Abstract
1. Introduction
- It tests for the first time ML models based on NIR spectroscopy as diagnostic support tools for GDM.
- It develops and evaluates a novel, simple, and rapid bioanalytical method for early detection and alternative screening of GDM, which avoids some of the disadvantages of OGTT, such as its unpleasant and time-consuming nature.
- It proposes an ML model based on NIR spectra of serum, which has similar or better predictive power than its literature counterparts, but with a shorter time of analysis, which makes it very attractive for use as an alternative screening tool to OGTT.
- It exhibits the potential of this new technology in obstetrics and gynecology, for example, for the prediction of other diseases and complications of pregnancy.
2. Materials and Methods
2.1. Ethical Aspects
2.2. Subjects Recruitment
2.3. Medical Data Collection
2.4. Blood Sample Collection
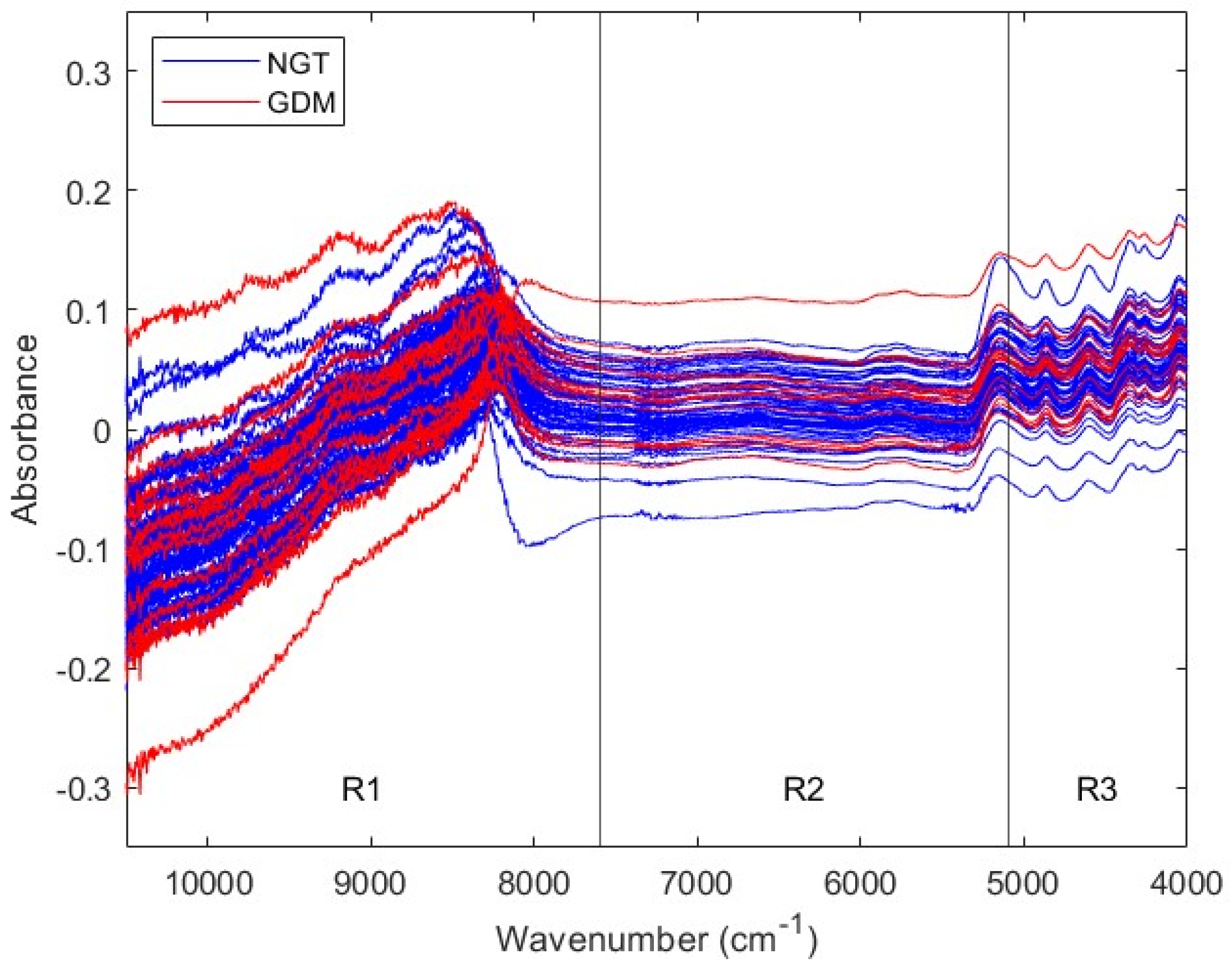
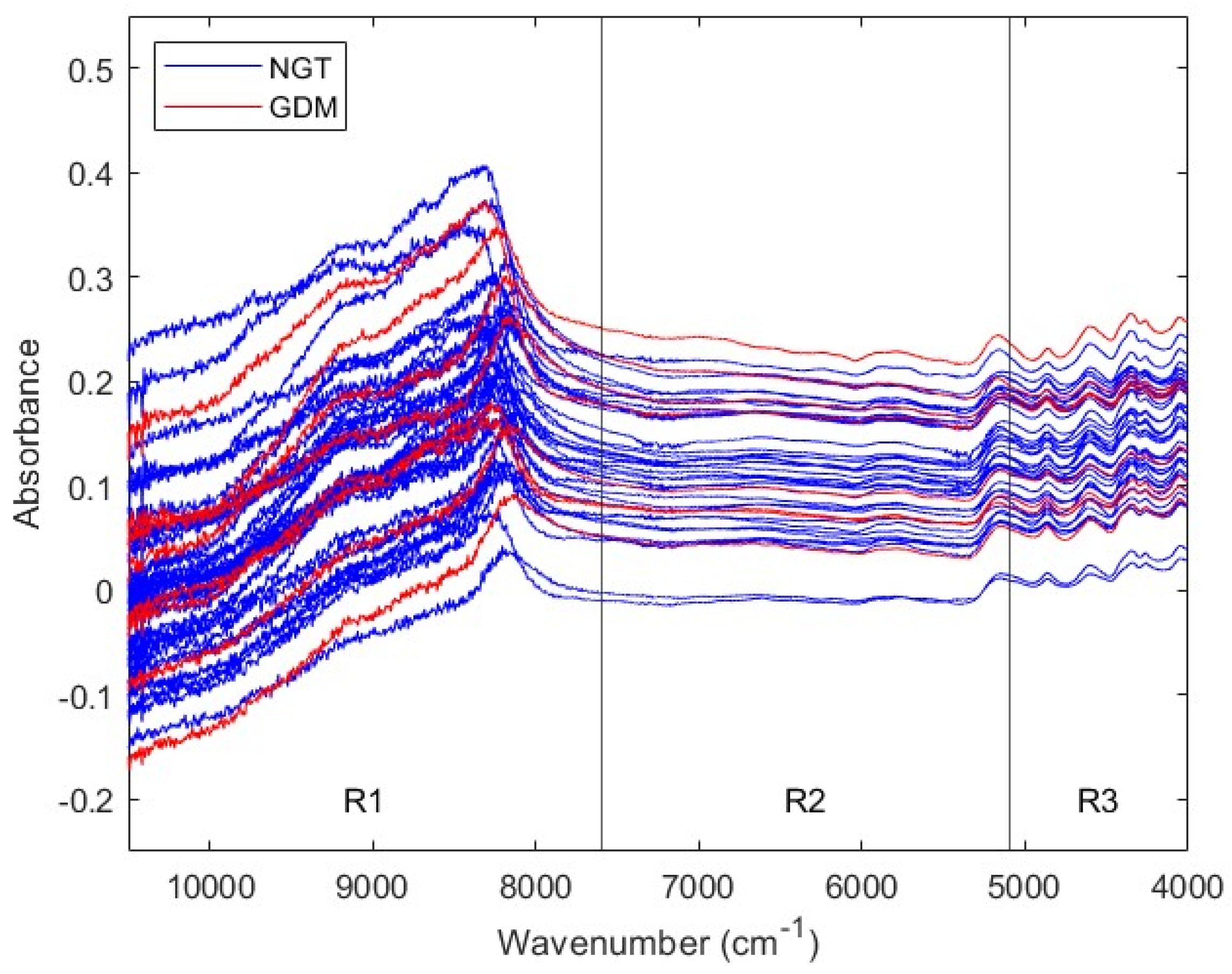
2.5. NIR Spectra Acquisition
2.6. GDM Diagnosis, Cohorts, and Study Groups
2.7. Classical Statistics Analyses
2.8. ML Analyses
2.8.1. Data Pretreatment
2.8.2. Single- and Multi-Block Analyses
- Calculate a PLS model between the binary-coded and the first predictor block : .
- Orthogonalize the second block with respect to : .
- Calculate a PLS model between the residuals of the first regression and the orthogonalized second predictor block : .
- The overall model can then be written as: , where collects the final predictions of the SO-PLS model.
- The classification model is obtained by applying LDA on .
2.8.3. Evaluation of Predictive Performance
2.8.4. Variable Importance and Selection
3. Results
3.1. First Trimester Cohort
3.1.1. Description of the First Trimester Cohort
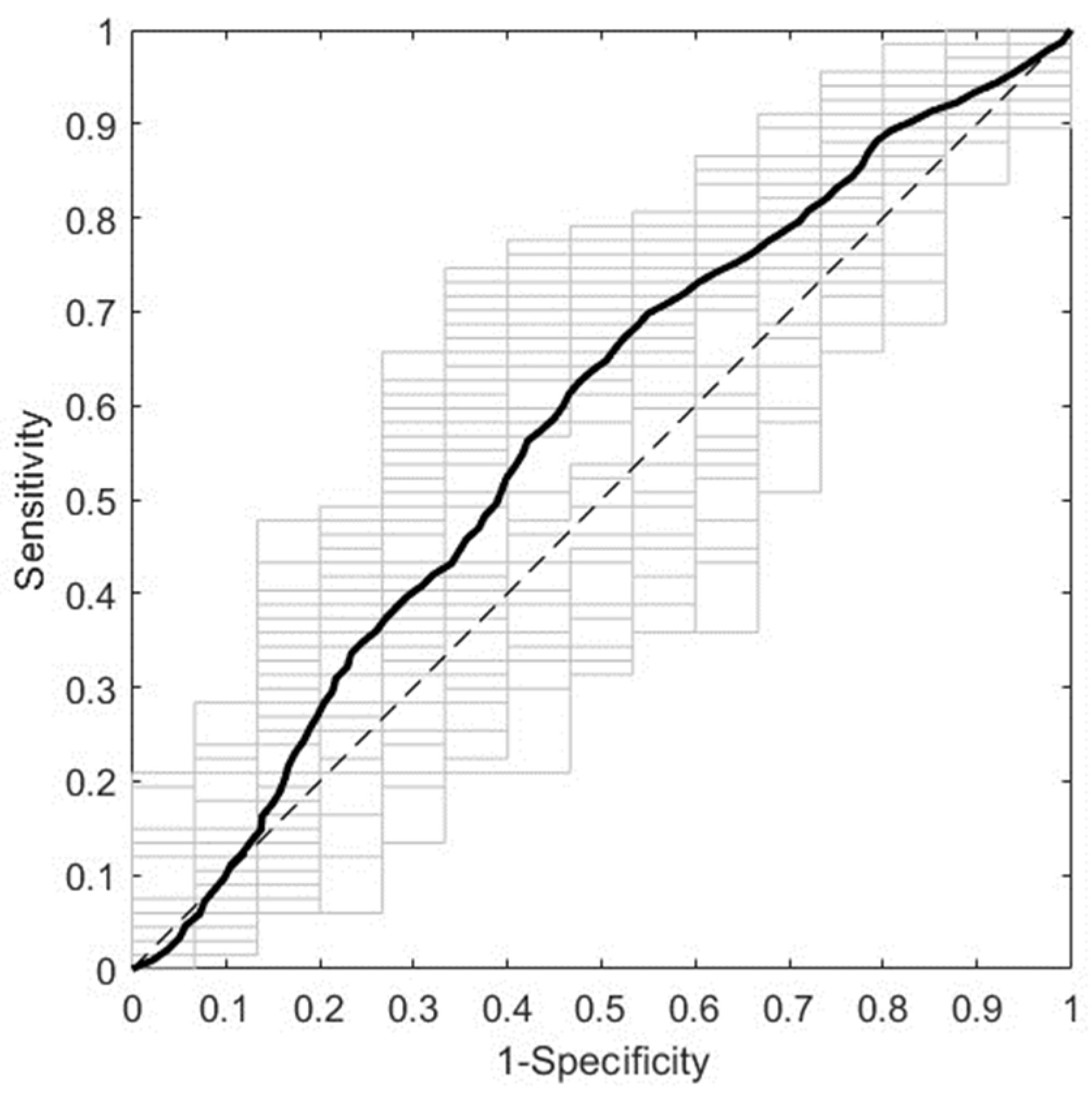
3.1.2. Prediction of GDM with First Trimester Serum NIR Spectral Data
3.2. Second Trimester Cohort
3.2.1. Description of the Second Trimester Cohort
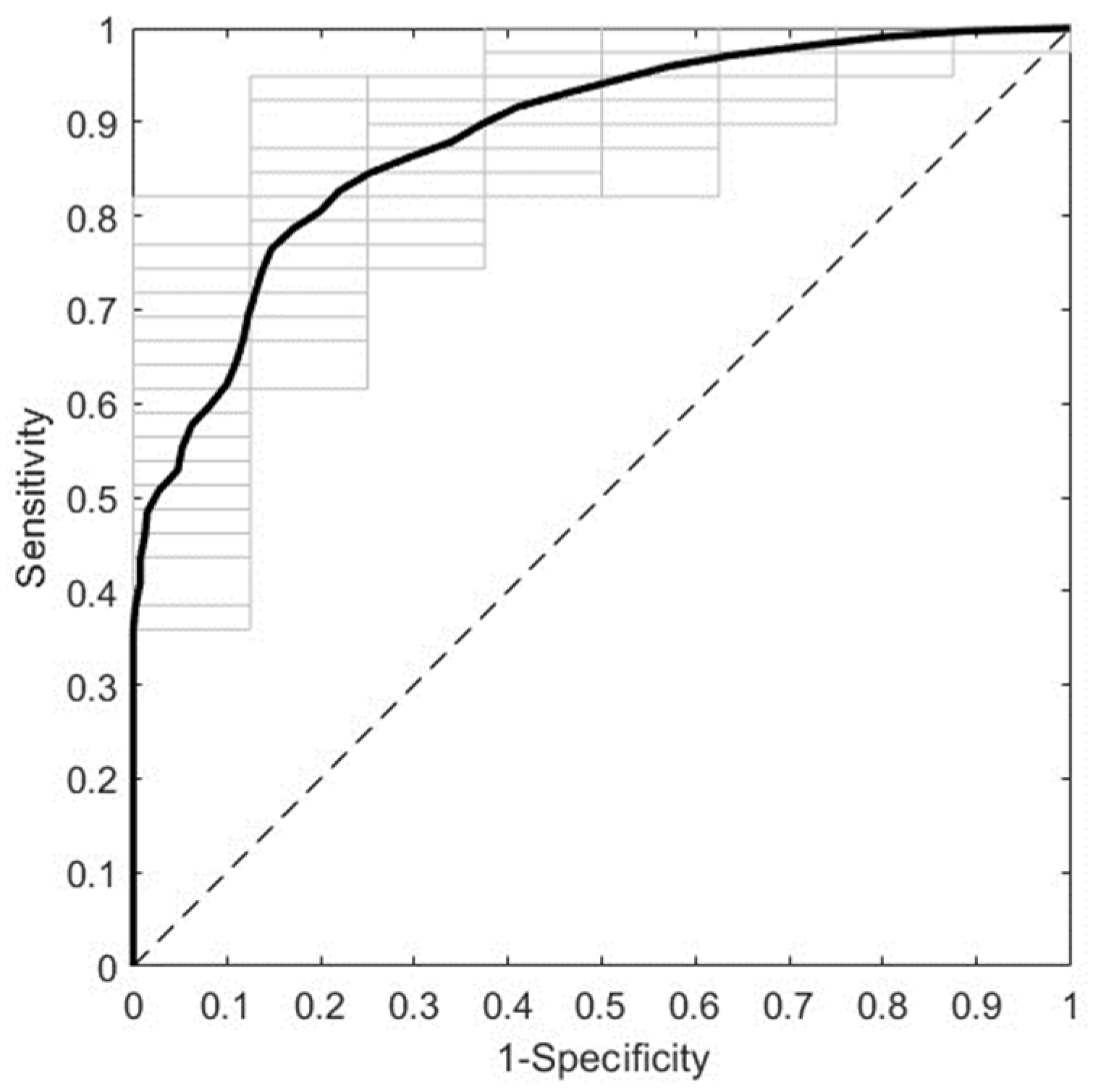
3.2.2. Prediction of GDM with Second Trimester Serum NIR Spectral Data
4. Discussion
4.1. The Addition of Medical Data Does Not Improve the Predictive Performance of NIR Data-Based Models
4.2. NIR Data-Based Prediction Has Advantages over Medical Data Prediction
4.3. NIR Spectral Data Pretreatment Is Essential to Maximize Predictive Power
4.4. Predictive Performance in the First and the Second Trimester Is Related to Biochemical Changes Occurring throughout GDM
4.5. NIR Data-Based Prediction Has Advantages over Other Instrumental Data-Based Prediction
4.6. The Presented Strategy Has Advantages over Other IR-Based Strategies
4.7. The Proposed NIR Data-Based Method Has Advantages over the OGTT
4.8. Strengths of This Study
4.9. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Prim. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Cao, Y.; Fadl, H.; Gustafson, H.; Simmons, D. Increasing Prevalence of Gestational Diabetes Mellitus When Implementing the IADPSG Criteria: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2021, 172, 108642. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, M.L.; Mondschein, S.; Montiel, B.; Kusanovic, J.P. Trends and Predictors of Gestational Diabetes Mellitus in Chile. Int. J. Gynecol. Obstet. 2020, 148, 210–218. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Giouleka, S.; Mamopoulos, A.; Kourtis, A.; Athanasiadis, A.; Filopoulou, D.; Dagklis, T. Diagnosis and Management of Gestational Diabetes Mellitus: An Overview of National and International Guidelines. Obstet. Gynecol. Surv. 2021, 76, 367–381. [Google Scholar] [CrossRef]
- Raets, L.; Vandewinkel, M.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; et al. Preference of Women for Gestational Diabetes Screening Method According to Tolerance of Tests and Population Characteristics. Front. Endocrinol. 2021, 12, 781384. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chang, P.-C.; Wang, C.-Y.; Wang, L.-C.; Shih, C.-L. Comparing the Effects of Water Temperature and Additives in Glucose Solution on Pregnant Women’s Taste, Side Effects, and Glycemic Levels during an Oral Glucose Tolerance Test: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. MFM 2023, 5, 100870. [Google Scholar] [CrossRef]
- Huhn, E.A.; Rossi, S.W.; Hoesli, I.; Göbl, C.S. Controversies in Screening and Diagnostic Criteria for Gestational Diabetes in Early and Late Pregnancy. Front. Endocrinol. 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Garonzi, C.; Maguolo, A.; Maffeis, C. Pros and Cons of Current Diagnostic Tools for Risk-Based Screening of Prediabetes and Type 2 Diabetes in Children and Adolescents with Overweight or Obesity. Horm. Res. Paediatr. 2023, 96, 356–365. [Google Scholar] [CrossRef]
- Bonongwe, P.; Lindow, S.W.; Coetzee, E.J. Reproducibility of a 75G Oral Glucose Tolerance Test in Pregnant Women. J. Perinat. Med. 2015, 43, 333–338. [Google Scholar] [CrossRef]
- Bogdanet, D.; O’Shea, P.; Lyons, C.; Shafat, A.; Dunne, F. The Oral Glucose Tolerance Test—Is It Time for a Change?—A Literature Review with an Emphasis on Pregnancy. J. Clin. Med. 2020, 9, 3451. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hinkle, S.N.; Grantz, K.L.; Kim, S.; Grewal, J.; Grobman, W.A.; Skupski, D.W.; Newman, R.B.; Chien, E.K.; Sciscione, A.; et al. Glycaemic Status during Pregnancy and Longitudinal Measures of Fetal Growth in a Multi-Racial US Population: A Prospective Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, S.K.; Kim, Y.L. Fetal Abdominal Overgrowth Is Already Present at 20–24 Gestational Weeks Prior to Diagnosis of Gestational Diabetes Mellitus. Sci. Rep. 2021, 11, 23821. [Google Scholar] [CrossRef] [PubMed]
- Yovera, L.; Zaharia, M.; Jachymski, T.; Velicu-Scraba, O.; Coronel, C.; de Paco Matallana, C.; Georgiopoulos, G.; Nicolaides, K.H.; Charakida, M. Impact of Gestational Diabetes Mellitus on Fetal Cardiac Morphology and Function: Cohort Comparison of Second- and Third-Trimester Fetuses. Ultrasound Obstet. Gynecol. 2021, 57, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Goecks, J.; Jalili, V.; Heiser, L.M.; Gray, J.W. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kline, A.; Wang, H.; Li, Y.; Dennis, S.; Hutch, M.; Xu, Z.; Wang, F.; Cheng, F.; Luo, Y. Multimodal Machine Learning in Precision Health: A Scoping Review. npj Digit. Med. 2022, 5, 171. [Google Scholar] [CrossRef] [PubMed]
- Mennickent, D.; Rodríguez, A.; Farías-Jofré, M.; Araya, J.; Guzmán-Gutiérrez, E. Machine Learning-Based Models for Gestational Diabetes Mellitus Prediction before 24–28 Weeks of Pregnancy: A Review. Artif. Intell. Med. 2022, 132, 102378. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, Y.; Ye, D.; Yu, X.; Huang, W.; Zang, S.; Jiang, G. Longitudinal Metabolomics Integrated with Machine Learning Identifies Novel Biomarkers of Gestational Diabetes Mellitus. Free Radic. Biol. Med. 2023, 209, 9–17. [Google Scholar] [CrossRef]
- Razo-Azamar, M.; Nambo-Venegas, R.; Meraz-Cruz, N.; Guevara-Cruz, M.; Ibarra-González, I.; Vela-Amieva, M.; Delgadillo-Velázquez, J.; Santiago, X.C.; Escobar, R.F.; Vadillo-Ortega, F.; et al. An Early Prediction Model for Gestational Diabetes Mellitus Based on Metabolomic Biomarkers. Diabetol. Metab. Syndr. 2023, 15, 116. [Google Scholar] [CrossRef]
- Sovio, U.; Clayton, G.L.; Cook, E.; Gaccioli, F.; Stephen Charnock-Jones, D.; Lawlor, D.A.; Smith, G.C.S. Metabolomic Identification of a Novel, Externally Validated Predictive Test for Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2022, 107, E3479–E3486. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Guo, Z.; Song, S.; Liu, S.; Yuan, T.; Fu, Y.; Dong, Y.; Sun, H.; Liu, X.; et al. Urinary Proteomic Analysis during Pregnancy and Its Potential Application in Early Prediction of Gestational Diabetes Mellitus and Spontaneous Abortion. J. Clin. Endocrinol. Med. 2022, 10, 736. [Google Scholar] [CrossRef]
- Zhu, Y.; Barupal, D.K.; Ngo, A.L.; Quesenberry, C.P.; Feng, J.; Fiehn, O.; Ferrara, A. Predictive Metabolomic Markers in Early to Mid-Pregnancy for Gestational Diabetes Mellitus: A Prospective Test and Validation Study. Diabetes 2022, 71, 1807–1817. [Google Scholar] [CrossRef]
- Piras, C.; Neri, I.; Pintus, R.; Noto, A.; Petrella, E.; Monari, F.; Dessì, A.; Fanos, V.; Atzori, L.; Facchinetti, F. First Trimester Metabolomics 1H-NMR Study of the Urinary Profile Predicts Gestational Diabetes Mellitus Development in Obese Women. J. Matern.-Fetal Neonatal Med. 2022, 35, 8275–8283. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Yang, L.; Zhong, P.; Huang, G.; Liang, Q.; Yu, X. Genetic Variants of ERBB4 Gene and Risk of Gestational Diabetes Mellitus: A Susceptibility and Diagnostic Nomogram Study. Front. Endocrinol. 2023, 14, 1283539. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.; Frishman, S.; Turjeman, S.; Eshel, A.; Nuriel-Ohayon, M.; Shrossel, O.; Ziv, O.; Walters, W.; Parsonnet, J.; Ley, C.; et al. Gestational Diabetes Is Driven by Microbiota-Induced Inflammation Months before Diagnosis. Gut 2023, 72, 918–928. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Zhou, M.; Liu, M.; Zhang, L.; Liang, Z.; Chen, D. An Early Prediction Model for Gestational Diabetes Mellitus Based on Genetic Variants and Clinical Characteristics in China. Diabetol. Metab. Syndr. 2022, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, M.; Gallardo-Rincón, H.; Martinez-Juarez, L.A.; Lomelin-Gascon, J.; Ortega-Montiel, J.; Montoya, A.; Mendizabal, L.; Arregi, M.; Martinez-Martinez, M.d.L.A.; Camarillo Romero, E.D.S.; et al. Development and Validation of a Multivariable Genotype-Informed Gestational Diabetes Prediction Algorithm for Clinical Use in the Mexican Population: Insights into Susceptibility Mechanisms. BMJ Open Diabetes Res. Care 2023, 11, e003046. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Huck, C. Near-Infrared Spectroscopy; Springer: Singapore, 2021; ISBN 978-981-15-8647-7. [Google Scholar]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Balan, V.; Mihai, C.T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef]
- Sakudo, A. Near-Infrared Spectroscopy for Medical Applications: Current Status and Future Perspectives. Clin. Chim. Acta 2016, 455, 181–188. [Google Scholar] [CrossRef]
- Vitorino, R.; Barros, A.S.; Guedes, S.; Caixeta, D.C.; Sabino-Silva, R. Diagnostic and Monitoring Applications Using near Infrared (NIR) Spectroscopy in Cancer and Other Diseases. Photodiagn. Photodyn. Ther. 2023, 42, 103633. [Google Scholar] [CrossRef] [PubMed]
- Villena Gonzales, W.; Mobashsher, A.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- MINSAL. Guía Perinatal; Ministerio de Salud de Chile (MINSAL): Santiago, Chile, 2015; ISBN 9789563480764. [Google Scholar]
- Morais, C.L.M.; Lima, K.M.G.; Singh, M.; Martin, F.L. Tutorial: Multivariate Classification for Vibrational Spectroscopy in Biological Samples. Nat. Protoc. 2020, 15, 2143–2162. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, Z.; Chen, X.; Fei, S. Preprocessing Methods for Near-Infrared Spectrum Calibration. J. Chemom. 2020, 34, e3306. [Google Scholar] [CrossRef]
- Biancolillo, A.; Måge, I.; Næs, T. Combining SO-PLS and Linear Discriminant Analysis for Multi-Block Classification. Chemom. Intell. Lab. Syst. 2015, 141, 58–67. [Google Scholar] [CrossRef]
- Cocchi, M.; Biancolillo, A.; Marini, F. Chemometric Methods for Classification and Feature Selection. Compr. Anal. Chem. 2018, 82, 265–299. [Google Scholar] [CrossRef]
- Ballabio, D.; Grisoni, F.; Todeschini, R. Multivariate Comparison of Classification Performance Measures. Chemom. Intell. Lab. Syst. 2018, 174, 33–44. [Google Scholar] [CrossRef]
- Mennickent, D.; Ortega-Contreras, B.; Gutiérrez-Vega, S.; Castro, E.; Rodríguez, A.; Araya, J.; Guzmán-Gutiérrez, E. Evaluation of First and Second Trimester Maternal Thyroid Profile on the Prediction of Gestational Diabetes Mellitus and Post Load Glycemia. PLoS ONE 2023, 18, e0280513. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas; Internacional Diabetes Federation (IDF): Brussels, Belgium, 2019; ISBN 9782930229874. Available online: https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 26 April 2024).
- Azcarate, S.M.; Ríos-Reina, R.; Amigo, J.M.; Goicoechea, H.C. Data Handling in Data Fusion: Methodologies and Applications. TrAC-Trends Anal. Chem. 2021, 143, 116355. [Google Scholar] [CrossRef]
- Post, M.J.; van der Putten, P.; van Rijn, J.N. Does Feature Selection Improve Classification? A Large Scale Experiment in OpenML. In Advances in Intelligent Data Analysis XV, Proceedings of the 15th International Symposium, IDA 2016, Stockholm, Sweden, 13–15 October 2016; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2016; pp. 158–170. [Google Scholar] [CrossRef]
- Cocchi, M. Data Fusion Methodology and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444639844. [Google Scholar]
- Workman, J., Jr.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429110511. [Google Scholar]
- Golic, M.; Walsh, K.; Lawson, P. Short-Wavelength Near-Infrared Spectra of Sucrose, Glucose, and Fructose with Respect to Sugar Concentration and Temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef]
- Goodarzi, M.; Saeys, W. Selection of the Most Informative near Infrared Spectroscopy Wavebands for Continuous Glucose Monitoring in Human Serum. Talanta 2016, 146, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhao, D.; Chen, X.; Khan, N.U.; Liu, X.; Zheng, Q.; Liang, Y.; Zhu, Y.; Iqbal, J.; et al. Potential Biomarkers Identified in Plasma of Patients with Gestational Diabetes Mellitus. Metabolomics 2021, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Han, Z.; Guo, L.; Liu, Y.; Li, G.; Li, H.; Zhang, J.; Bai, L.; Wu, H.; Chen, B. Identification of Urinary Biomarkers for the Prediction of Gestational Diabetes Mellitus in Early Second Trimester of Young Gravidae Based on ITRAQ Quantitative Proteomics. Endocr. J. 2018, 65, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, B.A.; Mojsak, P.; Rojo, D.; Telejko, B.; Paczkowska–Abdulsalam, M.; Hryniewicka, J.; Zielinska–Maciulewska, A.; Szelachowska, M.; Gorska, M.; Barbas, C.; et al. Gas Chromatography–Mass Spectroscopy-Based Metabolomics Analysis Reveals Potential Biochemical Markers for Diagnosis of Gestational Diabetes Mellitus. Front. Pharmacol. 2021, 12, 770240. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic Profiling, Metabolomic and Metabonomic Procedures for NMR Spectroscopy of Urine, Plasma, Serum and Tissue Extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- McBride, N.; Yousefi, P.; White, S.L.; Poston, L.; Farrar, D.; Sattar, N.; Nelson, S.M.; Wright, J.; Mason, D.; Suderman, M.; et al. Do Nuclear Magnetic Resonance (NMR)-Based Metabolomics Improve the Prediction of Pregnancy-Related Disorders? Findings from a UK Birth Cohort with Independent Validation. BMC Med. 2020, 18, 366. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Almeida, L.M.; Martins, A.S.; Duarte, D.; Barros, A.S.; Galhano, E.; Pita, C.; Almeida, M.D.C.; Carreira, I.M.; Gil, A.M. Prediction of Gestational Diabetes through NMR Metabolomics of Maternal Blood. J. Proteome Res. 2015, 14, 2696–2706. [Google Scholar] [CrossRef]
- Mokkala, K.; Vahlberg, T.; Pellonperä, O.; Houttu, N.; Koivuniemi, E.; Laitinen, K. Distinct Metabolic Profile in Early Pregnancy of Overweight and Obese Women Developing Gestational Diabetes. J. Nutr. 2020, 150, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Song, L.P.; Zheng, H.T.; Wei, S.D.; Wen, X.L.; Huang, B.; Liu, D. Bin Association between Functional Genetic Variants in Retinoid X Receptor-α/γ and the Risk of Gestational Diabetes Mellitus in a Southern Chinese Population. Biosci. Rep. 2021, 41, BSR20211338. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, J.; Jiang, T.; Shi, Z.; Yu, B.; Zhu, Y.; Chen, D.; Xu, J.; Huo, R.; Dai, J.; et al. Early Second-Trimester Serum MiRNA Profiling Predicts Gestational Diabetes Mellitus. PLoS ONE 2011, 6, e23925. [Google Scholar] [CrossRef]
- Dudzik, D.; Zorawski, M.; Skotnicki, M.; Zarzycki, W.; García, A.; Angulo, S.; Lorenzo, M.P.; Barbas, C.; Ramos, M.P. GC–MS Based Gestational Diabetes Mellitus Longitudinal Study: Identification of 2-and 3-Hydroxybutyrate as Potential Prognostic Biomarkers. J. Pharm. Biomed. Anal. 2017, 144, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Jia, Y.J.; Xing, B.H.; Shi, D.D.; Dong, X.J. Plasma MicroRNA-16-5p, -17-5p and -20a-5p: Novel Diagnostic Biomarkers for Gestational Diabetes Mellitus. J. Obstet. Gynaecol. Res. 2017, 43, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography–Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- Agena Bioscience, Inc. Single Nucleotide Polymorphism Detection with the IPLEX® Assay and the MassARRAY® System; Agena Bioscience: San Diego, CA, USA, 2015. [Google Scholar]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of Circulating MicroRNA Biomarkers in Plasma and Serum Using Quantitative Reverse Transcription-PCR (QRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Bernardes-Oliveira, E.; de Freitas, D.L.D.; Morais, C.d.L.M.d.; Cornetta, M.d.C.d.M.; Camargo, J.D.d.A.S.; de Lima, K.M.G.; Crispim, J.C.d.O. Spectrochemical Differentiation in Gestational Diabetes Mellitus Based on Attenuated Total Reflection Fourier-Transform Infrared (ATR-FTIR) Spectroscopy and Multivariate Analysis. Sci. Rep. 2020, 10, 19259. [Google Scholar] [CrossRef] [PubMed]
| Variable | Unit | NGT (n = 67) | GDM (n = 15) | p Value | All (n = 82) | |
|---|---|---|---|---|---|---|
| Age | Years | 30 ± 5 | 32 ± 7 | 0.394 | NS | 31 ± 6 |
| BMI | Kg/m2 | 27.6 (23.3–31.2) | 29.7 (26.6–31.6) | 0.051 | NS | 28.0 (24.1–31.5) |
| Supplement consumption | % | 64.2 (43/67) | 53.3 (8/15) | 0.557 | NS | 62.2 (51/82) |
| Hyperemesis | % | 26.9 (18/67) | 26.7 (4/15) | >0.999 | NS | 26.8 (22/82) |
| Vaginal bleeding | % | 9.0 (6/67) | 13.3 (2/15) | 0.634 | NS | 9.8 (8/82) |
| Drug use before pregnancy | % | |||||
| Cigarettes | 34.3 (23/67) | 53.3 (8/15) | 0.239 | NS | 37.8 (31/82) | |
| Alcohol | 53.7 (36/67) | 60.0 (9/15) | 0.777 | NS | 54.9 (45/82) | |
| Other drugs | 13.4 (9/67) | 13.3 (2/15) | >0.999 | NS | 13.4 (11/82) | |
| Prior pregnancy issues | % | |||||
| GDM | 1.5 (1/67) | 33.3 (5/15) | ˂0.001 | *** | 7.3 (6/82) | |
| Hypertensive disorder | 4.5 (3/67) | 6.7 (1/15) | 0.562 | NS | 4.9 (4/82) | |
| Preterm birth | 4.5 (3/67) | 6.7 (1/15) | 0.562 | NS | 4.9 (4/82) | |
| Other | 10.4 (7/67) | 6.7 (1/15) | >0.999 | NS | 9.8 (8/82) | |
| Prior non-viable pregnancy | % | 20.9 (14/67) | 20.0 (3/15) | >0.999 | NS | 20.7 (17/82) |
| Fertility problems | % | 14.9 (10/67) | 6.7 (1/15) | 0.679 | NS | 13.4 (11/82) |
| PCOS | % | 25.4 (17/67) | 13.3 (2/15) | 0.501 | NS | 23.2 (19/82) |
| First period age | Years | 13 (12–14) | 12 (11–13) | 0.078 | NS | 13 (12–13) |
| Last period month | % | 0.202 | NS | |||
| January | 7.5 (5/67) | 6.7 (1/15) | 7.3 (6/82) | |||
| February | 6.0 (4/67) | 20.0 (3/15) | 8.5 (7/82) | |||
| March | 7.5 (5/67) | 0.0 (0/15) | 6.1 (5/82) | |||
| April | 3.0 (2/67) | 6.7 (1/15) | 3.7 (3/82) | |||
| May | 13.4 (9/67) | 20.0 (3/15) | 14.6 (12/82) | |||
| June | 10.4 (7/67) | 13.3 (2/15) | 11.0 (9/82) | |||
| July | 9.0 (6/67) | 13.3 (2/15) | 9.8 (8/82) | |||
| August | 7.5 (5/67) | 0.0 (0/15) | 6.1 (5/82) | |||
| September | 6.0 (4/67) | 0.0 (0/15) | 4.9 (4/82) | |||
| October | 13.4 (9/67) | 6.7 (1/15) | 12.2 (10/82) | |||
| November | 10.4 (7/67) | 13.3 (2/15) | 11.0 (9/82) | |||
| December | 6.0 (4/67) | 0.0 (0/15) | 4.9 (4/82) | |||
| Personal morbid history | % | |||||
| Insulin resistance | 3.0 (2/67) | 6.7 (1/15) | 0.459 | NS | 3.7 (3/82) | |
| Thyroid dysfunction | 4.5 (3/67) | 6.7 (1/15) | 0.562 | NS | 4.9 (4/82) | |
| Asthma | 6.0 (4/67) | 0.0 (0/15) | >0.999 | NS | 4.9 (4/82) | |
| Other | 10.4 (7/67) | 20.0 (3/15) | 0.380 | NS | 12.2 (10/82) | |
| Family morbid history | % | |||||
| Insulin resistance or prediabetes | 3.0 (2/67) | 6.7 (1/15) | 0.459 | NS | 3.7 (3/82) | |
| DM | 32.8 (22/67) | 66.7 (10/15) | 0.020 | * | 39.0 (32/82) | |
| Hypertension | 41.8 (28/67) | 60.0 (9/15) | 0.255 | NS | 45.1 (37/82) | |
| Hypothyroidism | 17.9 (12/67) | 33.3 (5/15) | 0.287 | NS | 20.7 (17/82) | |
| Hyperthyroidism | 1.5 (1/67) | 13.3 (2/15) | 0.085 | NS | 3.7 (3/82) | |
| Asthma | 7.5 (5/67) | 0.0 (0/15) | 0.579 | NS | 6.1 (5/82) | |
| Other | 16.4 (11/67) | 13.3 (2/15) | >0.999 | NS | 15.9 (13/82) | |
| Range a | Pretreatment | Sp | Se | NER | |||
|---|---|---|---|---|---|---|---|
| Av | StD | Av | StD | Av | StD | ||
| Full | SM (W = 23) + N + MC | 0.6946 | 0.0456 | 0.4507 | 0.0681 | 0.5726 | 0.0410 |
| R1 | N + MC | 0.6722 | 0.0361 | 0.5920 | 0.0910 | 0.6321 | 0.0489 |
| R2 | SM (W = 3) + N + MC | 0.5678 | 0.0322 | 0.6480 | 0.1035 | 0.6079 | 0.0542 |
| R3 | SM (W = 23) + MC | 0.5931 | 0.0346 | 0.5133 | 0.0811 | 0.5532 | 0.0441 |
| Variable | Unit | NGT (n = 39) | GDM (n = 8) | p Value | All (n = 47) | |
|---|---|---|---|---|---|---|
| Age | Years | 29 ± 5 | 30 ± 7 | 0.606 | NS | 29 ± 5 |
| BMI | Kg/m2 | 27.0 ± 4.7 | 31.3 ± 6.5 | 0.034 | * | 27.7 ± 5.2 |
| Supplement consumption | % | 64.1 (25/39) | 62.5 (5/8) | >0.999 | NS | 63.8 (30/47) |
| Hyperemesis | % | 33.3 (13/39) | 25.0 (2/8) | >0.999 | NS | 31.9 (15/47) |
| Vaginal bleeding | % | 5.1 (2/39) | 25.0 (2/8) | 0.129 | NS | 8.5 (4/47) |
| Drug use before pregnancy | % | |||||
| Cigarettes | 33.3 (13/39) | 37.5 (3/8) | >0.999 | NS | 34.0 (16/47) | |
| Alcohol | 61.5 (24/39) | 50.0 (4/8) | 0.697 | NS | 59.6 (28/47) | |
| Other drugs | 25.6 (10/39) | 0.0 (0/8) | 0.174 | NS | 21.3 (10/47) | |
| Prior pregnancy issues | % | |||||
| GDM | 0.0 (0/39) | 37.5 (3/8) | 0.004 | ** | 6.4 (3/47) | |
| Hypertensive disorder | 7.7 (3/39) | 0.0 (0/8) | >0.999 | NS | 6.4 (3/47) | |
| Preterm birth | 5.1 (2/39) | 12.5 (1/8) | 0.436 | NS | 6.4 (3/47) | |
| Other | 7.7 (3/39) | 0.0 (0/8) | >0.999 | NS | 6.4 (3/47) | |
| Prior non-viable pregnancy | % | 17.9 (7/39) | 12.5 (1/8) | >0.999 | NS | 17.0 (8/47) |
| Fertility problems | % | 17.9 (7/39) | 0.0 (0/8) | 0.329 | NS | 14.9 (7/47) |
| PCOS | % | 25.6 (10/39) | 12.5 (1/8) | 0.659 | NS | 23.4 (11/47) |
| First period age | Years | 13 (12–14) | 12 (12–13) | 0.058 | NS | 13 (12–14) |
| Last period month | % | 0.729 | NS | |||
| January | 2.6 (1/39) | 0.0 (0/8) | 2.1 (1/47) | |||
| February | 7.7 (3/39) | 25.0 (2/8) | 10.6 (5/47) | |||
| March | 12.8 (5/39) | 0.0 (0/8) | 10.6 (5/47) | |||
| April | 5.1 (2/39) | 12.5 (1/8) | 6.4 (3/47) | |||
| May | 12.8 (5/39) | 0.0 (0/8) | 10.6 (5/47) | |||
| June | 10.3 (4/39) | 12.5 (1/8) | 10.6 (5/47) | |||
| July | 15.4 (6/39) | 25.0 (2/8) | 17.0 (8/47) | |||
| August | 12.8 (5/39) | 0.0 (0/8) | 10.6 (5/47) | |||
| September | 2.6 (1/39) | 12.5 (1/8) | 4.3 (2/47) | |||
| October | 10.3 (4/39) | 12.5 (1/8) | 10.6 (5/47) | |||
| November | 5.1 (2/39) | 0.0 (0/8) | 4.3 (2/47) | |||
| December | 2.6 (1/39) | 0.0 (0/8) | 2.1 (1/47) | |||
| Personal morbid history | % | |||||
| Insulin resistance | 5.1 (2/39) | 0.0 (0/8) | >0.999 | NS | 4.3 (2/47) | |
| Thyroid dysfunction | 10.3 (4/39) | 0.0 (0/8) | >0.999 | NS | 8.5 (4/47) | |
| Asthma | 7.7 (3/39) | 0.0 (0/8) | >0.999 | NS | 6.4 (3/47) | |
| Other | 10.3 (4/39) | 37.5 (3/8) | 0.084 | NS | 14.9 (7/47) | |
| Family morbid history | % | |||||
| Insulin resistance or prediabetes | 7.7 (3/39) | 12.5 (1/8) | 0.539 | NS | 8.5 (4/47) | |
| DM | 35.9 (14/39) | 62.5 (5/8) | 0.240 | NS | 40.4 (19/47) | |
| Hypertension | 48.7 (19/39) | 62.5 (5/8) | 0.701 | NS | 51.1 (24/47) | |
| Hypothyroidism | 17.9 (7/39) | 25.0 (2/8) | 0.639 | NS | 19.1 (9/47) | |
| Hyperthyroidism | 5.1 (2/39) | 12.5 (1/8) | 0.436 | NS | 6.4 (3/47) | |
| Asthma | 10.3 (4/39) | 0.0 (0/8) | >0.999 | NS | 8.5 (4/47) | |
| Other | 12.8 (5/39) | 12.5 (1/8) | >0.999 | NS | 12.8 (6/47) | |
| Range a | Pretreatment | Sp | Se | NER | |||
|---|---|---|---|---|---|---|---|
| Av | StD | Av | StD | Av | StD | ||
| Full | 2D (W = 15) + N + MC | 0.8133 | 0.0324 | 0.1150 | 0.0556 | 0.4642 | 0.0321 |
| R1 | WLS + N + MC | 0.8754 | 0.0414 | 0.1625 | 0.1218 | 0.5189 | 0.0643 |
| R2 | 2D (W = 3) + N + MC | 0.6821 | 0.0288 | 0.3875 | 0.1191 | 0.5348 | 0.0613 |
| R3 | 1D (W = 15) + MC | 0.8713 | 0.0361 | 0.7075 | 0.0783 | 0.7894 | 0.0431 |
| Time of Application | Study | Instrumental Technique | Predictive Power | Duration of Analysis |
|---|---|---|---|---|
| Before diagnosis of GDM by OGTT | This study | NIRS | AUROC: 0.5768 ± 0.0635 NER: 0.6321 ± 0.0489 | 32 min |
| [21] | LC-MS | AUROC: 0.724–0.902 | >8 h | |
| [48] | LC-MS | AUROC: 0.7075 | 1.5 h | |
| [49] | LC-MS | AUROC: 0.729–0.906 | >4 h | |
| [22] | GC-MS | AUROC: 0.771–0.907 | >1.5 h | |
| [50] | GC-MS | AUROC: 0.745–0.797 | >16 h | |
| [23] | NMRS | AUROC: 0.796 | Not mentioned. Typically 1–1.5 h [51] | |
| [52] | NMRS | AUROC: 0.59 | ||
| [53] | NMRS | NER: 0.635–0.825 | ||
| [54] | NMRS | AUROC: 0.610–0.719 | ||
| [27] | PCR | AUROC: 0.7694 | >8 h | |
| [55] | PCR | NER: 0.531–0.552 | >2 h | |
| [56] | PCR | AUROC: 0.600–0.669 | >2.5 h | |
| At the time of diagnosis of GDM by OGTT | This study | NIRS | AUROC: 0.8836 ± 0.0259 NER: 0.7894 ± 0.0431 | 32 min |
| [48] | LC-MS | AUROC: 0.7800 | 1.5 h | |
| [50] | GC-MS | AUROC: 0.745–0.828 | >16 h | |
| [57] | GC-MS | AUROC: 0.83–0.90 | >16 h | |
| [52] | NMRS | AUROC: 0.62 | Not mentioned. Typically 1–1.5 h [51] | |
| [53] | NMRS | NER: 0.695–0.885 | ||
| [27] | PCR | AUROC: 0.7694 | >8 h | |
| [55] | PCR | NER: 0.531–0.552 | >2 h | |
| [58] | PCR | AUROC: 0.74–0.92 | >2.5 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennickent, D.; Romero-Albornoz, L.; Gutiérrez-Vega, S.; Aguayo, C.; Marini, F.; Guzmán-Gutiérrez, E.; Araya, J. Simple and Fast Prediction of Gestational Diabetes Mellitus Based on Machine Learning and Near-Infrared Spectra of Serum: A Proof of Concept Study at Different Stages of Pregnancy. Biomedicines 2024, 12, 1142. https://doi.org/10.3390/biomedicines12061142
Mennickent D, Romero-Albornoz L, Gutiérrez-Vega S, Aguayo C, Marini F, Guzmán-Gutiérrez E, Araya J. Simple and Fast Prediction of Gestational Diabetes Mellitus Based on Machine Learning and Near-Infrared Spectra of Serum: A Proof of Concept Study at Different Stages of Pregnancy. Biomedicines. 2024; 12(6):1142. https://doi.org/10.3390/biomedicines12061142
Chicago/Turabian StyleMennickent, Daniela, Lucas Romero-Albornoz, Sebastián Gutiérrez-Vega, Claudio Aguayo, Federico Marini, Enrique Guzmán-Gutiérrez, and Juan Araya. 2024. "Simple and Fast Prediction of Gestational Diabetes Mellitus Based on Machine Learning and Near-Infrared Spectra of Serum: A Proof of Concept Study at Different Stages of Pregnancy" Biomedicines 12, no. 6: 1142. https://doi.org/10.3390/biomedicines12061142
APA StyleMennickent, D., Romero-Albornoz, L., Gutiérrez-Vega, S., Aguayo, C., Marini, F., Guzmán-Gutiérrez, E., & Araya, J. (2024). Simple and Fast Prediction of Gestational Diabetes Mellitus Based on Machine Learning and Near-Infrared Spectra of Serum: A Proof of Concept Study at Different Stages of Pregnancy. Biomedicines, 12(6), 1142. https://doi.org/10.3390/biomedicines12061142









