Comprehensive Molecular Analysis of Disease-Related Genes as First-Tier Test for Early Diagnosis, Classification, and Management of Patients Affected by Nonsyndromic Ichthyosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Molecular Analyses
2.3. Molecular Cloning in the Minigene Vector
2.4. Cell Culture, Minigene Expression and Transcript Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vahlquist, A.; Törmä, H. Ichthyosis: A Road Model for Skin Research. Acta Derm. Venereol. 2020, 100, adv00097. [Google Scholar] [CrossRef] [PubMed]
- Vahlquist, A.; Fischer, J.; Törmä, H. Inherited Nonsyndromic Ichthyoses: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Youssefian, L.; Saeidian, A.; Vahidnezhad, H. Molecular Genetics of Keratinization Disorders—What’s New About Ichthyosis. Acta Derm. Venereol. 2020, 100, adv00095-185. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Falco, F.; Neri, I.; Graziano, C.; Toschi, B.; Auricchio, L.; Gouveia, C.; Sousa, A.B.; Salvatore, F. Different TGM1 mutation spectra in Italian and Portuguese patients with autosomal recessive congenital ichthyosis: Evidence of founder effects in Portugal. Br. J. Dermatol. 2013, 168, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Abeni, D.; Rotunno, R.; Diociaiuti, A.; Giancristoforo, S.; Bonamonte, D.; Schepis, C.; Neri, I.; Castiglia, D.; Zambruno, G.; El Hachem, M. A multicenter study on quality of life of the “greater patient” in congenital ichthyoses. Orphanet J. Rare Dis. 2021, 16, 440. [Google Scholar] [CrossRef]
- Gutiérrez-Cerrajero, C.; Sprecher, E.; Paller, A.S.; Akiyama, M.; Mazereeuw-Hautier, J.; Hernández-Martín, A.; González-Sarmiento, R. Ichthyosis. Nat. Rev. Dis. Primers 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Carsana, A. Metabolic Alterations in Cardiomyocytes of Patients with Duchenne and Becker Muscular Dystrophies. J. Clin. Med. 2019, 8, 2151. [Google Scholar] [CrossRef] [PubMed]
- Caso, V.M.; Manzo, V.; Pecchillo Cimmino, T.; Conti, V.; Caso, P.; Esposito, G.; Russo, V.; Filippelli, A.; Ammendola, R.; Cattaneo, F. Regulation of Inflammation and Oxidative Stress by Formyl Peptide Receptors in Cardiovascular Disease Progression. Life 2021, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Ohno, Y.; Nara, A.; Nakamichi, S.; Kihara, A. Molecular mechanism of the ichthyosis pathology of Chanarin-Dorfman syndrome: Stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD. J. Dermatol. Sci. 2018, 92, 245–253. [Google Scholar] [CrossRef]
- Sarret, C.; Ashkavand, Z.; Paules, E.; Dorboz, I.; Pediaditakis, P.; Sumner, S.; Eymard-Pierre, E.; Francannet, C.; Krupenko, N.I.; Boespflug-Tanguy, O.; et al. Deleterious mutations in ALDH1L2 suggest a novel cause for neuro-ichthyotic syndrome. NPJ Genom. Med. 2019, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting deregulated oxidative stress in skin inflammatory diseases: An update on clinical importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.-U.; Kwon, Y.-J.; Baek, H.-S.; Park, H.; Lee, H.; Chun, Y.-J. Unraveling the molecular mechanisms of cell migration impairment and apoptosis associated with steroid sulfatase deficiency: Implications for X-linked ichthyosis. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2024, 1870, 167004. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Bourrat, E. Genetics of Inherited Ichthyoses and Related Diseases. Acta Derm. Venereol. 2020, 100, adv00096-196. [Google Scholar] [CrossRef] [PubMed]
- Metze, D.; Traupe, H.; Süßmuth, K. Ichthyoses—A Clinical and Pathological Spectrum from Heterogeneous Cornification Disorders to Inflammation. Dermatopathology 2021, 8, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, A.; El Hachem, M.; Pisaneschi, E.; Giancristoforo, S.; Genovese, S.; Sirleto, P.; Boldrini, R.; Angioni, A. Role of molecular testing in the multidisciplinary diagnostic approach of ichthyosis. Orphanet J. Rare Dis. 2016, 11, 4. [Google Scholar] [CrossRef]
- Fioretti, T.; Auricchio, L.; Piccirillo, A.; Vitiello, G.; Ambrosio, A.; Cattaneo, F.; Ammendola, R.; Esposito, G. Multi-Gene Next-Generation Sequencing for Molecular Diagnosis of Autosomal Recessive Congenital Ichthyosis: A Genotype-Phenotype Study of Four Italian Patients. Diagnostics 2020, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.P.-C.; Doolan, B.J.; McGrath, J.A.; Onoufriadis, A. A decade of next-generation sequencing in genodermatoses: The impact on gene discovery and clinical diagnostics*. Br. J. Dermatol. 2021, 184, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Zhang, V.W.; Tian, X.; Feng, Y.; Wang, G.; Gorman, E.; Wang, H.; Lutz, R.E.; Schmitt, E.S.; et al. Capture-based high-coverage NGS: A powerful tool to uncover a wide spectrum of mutation types. Genet. Med. 2016, 18, 513–521. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Gaildrat, P.; Killian, A.; Martins, A.; Tournier, I.; Frébourg, T.; Tosi, M. Use of splicing reporter minigene assay to evaluate the effect on splicing of unclassified genetic variants. In Cancer Susceptibility: Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 653, pp. 249–257. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Bellia, C.; Cardillo, G.; Castaldo, G.; Ciaccio, M.; Elce, A.; Lembo, F.; Tomaiuolo, R. Extensive Molecular Analysis of Patients Bearing CFTR-Related Disorders. J. Mol. Diagn. 2012, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, A.; Iorio, M.; Amato, F.; Elce, A.; Ingino, R.; Filocamo, M.; Castaldo, G.; Salvatore, F.; Tomaiuolo, R. A Novel DHPLC-Based Procedure for the Analysis of COL1A1 and COL1A2 Mutations in Osteogenesis Imperfecta. J. Mol. Diagn. 2011, 13, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Severino-Freire, M.; Tournier, C.G.; Chiaverini, C.; Audouze, A.; Morice-Picard, F.; Texier, H.; Dreyfus, I.; Bing-Lecointe, A.-C.; Mallet, S.; Bodemer, C.; et al. French national protocol for the management of congenital ichthyosis. Ann. De Dermatol. Et De Venereol. 2024, 151, 103247. [Google Scholar] [CrossRef]
- Hotz, A.; Fölster-Holst, R.; Oji, V.; Bourrat, E.; Frank, J.; Marrakchi, S.; Ennouri, M.; Wankner, L.; Komlosi, K.; Alter, S.; et al. Erythrokeratodermia Variabilis-like Phenotype in Patients Carrying ABCA12 Mutations. Genes 2024, 15, 288. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S.E.; Hambuch, T.; Zook, J.M.; Bristow, S.L.; Hatchell, K.; Truty, R.; Kennemer, M.; Shirts, B.H.; Fellowes, A.; Chowdhury, S.; et al. One in seven pathogenic variants can be challenging to detect by NGS: An analysis of 450,000 patients with implications for clinical sensitivity and genetic test implementation. Genet. Med. 2021, 23, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, Y.G.; Kim, J.K.; Kim, H.H. STS and PUDP Deletion Identified by Targeted Panel Sequencing with CNV Analysis in X-Linked Ichthyosis: A Case Report and Literature Review. Genes 2023, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, F. Molecular analysis of Duchenne Becker muscular dystrophy. Front. Biosci. 2010, E2, 113. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, T.; Di Iorio, V.; Lombardo, B.; De Falco, F.; Cevenini, A.; Cattaneo, F.; Testa, F.; Pastore, L.; Simonelli, F.; Esposito, G. Molecular Characterization of Choroideremia-Associated Deletions Reveals an Unexpected Regulation of CHM Gene Transcription. Genes 2021, 12, 1111. [Google Scholar] [CrossRef]
- Diociaiuti, A.; Angioni, A.; Pisaneschi, E.; Alesi, V.; Zambruno, G.; Novelli, A.; El Hachem, M. X-linked ichthyosis: Clinical and molecular findings in 35 Italian patients. Exp. Dermatol. 2019, 28, 1156–1163. [Google Scholar] [CrossRef]
- Chouk, H.; Saad, S.; Dimassi, S.; Fetoui, N.G.; Bennour, A.; Gammoudi, R.; Elmabrouk, H.; Saad, A.; Denguezli, M.; H’mida, D. X-linked recessive ichthyosis in 8 Tunisian patients: Awareness of misdiagnosis due to the technical trap of the STS pseudogene. BMC Med. Genom. 2022, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Cavenagh, A.; Chatterjee, S.; Davies, W. Behavioural and psychiatric phenotypes in female carriers of genetic mutations associated with X-linked ichthyosis. PLoS ONE 2019, 14, e0212330. [Google Scholar] [CrossRef] [PubMed]
- Brcic, L.; Underwood, J.F.; Kendall, K.M.; Caseras, X.; Kirov, G.; Davies, W. Medical and neurobehavioural phenotypes in carriers of X-linked ichthyosis-associated genetic deletions in the UK Biobank. J. Med. Genet. 2020, 57, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chulpanova, D.S.; Shaimardanova, A.A.; Ponomarev, A.S.; Elsheikh, S.; Rizvanov, A.A.; Solovyeva, V.V. Current Strategies for the Gene Therapy of Autosomal Recessive Congenital Ichthyosis and Other Types of Inherited Ichthyosis. Int. J. Mol. Sci. 2022, 23, 2506. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Rescigno, G.; Salvatore, F.; Auricchio, L.; Paparo, F.; Rinaldi, M. Transglutaminase 1 Gene Mutations in Italian Patients with Autosomal Recessive Lamellar Ichthyosis. J. Investig. Dermatol. 2001, 116, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Falco, F.; Brazzelli, V.; Montanari, L.; Larizza, D.; Salvatore, F. Autosomal recessive congenital ichthyosis and congenital hypothyroidism in a Tunisian patient with a nonsense mutation in TGM. J. Dermatol. Sci. 2009, 55, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Tadini, G.; Paparo, F.; Viola, A.; Ieno, L.; Pennacchia, W.; Messina, F.; Giordano, L.; Piccirillo, A.; Auricchio, L. Transglutaminase 1 deficiency and corneocyte collapse: An indication for targeted molecular screening in autosomal recessive congenital ichthyosis. Br. J. Dermatol. 2007, 157, 808–810. [Google Scholar] [CrossRef]
- Gumus, E. Case report of two brothers with a novel homozygous mutation in ALOX12B leads to autosomal recessive congenital ichthyosis: Which type and which subtype? Two siblings with a novel homozygous mutation in ALOX12B. Dermatol. Sin. 2019, 37, 150. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Shen, N.; Yin, H.; Qiao, J. Case report of self-improving collodion ichthyosis in the newborn. J. Int. Med. Res. 2023, 51, 3000605231204491. [Google Scholar] [CrossRef]
- Zwara, A.; Wertheim-Tysarowska, K.; Mika, A. Alterations of Ultra Long-Chain Fatty Acids in Hereditary Skin Diseases—Review Article. Front. Med. 2021, 8, 730855. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Lu, J.; Zhao, P.; Zhang, X.; Tan, L.; Li, J.; Xiao, C.; Zeng, L.; He, X. Identification of the first congenital ichthyosis case caused by a homozygous deletion in the ALOX12B gene due to chromosome 17 mixed uniparental disomy. Front. Genet. 2022, 13, 1833. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, A.; Corbeddu, M.; Rossi, S.; Pisaneschi, E.; Cesario, C.; Condorelli, A.G.; Samela, T.; Giancristoforo, S.; Angioni, A.; Zambruno, G.; et al. Cross-sectional study on autosomal recessive congenital ichthyoses: Association of genotype with disease severity, phenotypic and ultrastructural features in 74 Italian patients. Dermatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Loriè, E.P.; Fischer, J.; Vahlquist, A.; Törmä, H. The Expression of Epidermal Lipoxygenases and Transglutaminase-1 Is Perturbed by NIPAL4 Mutations: Indications of a Common Metabolic Pathway Essential for Skin Barrier Homeostasis. J. Investig. Dermatol. 2012, 132, 2368–2375. [Google Scholar] [CrossRef]
- Herman, M.L.; Farasat, S.; Steinbach, P.J.; Wei, M.-H.; Toure, O.; Fleckman, P.; Blake, P.; Bale, S.J.; Toro, J.R. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: Summary of mutations (including 23 novel) and modeling of TGase-1. Hum. Mutat. 2009, 30, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, J.; Samuelov, L.; Malchin, N.; Rabinowitz, T.; Assaf, S.; Malki, L.; Malovitski, K.; Israeli, S.; Grafi-Cohen, M.; Bitterman-Deutsch, O.; et al. Molecular epidemiology of non-syndromic autosomal recessive congenital ichthyosis in a Middle-Eastern population. Exp. Dermatol. 2021, 30, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Aufenvenne, K.; Rice, R.H.; Hausser, I.; Oji, V.; Hennies, H.C.; Del Rio, M.; Traupe, H.; Larcher, F. Long-Term Faithful Recapitulation of Transglutaminase 1–Deficient Lamellar Ichthyosis in a Skin-Humanized Mouse Model, and Insights from Proteomic Studies. J. Investig. Dermatol. 2012, 132, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Aufenvenne, K.; Larcher, F.; Hausser, I.; Duarte, B.; Oji, V.; Nikolenko, H.; Del Rio, M.; Dathe, M.; Traupe, H. Topical Enzyme-Replacement Therapy Restores Transglutaminase 1 Activity and Corrects Architecture of Transglutaminase-1-Deficient Skin Grafts. Am. J. Hum. Genet. 2013, 93, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Shearer, Z.; White, G.; Steed, J.Z.; Brown, C.; Venable, T.; Baber, M. A novel combination of mutations leading to congenital ichthyosis and ichthyosis vulgaris. Clin. Case Rep. 2023, 11, e7910. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, S.; Suga, Y.; Kon, J.; Matsuba, S.; Hashimoto, Y.; Ogawa, H. A Japanese patient with a mild form of lamellar ichthyosis harbouring two missense mutations in the core domain of the transglutaminase 1 gene. Br. J. Dermatol. 2004, 150, 390–392. [Google Scholar] [CrossRef]
- Youssefian, L.; Vahidnezhad, H.; Saeidian, A.H.; Touati, A.; Sotoudeh, S.; Mahmoudi, H.; Mansouri, P.; Daneshpazhooh, M.; Aghazadeh, N.; Hesari, K.K.; et al. Autosomal recessive congenital ichthyosis: Genomic landscape and phenotypic spectrum in a cohort of 125 consanguineous families. Hum. Mutat. 2019, 40, 288–298. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.-C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the Roles of Filaggrin in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.D.; Irvine, A.D.; Terron-Kwiatkowski, A.; Sandilands, A.; E Campbell, L.; Zhao, Y.; Liao, H.; Evans, A.T.; Goudie, D.R.; Lewis-Jones, S.; et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat. Genet. 2006, 38, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Hoober, J.K.; Eggink, L.L. The Discovery and Function of Filaggrin. Int. J. Mol. Sci. 2022, 23, 1455. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, B.; He, X.; Zou, P.; Zeng, Z.; Xiao, R. Nonsense Suppression Therapy: An Emerging Treatment for Hereditary Skin Diseases. Acta Derm. Venereol. 2022, 102, adv00658. [Google Scholar] [CrossRef]
- Bouaziz, M.; Mullaert, J.; Bigio, B.; Seeleuthner, Y.; Casanova, J.-L.; Alcais, A.; Abel, L.; Cobat, A. Controlling for human population stratification in rare variant association studies. Sci. Rep. 2021, 11, 19015. [Google Scholar] [CrossRef]
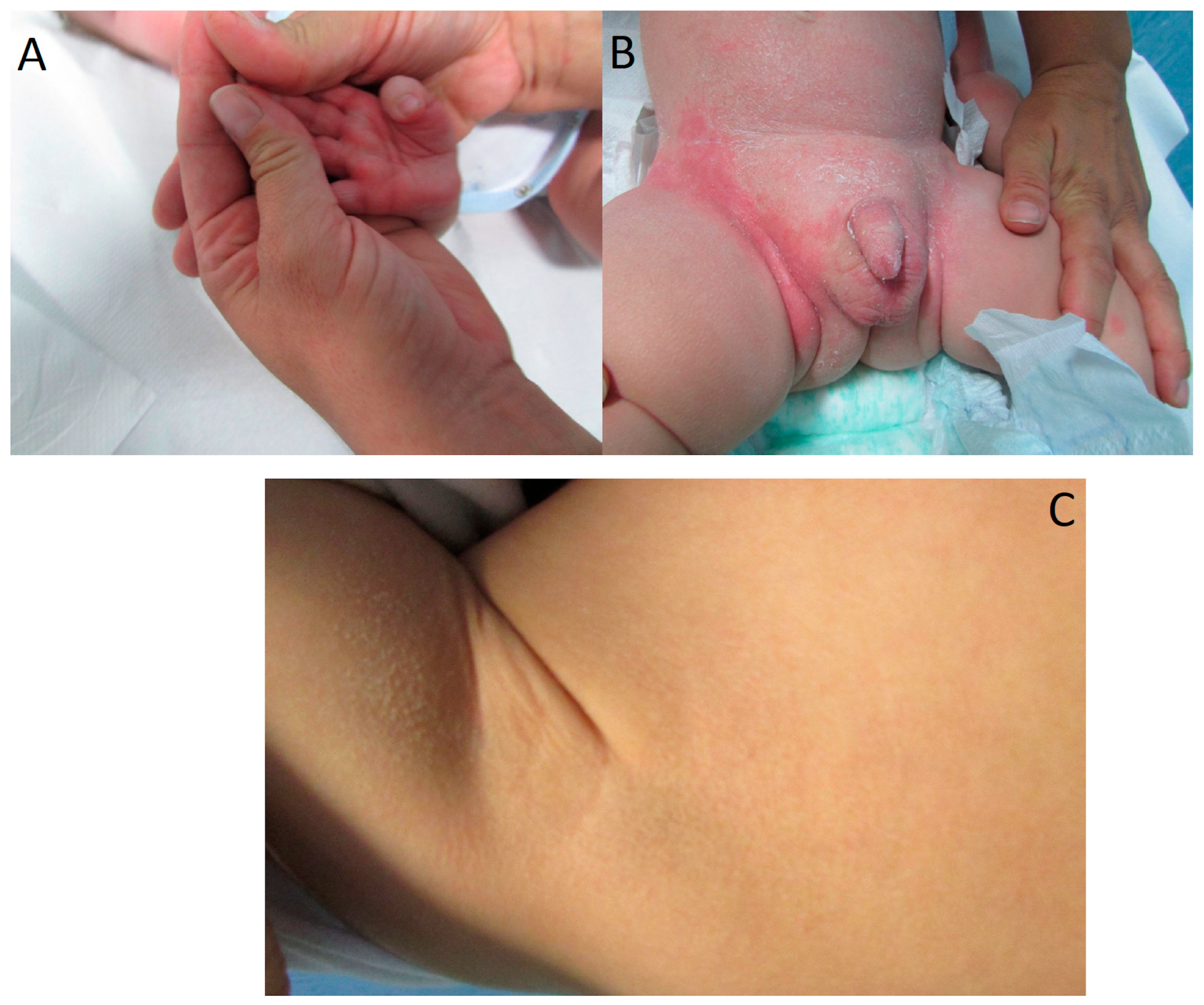
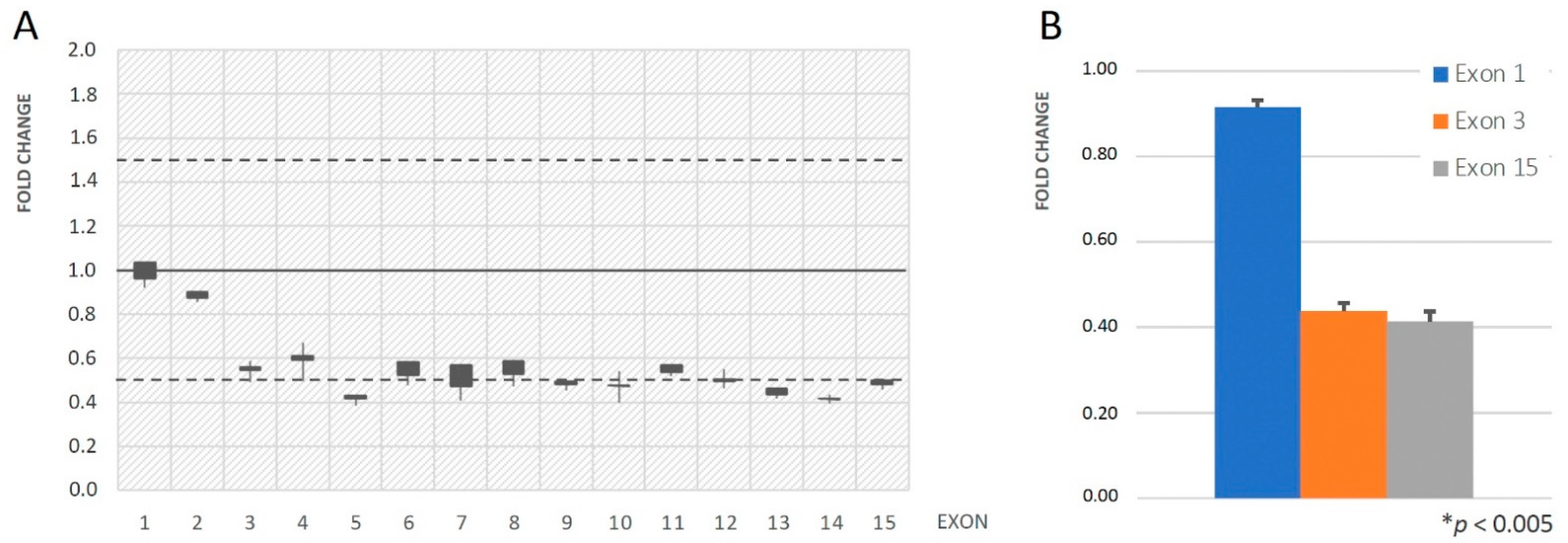
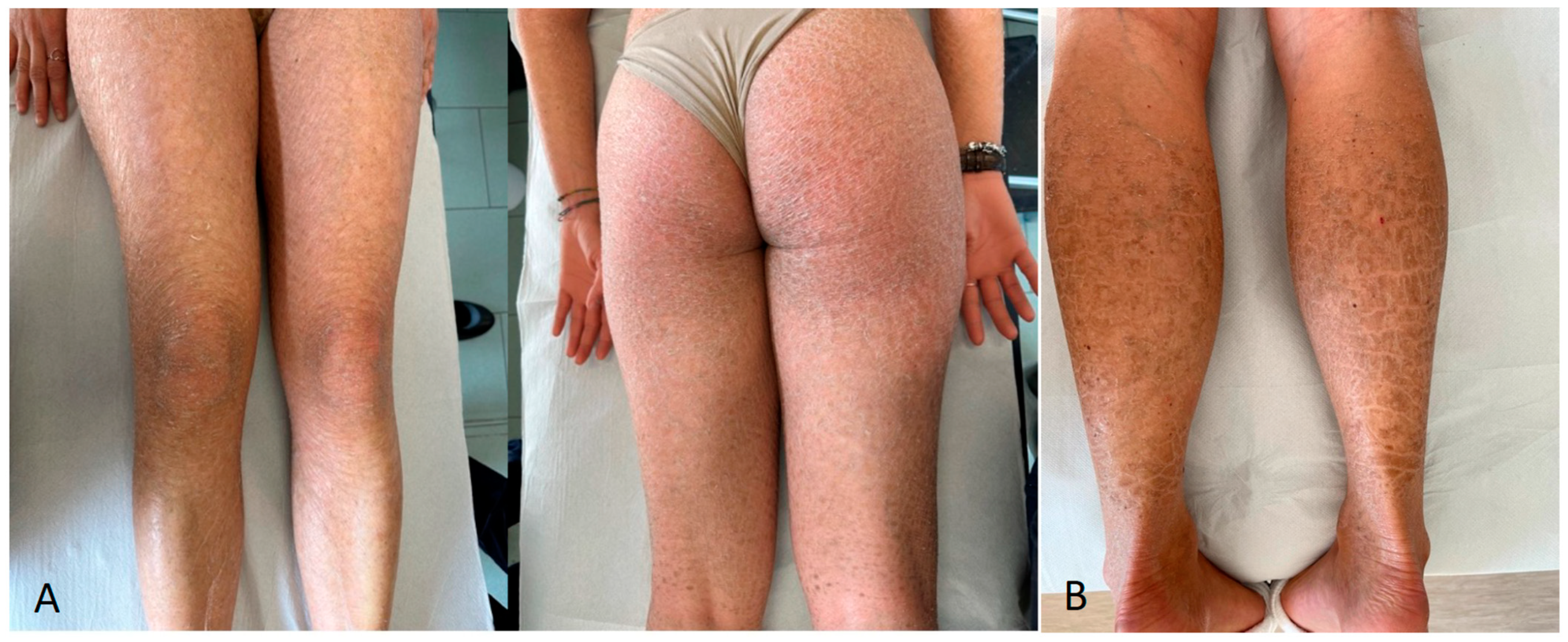
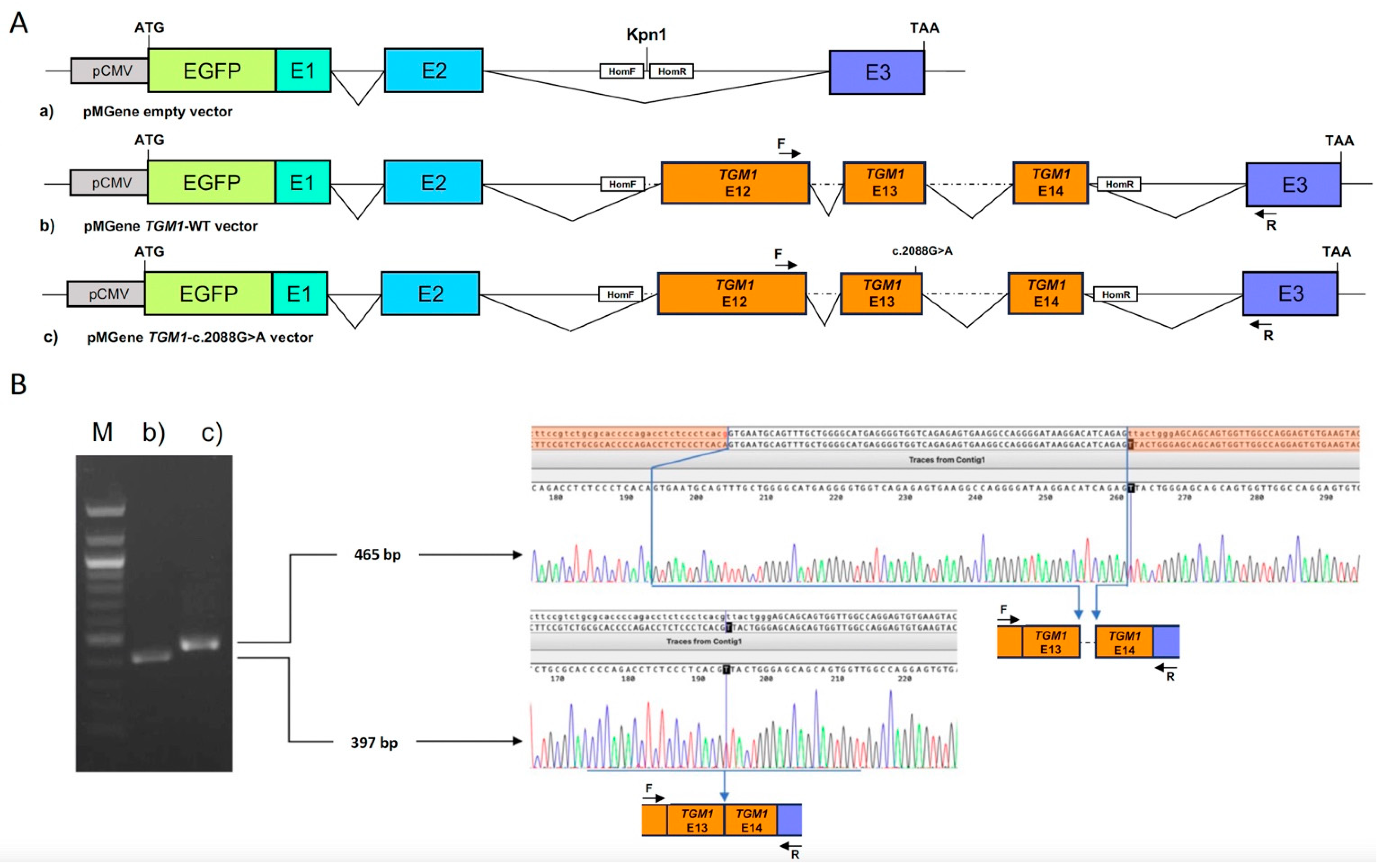
| Patient ID | Age (Years) | Sex | Phenotype | Gene | Variant Allele | ACMG |
|---|---|---|---|---|---|---|
| 01 | 3 | F | CB–SICI with normal skin | ALOX12B | c.47C>T p.(Ser16Leu) | Class 5 |
| c.1798C>T p.(Arg600Trp) | Class 4 | |||||
| 02 | 3 | M | CB–SICI—Familial | ALOX12B | c.1907G>T p.(Ser636Ile) † | Class 4 |
| Palmar-plantar keratoderma | c.1192C>T p.(His398Tyr) | Class 5 | ||||
| ectropion, trunk/face scaling | TGM1 | c.1033G>A p.(Asp345Asn) | Class 3 | |||
| 03 | 50 | F | CB–CIE—generalized dryness | ALOX12B | c.1259G>A p.(Cys420Tyr) † | Class 4 |
| face erythroderma, widespread whitish | c.1642C>T p.(Arg548Trp) | Class 5 | ||||
| fine scales | RIN2 | c.364C>T p.(His122Trp) | Class 3 | |||
| SULT2B1 | c.912G>A p.(Met304Ile) | Class 2 | ||||
| 04 | 3 | M | CIE—congenital dyskeratosis | ALOX12B | c.1909C>G p.(Arg637Gly) † | Class 3 |
| cutaneous xerosis, mild face erythroderma | EX3_15del | Class 5 | ||||
| TGM5 | c.850G>A p.(Val284Ile) | Class 3 | ||||
| RTEL1 ‡ | c.2920G>A p.(Gly974Ser) | Class 3 | ||||
| 05 | 33 | F | CIE—Dark brownish, polygonal scales | FLG ‡ | c.6034C>T p.(Gln2012*) | Class 4 |
| generalized dryness | c.2282_2285del p.(Ser761Cysfs*) | Class 4 | ||||
| TGM1 | c.2405A>T p.(Asp802Val) | Class 1 | ||||
| 06 | 71 | M | LI—Widespread dark brown, | FLG ‡ | c.1501C>T p.(Arg501*) | Class 5 |
| polygonal scales impetigo/familial, | c.5186C>G p.(Ser1729*) | Class 5 | ||||
| frequent skin infections, nail dystrophy | ALOXE3 | c.122C>A p.(Ala41Asp) | Class 3 | |||
| 07 | 5 | F | CI—ichthyosiform xerosis, spread to the trunk and limbs, | FLG ‡ | c.5980delC p.(His1994Metfs*) † | Class 5 |
| palmar-plantar hyperkeratosis | c.1951G>T p.(Glu651*) | Class 5 | ||||
| 08 | 50 | M | CB–CIE | NIPAL4 | c.398C>A p.(Pro133His) | Class 5 |
| c.514_515dup p.(Met172Ilefs*) † | Class 5 | |||||
| TCHH | c.4005_408del p.(Asp1335Glufs*) | Class 5 | ||||
| 09 | 29 | F | No CB—congenital widespread, | NIPAL4 | c.137G>A p.(Trp46*) † | Class 5 |
| dark brown, polygonal scales; | c.137G>A p.(Trp46*) † | Class 5 | ||||
| generalized dryness, nail dystrophy, | ABCA12 | c.6593C>T p.(Thr2198Ile) | Class 3 | |||
| frequent skin infections | SERPINB8 | c.988G>A p.(Ala330Thr) | Class 3 | |||
| 10 | 2 | M | CB—SICI | TGM1 | c.377G>A p.(Arg126His) | Class 5 |
| c.968G>A p.(Arg323Gln) | Class 5 | |||||
| FLG ‡ | c.1501C>T p.(Arg501*) | Class 5 | ||||
| LSS | c.1307A>G p.(Asn436Ser) | Class 3 | ||||
| 11 | 22 | F | CB–LI—Large scales and plates, adherent | TGM1 | c.1166G>A p.(Arg389His) | Class 5 |
| to most of the body surface, ectropion, | c.2088G>A p.(Thr696=) † | Class 5 | ||||
| ear anomalies, nail dystrophy | SUMF1 | c.376A>C p.(Met126Leu) | Class 3 | |||
| palmar-plantar hyperkeratosis, erythroderma | GJB2 ‡ | c.663G>C p.Lys221Asn | Class 3 | |||
| 12 | 14 | M | CI—xerosis of the face | STS | EX1-3del | Class 5 |
| elbows/knees hyperkeratosis | KRT1 ‡ | c.1724A>G p.(His575Arg) | Class 3 | |||
| 13 | 32 | M | CI—generalized dryness; widespread, | STS | EX10-11del | Class 5 |
| dark brown, polygonal scales; mother affected | GJB4 ‡ | c.500A>G p.(Glu167Gly) | Class 2 | |||
| 14 | 6 | M | CI | STS | EX1-12del | Class 5 |
| ALOX3 | c.122C>A p.(Ala41Asp) | Class 3 | ||||
| 15 | M | CI | STS | EX1-12del | Class 5 | |
| ST14 | c.2146G>C p.(Glu716Gln) | Class 3 | ||||
| 16 | 9 | M | CI—face and trunk desquamation | STS | EX1-12del | Class 5 |
| elbows/knees hyperkeratosis | ABCA12 | c.6704A>C p.(Glu2235Ala) | Class 1 | |||
| mild erythema improved with growth | ||||||
| 17 | 22 | M | CI—Familial | STS | EX1-12del | Class 5 |
| PNPLA1 | c.1464T>A p.(Tyr488*) | Class 1 | ||||
| KRT1 ‡ | c.1669A>G p.(Ser557Gly) | Class 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioretti, T.; Martora, F.; De Maggio, I.; Ambrosio, A.; Piscopo, C.; Vallone, S.; Amato, F.; Passaro, D.; Acquaviva, F.; Gaudiello, F.; et al. Comprehensive Molecular Analysis of Disease-Related Genes as First-Tier Test for Early Diagnosis, Classification, and Management of Patients Affected by Nonsyndromic Ichthyosis. Biomedicines 2024, 12, 1112. https://doi.org/10.3390/biomedicines12051112
Fioretti T, Martora F, De Maggio I, Ambrosio A, Piscopo C, Vallone S, Amato F, Passaro D, Acquaviva F, Gaudiello F, et al. Comprehensive Molecular Analysis of Disease-Related Genes as First-Tier Test for Early Diagnosis, Classification, and Management of Patients Affected by Nonsyndromic Ichthyosis. Biomedicines. 2024; 12(5):1112. https://doi.org/10.3390/biomedicines12051112
Chicago/Turabian StyleFioretti, Tiziana, Fabrizio Martora, Ilaria De Maggio, Adelaide Ambrosio, Carmelo Piscopo, Sabrina Vallone, Felice Amato, Diego Passaro, Fabio Acquaviva, Francesca Gaudiello, and et al. 2024. "Comprehensive Molecular Analysis of Disease-Related Genes as First-Tier Test for Early Diagnosis, Classification, and Management of Patients Affected by Nonsyndromic Ichthyosis" Biomedicines 12, no. 5: 1112. https://doi.org/10.3390/biomedicines12051112
APA StyleFioretti, T., Martora, F., De Maggio, I., Ambrosio, A., Piscopo, C., Vallone, S., Amato, F., Passaro, D., Acquaviva, F., Gaudiello, F., Di Girolamo, D., Maiolo, V., Zarrilli, F., Esposito, S., Vitiello, G., Auricchio, L., Sammarco, E., De Brasi, D., Petillo, R., ... Esposito, G. (2024). Comprehensive Molecular Analysis of Disease-Related Genes as First-Tier Test for Early Diagnosis, Classification, and Management of Patients Affected by Nonsyndromic Ichthyosis. Biomedicines, 12(5), 1112. https://doi.org/10.3390/biomedicines12051112








