N-Acetylcysteine Attenuates Sepsis-Induced Muscle Atrophy by Downregulating Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Drug Treatment
2.3. Cell Culture and Drug Treatment
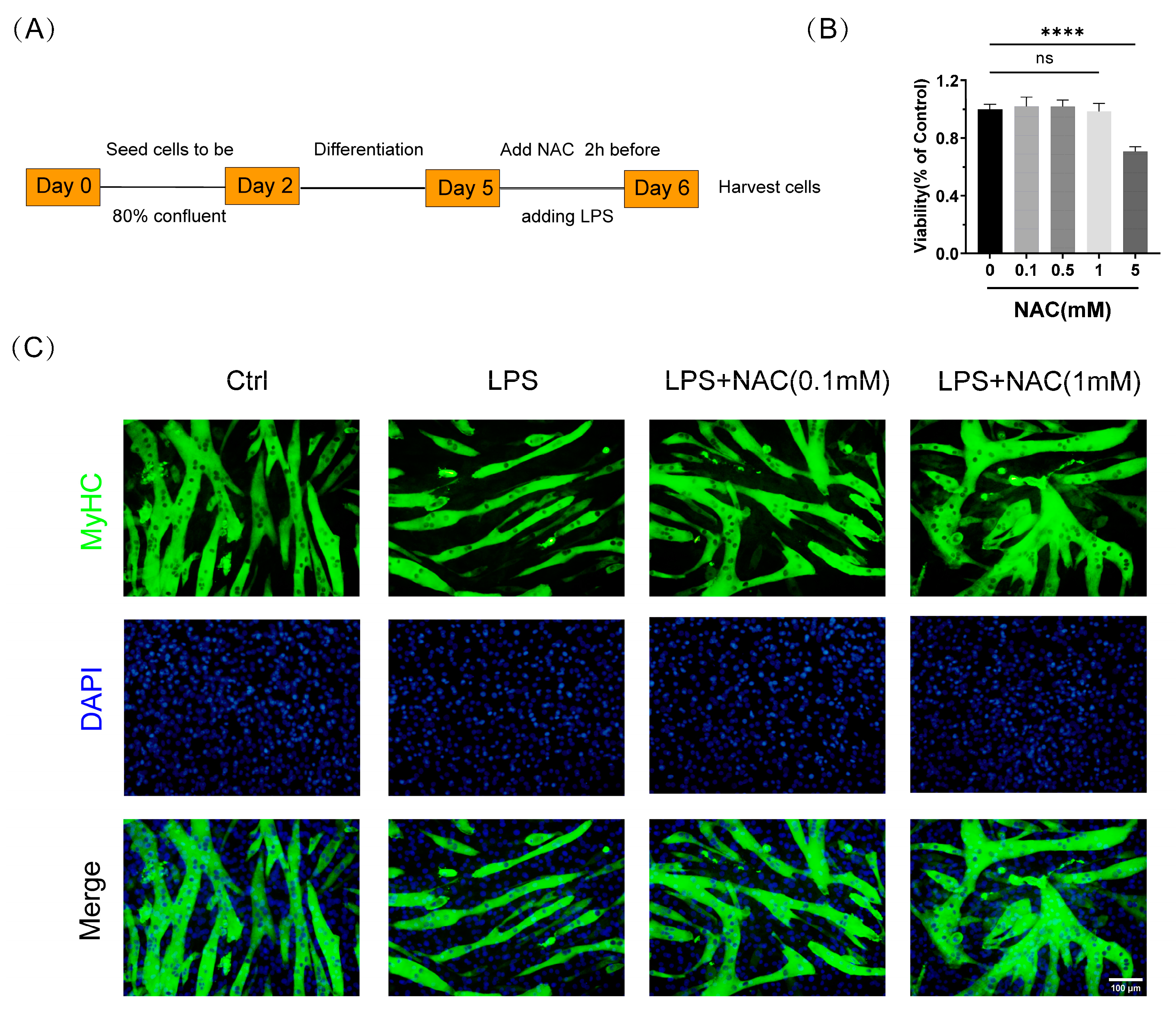
2.4. Cell Viability
2.5. Western Blot
2.6. Immunofluorescence Staining of Myofibers
2.7. Cell Immunofluorescence
2.8. Statistical Analysis
3. Results
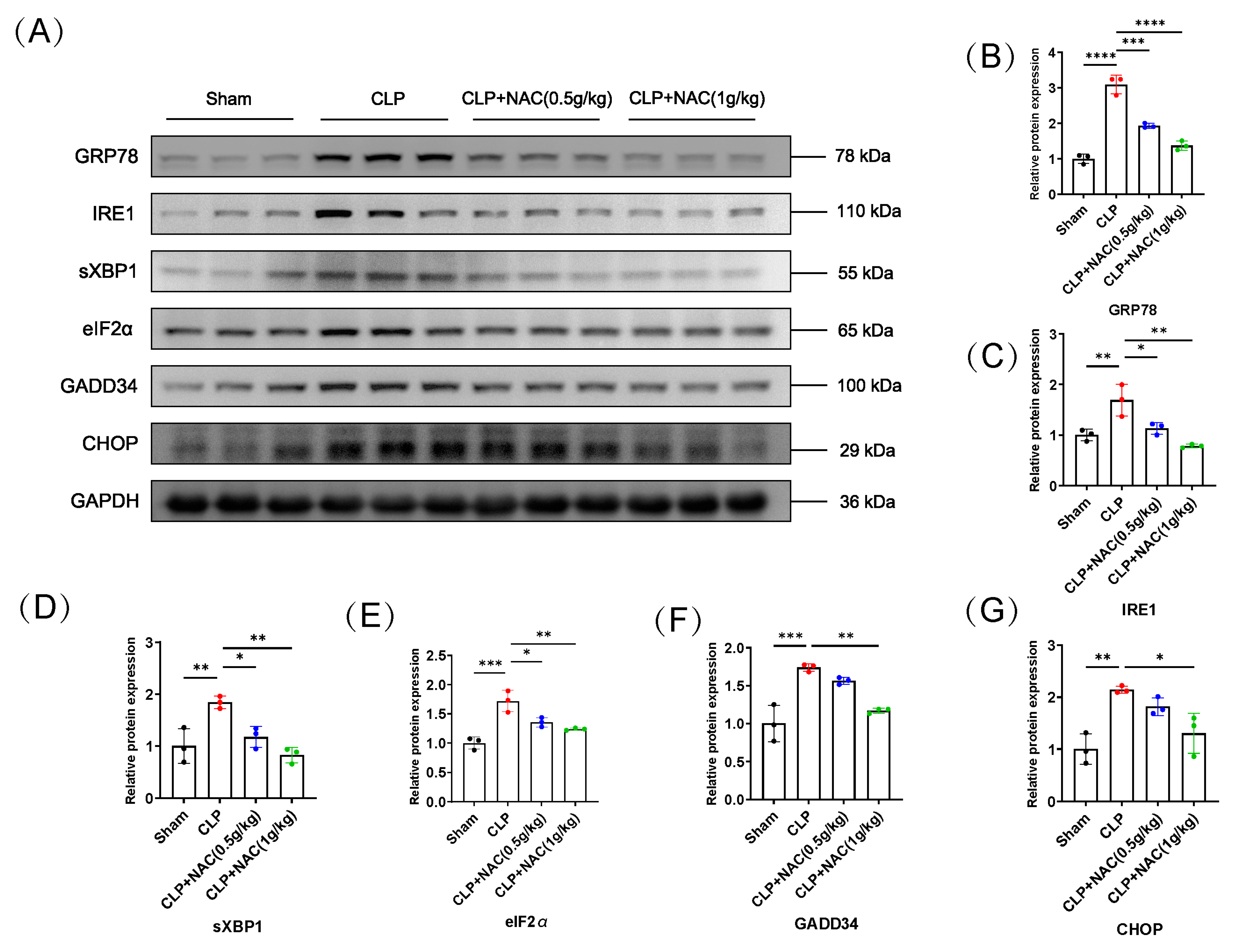
3.1. N-Acetylcysteine Prevents Sepsis-Induced Muscle Atrophy In Vivo
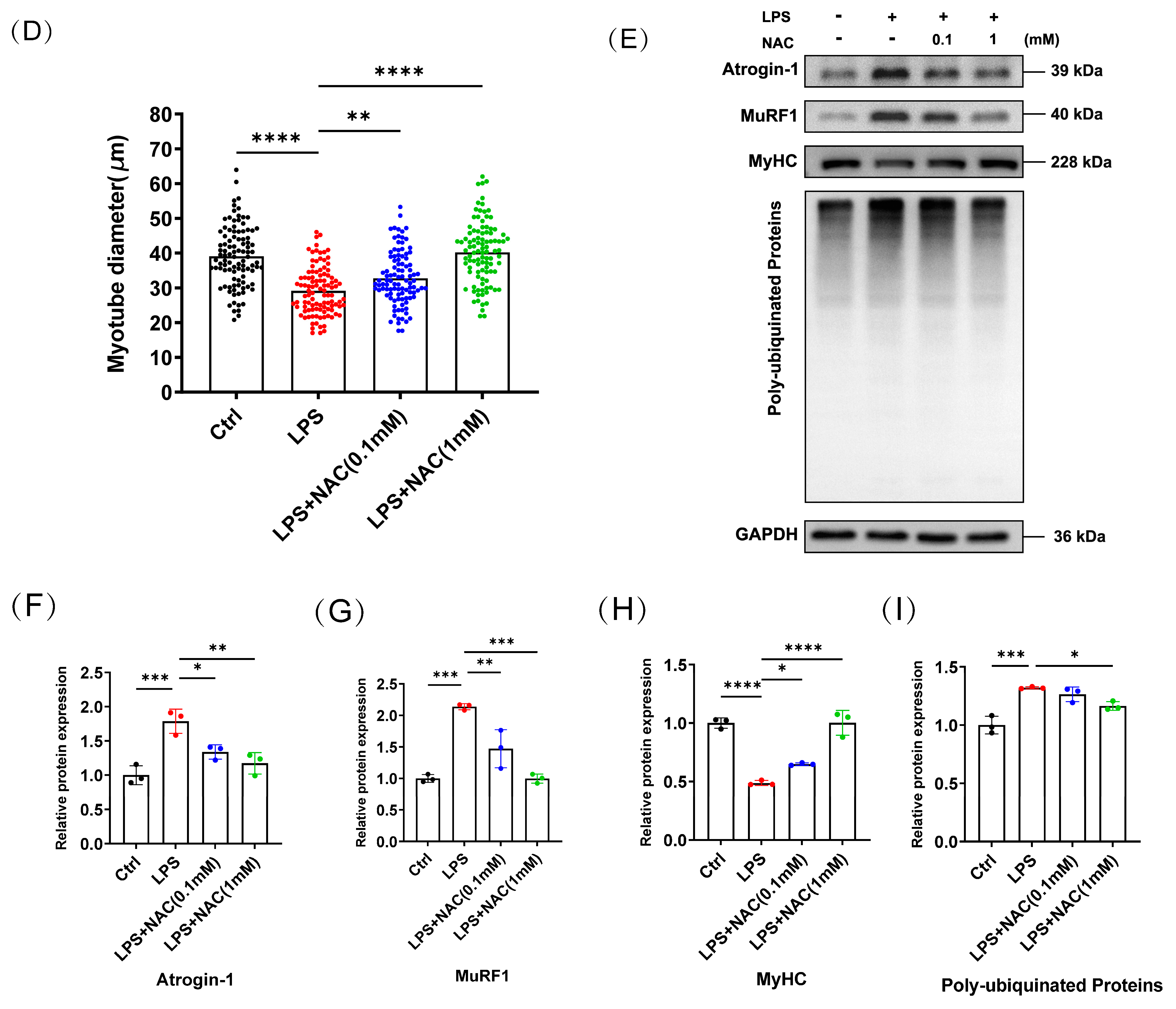
3.2. N-Acetylcysteine Attenuates LPS-Induced Myotube Atrophy In Vitro
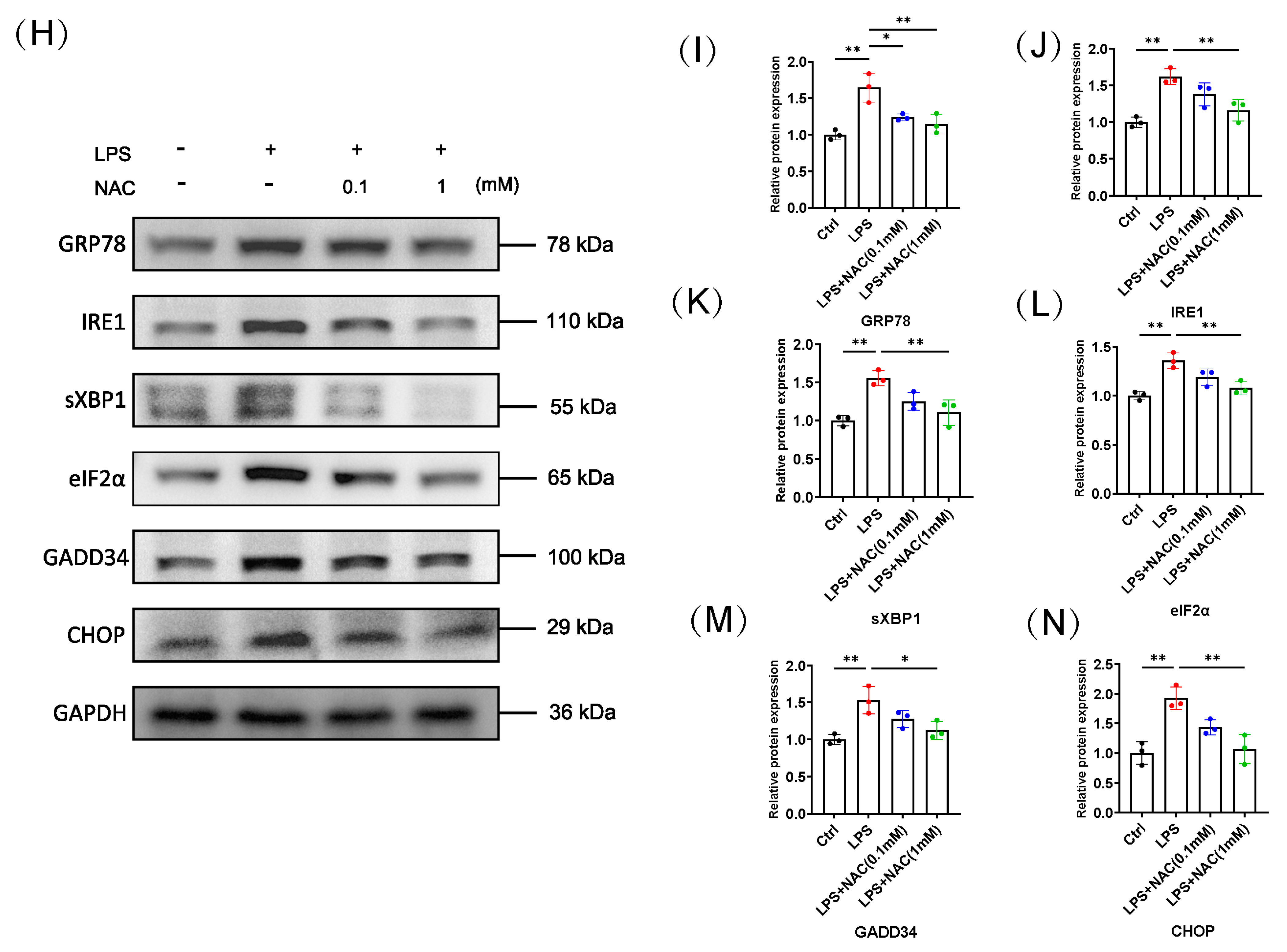
3.3. N-Acetylcysteine Inhibits the Activation of Sepsis-Induced Endoplasmic Reticulum Stress
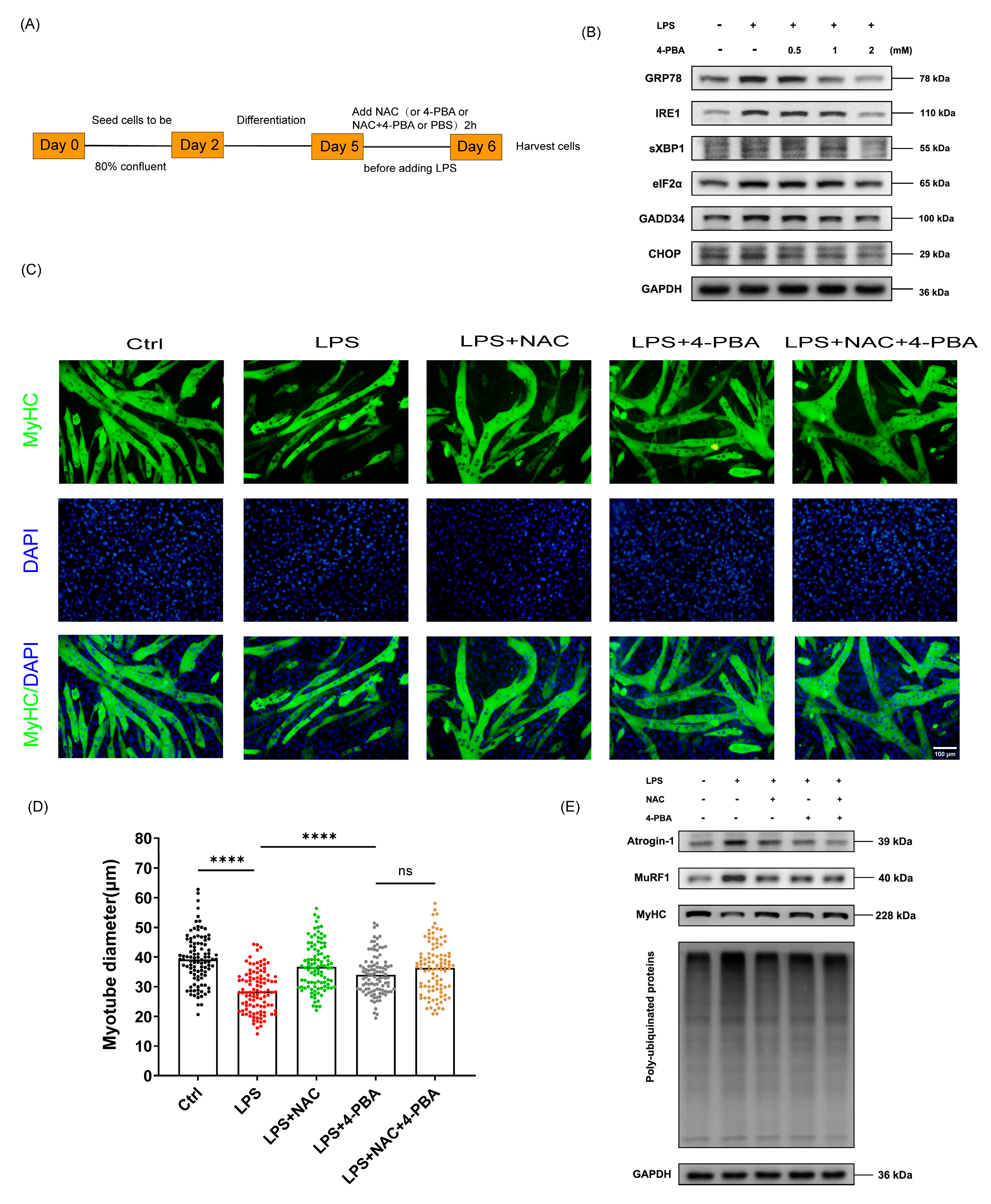
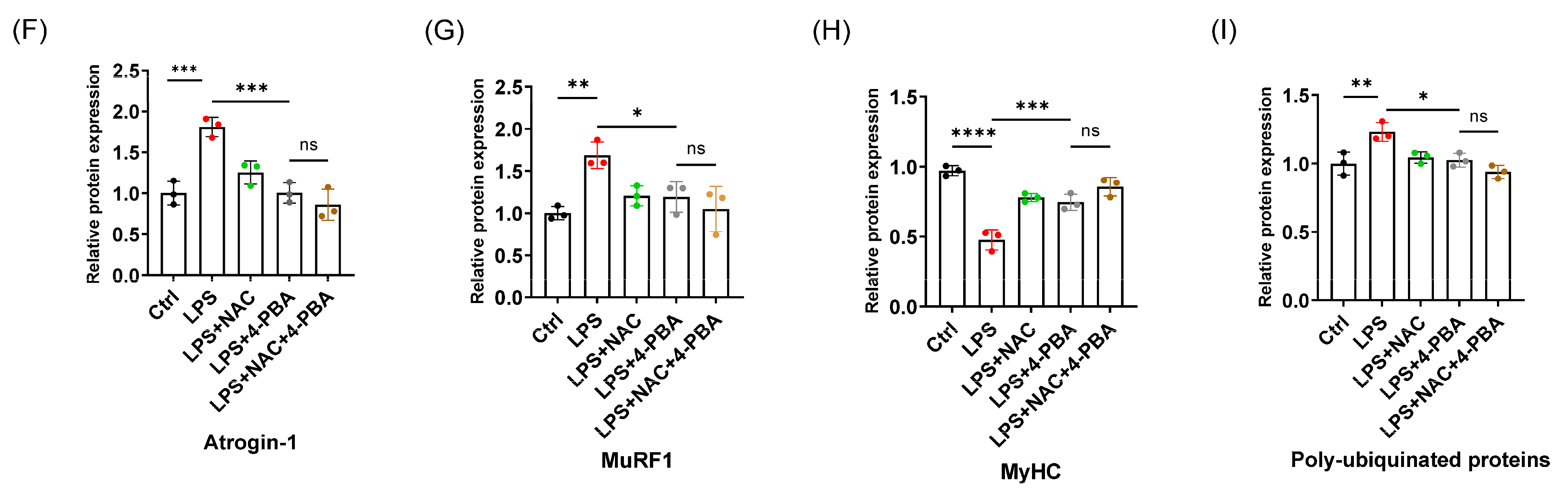
3.4. The Protective Effect of NAC on Sepsis-Induced Muscle Atrophy Is Partly Mediated by ER Stress
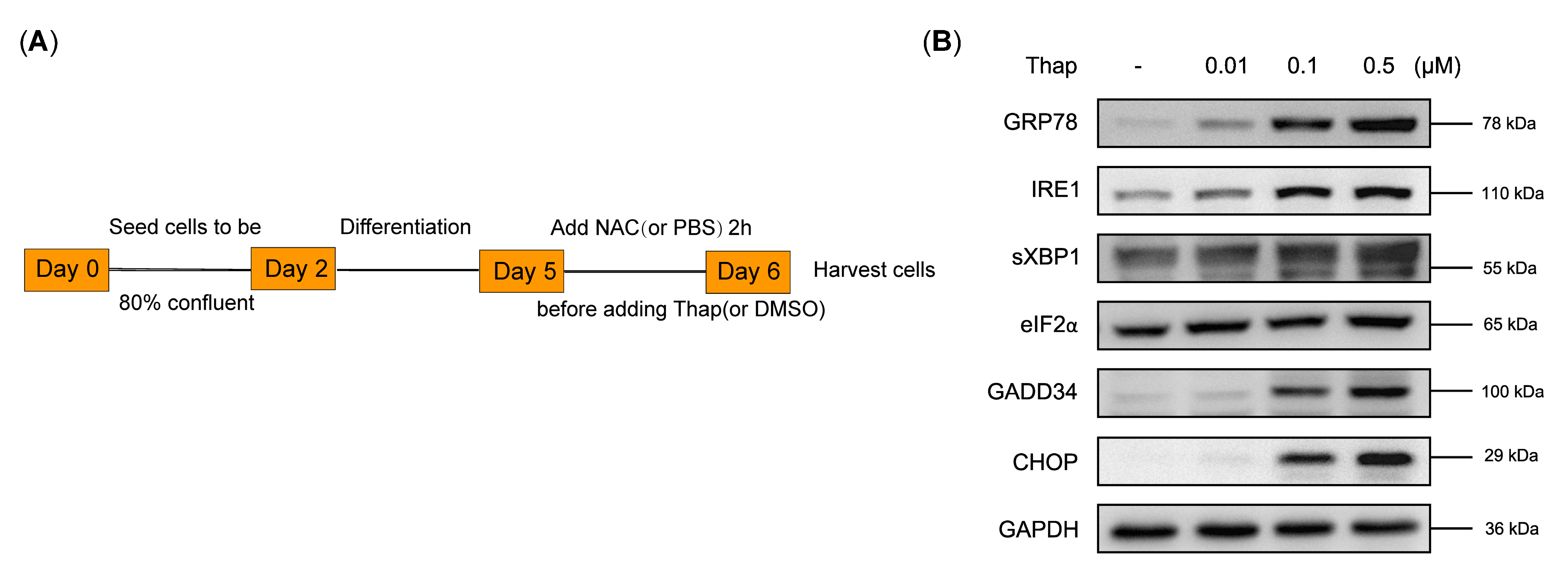
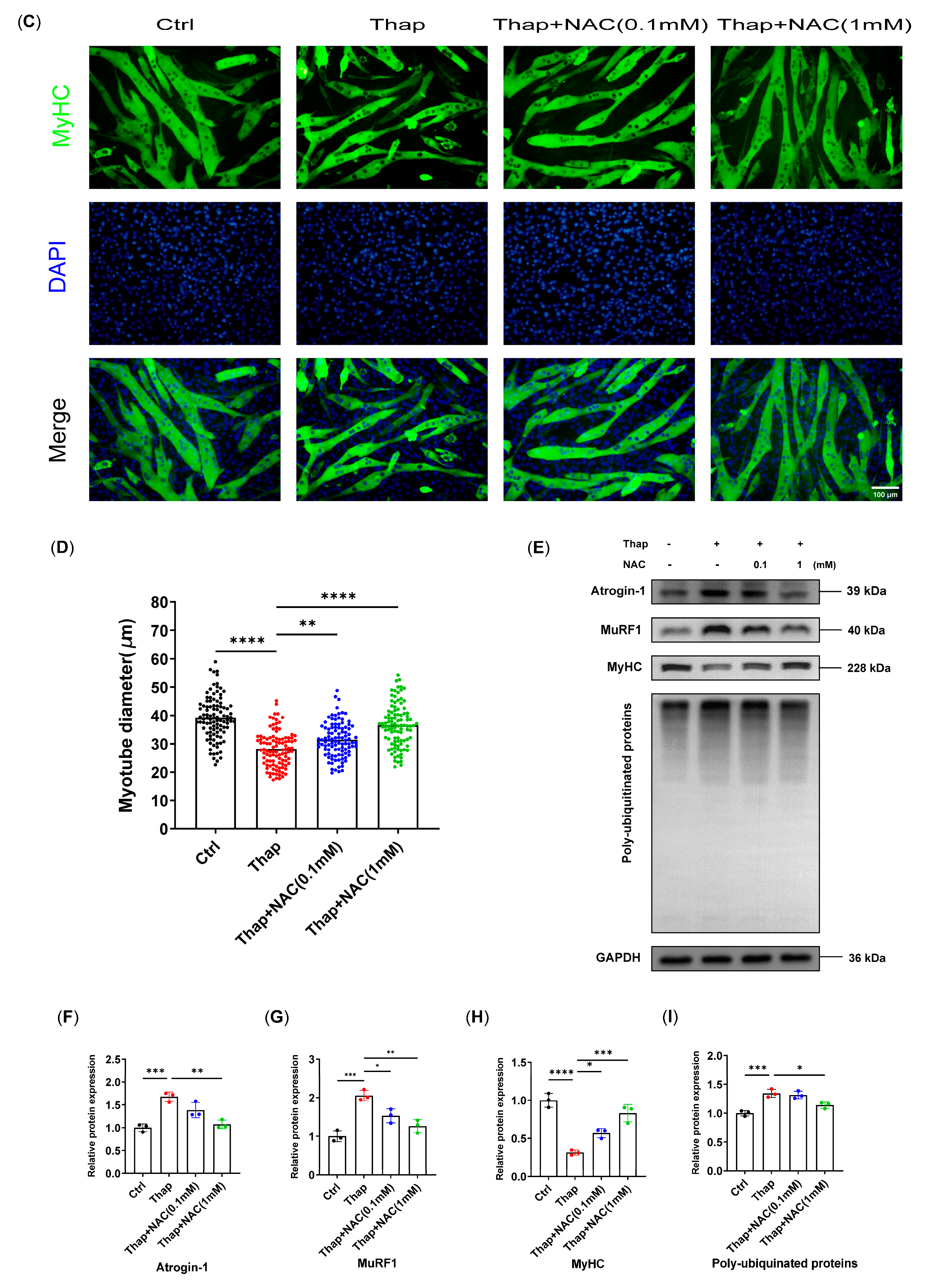
3.5. N-Acetylcysteine Attenuates ER Stress-Induced Myotube Atrophy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Laitano, O.; Clanton, T.L.; Brakenridge, S.C. Pathophysiology and Treatment Strategies of Acute Myopathy and Muscle Wasting after Sepsis. J. Clin. Med. 2021, 10, 1874. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.J.; Mammen, J.M.; Hershko, D.D.; Hasselgren, P.-O. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int. J. Biochem. Cell Biol. 2003, 35, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.-L.; Lo, Y.-C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zheng, Y.; Chen, R.; Wang, Y.; Zhong, Y.; Zhou, C.; Zhan, C.; Luo, J. Astragaloside IV alleviates sepsis-induced muscle atrophy by inhibiting the TGF-β1/Smad signaling pathway. Int. Immunopharmacol. 2023, 115, 109640. [Google Scholar] [CrossRef] [PubMed]
- Afroze, D.; Kumar, A. ER stress in skeletal muscle remodeling and myopathies. FEBS J. 2019, 286, 379–398. [Google Scholar] [CrossRef]
- Gallot, Y.S.; Bohnert, K.R. Confounding Roles of ER Stress and the Unfolded Protein Response in Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2021, 22, 2567. [Google Scholar] [CrossRef]
- Bohnert, K.R.; McMillan, J.D.; Kumar, A. Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell Physiol. 2018, 233, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Rayavarapu, S.; Coley, W.; Nagaraju, K. Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr. Rheumatol. Rep. 2012, 14, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Zito, E. Targeting ER stress/ER stress response in myopathies. Redox Biol. 2019, 26, 101232. [Google Scholar] [CrossRef] [PubMed]
- Deldicque, L.; Hespel, P.; Francaux, M. Endoplasmic Reticulum Stress in Skeletal Muscle: Origin and Metabolic Consequences. Exerc. Sport Sci. Rev. 2012, 40, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Yang, W.L.; Wang, P. Endoplasmic reticulum stress in sepsis. Shock 2015, 44, 294–304. [Google Scholar] [CrossRef]
- Wang, L.; Pang, X.; Li, S.; Li, W.; Chen, X.; Zhang, P. ER Stress is Activated and Involved in Disuse-Induced Muscle Atrophy. Front. Biosci. 2023, 28, 136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dai, H.; Chen, R.; Zhong, Y.; Zhou, C.; Wang, Y.; Zhan, C.; Luo, J. Endoplasmic reticulum stress promotes sepsis-induced muscle atrophy via activation of STAT3 and Smad3. J. Cell Physiol. 2023, 238, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Pallecchi, L.; Rossolini, G.M.; Travaglino, F.; Zanatta, P. Rationale and evidence for the adjunctive use of N-acetylcysteine in multidrug-resistant infections. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4316–4325. [Google Scholar] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Le, J.-W.; Sun, M.; Zhu, J.-H.; Fan, H. Protective effect of N-acetylcysteine on acute lung injury in septic rats by inhibiting inflammation, oxidation, and apoptosis. Iran. J. Basic. Med. Sci. 2022, 25, 859–864. [Google Scholar] [PubMed]
- Fan, H.; Le, J.W.; Zhu, J.H. Protective Effect of N-Acetylcysteine Pretreatment on Acute Kidney Injury in Septic Rats. J. Surg. Res. 2020, 254, 125–134. [Google Scholar] [CrossRef]
- Zhong, Y.; Guan, J.; Ma, Y.; Xu, M.; Cheng, Y.; Xu, L.; Lin, Y.; Zhang, X.; Wu, R. Role of Imaging Modalities and N-Acetylcysteine Treatment in Sepsis-Associated Encephalopathy. ACS Chem. Neurosci. 2023, 14, 2172–2182. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, B.; Wang, M.; Li, T.; Li, H.; Han, Q.; Liao, J.; Tang, Z. N-acetylcysteine alleviates oxidative stress and apoptosis and prevents skeletal muscle atrophy in type 1 diabetes mellitus through the NRF2/HO-1 pathway. Life Sci. 2023, 329, 121975. [Google Scholar] [CrossRef]
- Moroz, N.; Maes, K.; Leduc-Gaudet, J.-P.; Goldberg, P.; Petrof, B.J.; Mayaki, D.; Vassilakopoulos, T.; Rassier, D.; Gayan-Ramirez, G.; Hussain, S.N. Oxidants Regulated Diaphragm Proteolysis during Mechanical Ventilation in Rats. Anesthesiology 2019, 131, 605–618. [Google Scholar] [CrossRef]
- Galbes, O.; Bourret, A.; Nouette-Gaulain, K.; Pillard, F.; Matecki, S.; Py, G.; Mercier, J.; Capdevila, X.; Philips, A. N-acetylcysteine protects against bupivacaine-induced myotoxicity caused by oxidative and sarcoplasmic reticulum stress in human skeletal myotubes. Anesthesiology 2010, 113, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Huber-Lang, M.S.; A Flierl, M.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Kny, M.; Fielitz, J. Hidden Agenda—The Involvement of Endoplasmic Reticulum Stress and Unfolded Protein Response in Inflammation-Induced Muscle Wasting. Front. Immunol. 2022, 13, 878755. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.; Supinski, G.S. Sepsis-induced myopathy. Crit. Care Med. 2009, 37 (Suppl. S10), S354–S367. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, P.-O.; Menconi, M.J.; Fareed, M.U.; Yang, H.; Wei, W.; Evenson, A. Novel aspects on the regulation of muscle wasting in sepsis. Int. J. Biochem. Cell Biol. 2005, 37, 2156–2168. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The ubiquitin-proteasome system in regulation of the skeletal muscle homeostasis and atrophy: From basic science to disorders. J. Physiol. Sci. 2020, 70, 40. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Phogat, J.; Yadav, A.; Dabur, R. The dependency of autophagy and ubiquitin proteasome system during skeletal muscle atrophy. Biophys. Rev. 2021, 13, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, P.A.; Coyne, E.S.; Wing, S.S. The ubiquitin proteasome system in atrophying skeletal muscle: Roles and regulation. Am. J. Physiol. Cell Physiol. 2016, 311, C392–C403. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Chen, X.; Lee, A.-H.; Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010, 11, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; Chen, X.; Delmotte, P.; Sieck, G.C. TNFα selectively activates the IRE1α/XBP1 endoplasmic reticulum stress pathway in human airway smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L483–L493. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, J.; Wang, J.; Chen, M.; Xia, H.; Yao, S.; Zhang, D. SIRT1 ameliorated septic associated-lung injury and macrophages apoptosis via inhibiting endoplasmic reticulum stress. Cell. Signal. 2022, 97, 110398. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Li, C.; Wu, X.; He, C.; Zhu, X.; Zhao, H.; Mu, L. Toll-like receptor 4-mediated endoplasmic reticulum stress induces intestinal paneth cell damage in mice following CLP-induced sepsis. Sci. Rep. 2022, 12, 15256. [Google Scholar] [CrossRef]
- Li, F.; Lin, Q.; Shen, L.; Zhang, Z.; Wang, P.; Zhang, S.; Xing, Q.; Xia, Z.; Zhao, Z.; Zhang, Y.; et al. The diagnostic value of endoplasmic reticulum stress-related specific proteins GRP78 and CHOP in patients with sepsis: A diagnostic cohort study. Ann. Transl. Med. 2022, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [PubMed]
- Li, W.; Cao, T.; Luo, C.; Cai, J.; Zhou, X.; Xiao, X.; Liu, S. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 6129–6140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhang, Z.X.; Xu, Y.J.; Ni, W.; Chen, S.X.; Yang, Z.; Ma, D. N-Acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibited nuclear factor-κB activation in alveolar macrophages by different mechanisms. Acta Pharmacol. Sin. 2006, 27, 339–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferlito, M.; Wang, Q.; Fulton, W.B.; Colombani, P.M.; Marchionni, L.; Fox-Talbot, K.; Paolocci, N.; Steenbergen, C. Hydrogen sulfide increases survival during sepsis: Protective effect of CHOP inhibition. J. Immunol. 2014, 192, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Baulies, A.; Insausti-Urkia, N.; Alarcón-Vila, C.; Fucho, R.; Solsona-Vilarrasa, E.; Núñez, S.; Robles, D.; Ribas, V.; Wakefield, L.; et al. Endoplasmic Reticulum Stress-Induced Upregulation of STARD1 Promotes Acetaminophen-Induced Acute Liver Failure. Gastroenterology 2019, 157, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Engin, F.; Hotamisligil, G.S. Restoring endoplasmic reticulum function by chemical chaperones: An emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 2010, 12 (Suppl. S2), 108–115. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, P.; Yap, J.Q.; Dasgupta, D.; Sieck, G.C. Chemical Chaperone 4-PBA Mitigates Tumor Necrosis Factor Alpha-Induced Endoplasmic Reticulum Stress in Human Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 15816. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin—From Traditional Medicine to Anticancer Drug. Int. J. Mol. Sci. 2020, 22, 4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Zheng, Y.; Zhou, C.; Dai, H.; Wang, Y.; Chu, Y.; Luo, J. N-Acetylcysteine Attenuates Sepsis-Induced Muscle Atrophy by Downregulating Endoplasmic Reticulum Stress. Biomedicines 2024, 12, 902. https://doi.org/10.3390/biomedicines12040902
Chen R, Zheng Y, Zhou C, Dai H, Wang Y, Chu Y, Luo J. N-Acetylcysteine Attenuates Sepsis-Induced Muscle Atrophy by Downregulating Endoplasmic Reticulum Stress. Biomedicines. 2024; 12(4):902. https://doi.org/10.3390/biomedicines12040902
Chicago/Turabian StyleChen, Renyu, Yingfang Zheng, Chenchen Zhou, Hongkai Dai, Yurou Wang, Yun Chu, and Jinlong Luo. 2024. "N-Acetylcysteine Attenuates Sepsis-Induced Muscle Atrophy by Downregulating Endoplasmic Reticulum Stress" Biomedicines 12, no. 4: 902. https://doi.org/10.3390/biomedicines12040902
APA StyleChen, R., Zheng, Y., Zhou, C., Dai, H., Wang, Y., Chu, Y., & Luo, J. (2024). N-Acetylcysteine Attenuates Sepsis-Induced Muscle Atrophy by Downregulating Endoplasmic Reticulum Stress. Biomedicines, 12(4), 902. https://doi.org/10.3390/biomedicines12040902





