Therapeutic Drug Monitoring as a Tool for the Clinical Outcome Prediction in Vedolizumab-Treated Patients: An Italian Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- For Crohn’s disease, at least a 3-point decrease in Harvey–Bradshaw Index (HBI) from before treatment initiation or HBI ≤ 4 at the time of assessment;
- -
- For ulcerative colitis, a decrease of at least 2 points in partial MAYO score (PMS) or PMS ≤ 1.
2.2. Vedolizumab and Antibody–Anti Vedolizumab Quantification
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Actis, G.C.; Pellicano, R.; Fagoonee, S.; Ribaldone, D.G. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef] [PubMed]
- GBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Annese, M. Precision Medicine in Inflammatory Bowel Disease. Diagnostics 2023, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Rosso, C.; Stalla, F.; Rizzo, M.; Massano, A.; Abate, M.L.; Olivero, A.; Armandi, A.; Vanni, E.; Younes, R.; et al. On-Treatment Decrease of Serum Interleukin-6 as a Predictor of Clinical Response to Biologic Therapy in Patients with Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 800. [Google Scholar] [CrossRef]
- Bertani, L.; Trico, D.; Pugliese, D.; Privitera, G.; Linsalata, G.; Zanzi, F.; Gloria Mumolo, M.; Barberio, B.; Monzani, F.; Marchi, S.; et al. Serum triiodothyronine-to-thyroxine (T3/T4) ratio predicts therapeutic outcome to biological therapies in elderly IBD patients. Aliment. Pharmacol. Ther. 2020, 53, 273–280. [Google Scholar] [CrossRef]
- Bertani, L.; Rossari, F.; Barberio, B.; Demarzo, M.G.; Tapete, G.; Albano, E.; Baiano Svizzero, G.; Ceccarelli, L.; Mumolo, M.G.; Brombin, C.; et al. Novel Prognostic Biomarkers of Mucosal Healing in Ulcerative Colitis Patients Treated With Anti-TNF: Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio. Inflamm. Bowel Dis. 2020, 26, 1579–1587. [Google Scholar] [CrossRef]
- Dragoni, G.; Innocenti, T.; Galli, A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig. Dis. 2020, 39, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Bangma, A.; Voskuil, M.D.; Uniken Venema, W.T.C.; Brugge, H.; Hu, S.; Lanting, P.; Franke, L.; Dijkstra, G.; Festen, E.A.M.; Weersma, R.K. Predicted efficacy of a pharmacogenetic passport for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 51, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Zhang, L.Y.; Han, W.; Shao, Y.; Chen, M.; Ni, R.; Wang, G.N.; Wei, F.X.; Zhang, Y.W.; Xu, X.D.; et al. PRISMA--efficacy and safety of vedolizumab for inflammatory bowel diseases: A systematic review and meta-analysis of randomized controlled trials. Medicine 2014, 93, e326. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Ventimiglia, M.; Orlando, A. Effectiveness and Safety of Vedolizumab in Inflammatory Bowel Disease: A Comprehensive Meta-analysis of Observational Studies. J. Crohn’s Colitis 2023, 17, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Restellini, S.; Afif, W. Update on TDM (Therapeutic Drug Monitoring) with Ustekinumab, Vedolizumab and Tofacitinib in Inflammatory Bowel Disease. J. Clin. Med. 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Rosario, M.; Dirks, N.L.; Milch, C.; Parikh, A.; Bargfrede, M.; Wyant, T.; Fedyk, E.; Fox, I. A Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Vedolizumab. Clin. Pharmacokinet. 2017, 56, 1287–1301. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Baldelli, S.; Cozzi, V.; Clementi, E.; Marriott, D.J.E.; Gervasoni, C. Impact of Therapeutic Drug Monitoring of Antiretroviral Drugs in Routine Clinical Management of People Living with HIV: A Narrative Review. Ther. Drug Monit. 2019, 42, 64–74. [Google Scholar] [CrossRef]
- Punyawudho, B.; Singkham, N.; Thammajaruk, N.; Dalodom, T.; Kerr, S.J.; Burger, D.M.; Ruxrungtham, K. Therapeutic drug monitoring of antiretroviral drugs in HIV-infected patients. Expert. Rev. Clin. Pharmacol. 2016, 9, 1583–1595. [Google Scholar] [CrossRef]
- Cheifetz, A.S.; Abreu, M.T.; Afif, W.; Cross, R.K.; Dubinsky, M.C.; Loftus, E.V., Jr.; Osterman, M.T.; Saroufim, A.; Siegel, C.A.; Yarur, A.J.; et al. A Comprehensive Literature Review and Expert Consensus Statement on Therapeutic Drug Monitoring of Biologics in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 2014–2025. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-Lopez, O.; Centelles-Oria, M.; Marques-Minana, M.R.; Megias-Vericat, J.E.; Poveda-Andres, J.L. Proactive therapeutic drug monitoring and pharmacogenetic analysis in inflammatory bowel disease: A systematic review. Farm. Hosp. 2022, 45, 56–63. [Google Scholar]
- Mitrev, N.; Vande Casteele, N.; Seow, C.H.; Andrews, J.M.; Connor, S.J.; Moore, G.T.; Barclay, M.; Begun, J.; Bryant, R.; Chan, W.; et al. Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 46, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Heron, V.; Afif, W. Update on Therapeutic Drug Monitoring in Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Nguyen, G.C.; Kupfer, S.S.; Falck-Ytter, Y.; Singh, S. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017, 153, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Levartovsky, A.; Cohen, I.; Abitbol, C.M.; Yavzori, M.; Fudim, E.; Picard, O.; Kopylov, U.; Ben-Horin, S.; Ungar, B. Do Vedolizumab trough Levels Predict the Outcome of Subsequent Therapy in Inflammatory Bowel Disease? Biomedicines 2023, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.G.; Sparrow, M.P.; Roblin, X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: Current data and future directions. Therap Adv. Gastroenterol. 2018, 11, 1756284818772786. [Google Scholar] [CrossRef]
- Al-Bawardy, B.; Ramos, G.P.; Willrich, M.A.V.; Jenkins, S.M.; Park, S.H.; Aniwan, S.; Schoenoff, S.A.; Bruining, D.H.; Papadakis, K.A.; Raffals, L.; et al. Vedolizumab Drug Level Correlation With Clinical Remission, Biomarker Normalization, and Mucosal Healing in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 580–586. [Google Scholar] [CrossRef]
- Huttemann, E.; Muzalyova, A.; Grohl, K.; Nagl, S.; Fleischmann, C.; Ebigbo, A.; Classen, J.; Wanzl, J.; Prinz, F.; Mayr, P.; et al. Efficacy and Safety of Vedolizumab in Patients with Inflammatory Bowel Disease in Association with Vedolizumab Drug Levels. J. Clin. Med. 2024, 13. [Google Scholar] [CrossRef]
- Plevris, N.; Jenkinson, P.W.; Chuah, C.S.; Lyons, M.; Merchant, L.M.; Pattenden, R.J.; Arnott, I.D.; Jones, G.R.; Lees, C.W. Association of trough vedolizumab levels with clinical, biological and endoscopic outcomes during maintenance therapy in inflammatory bowel disease. Frontline Gastroenterol. 2020, 11, 117–123. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Williet, N.; Boschetti, G.; Fovet, M.; Di Bernado, T.; Claudez, P.; Del Tedesco, E.; Jarlot, C.; Rinaldi, L.; Berger, A.; Phelip, J.M.; et al. Association Between Low Trough Levels of Vedolizumab During Induction Therapy for Inflammatory Bowel Diseases and Need for Additional Doses Within 6 Months. Clin. Gastroenterol. Hepatol. 2016, 15, 1750–1757.e3. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, E.; Verstockt, B.; Bian, S.; de Bruyn, M.; Compernolle, G.; Tops, S.; Noman, M.; Van Assche, G.; Ferrante, M.; Gils, A.; et al. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2018, 16, 1937–1946.e8. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Pugliese, D.; Panici Tonucci, T.; Bertani, L.; Costa, F.; Privitera, G.; Tolusso, B.; Di Mario, C.; Albano, E.; Tapete, G.; et al. Early vedolizumab trough levels predict treatment persistence over the first year in inflammatory bowel disease. United European Gastroenterol. J. 2019, 7, 1189–1197. [Google Scholar] [CrossRef]
- Schulze, H.; Esters, P.; Hartmann, F.; Stein, J.; Christ, C.; Zorn, M.; Dignass, A. A prospective cohort study to assess the relevance of vedolizumab drug level monitoring in IBD patients. Scand. J. Gastroenterol. 2018, 53, 670–676. [Google Scholar] [CrossRef]

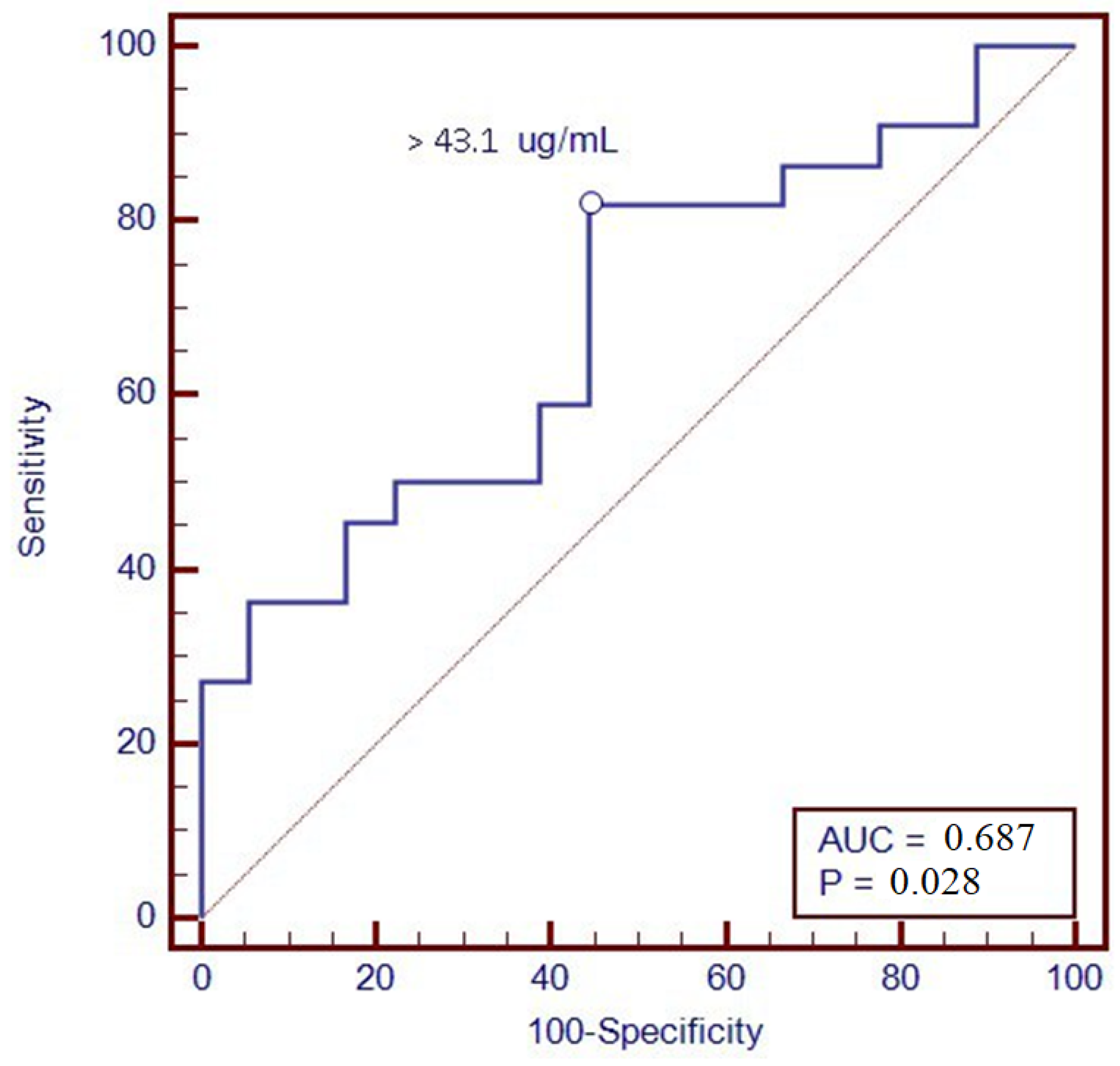
| Variables | |
|---|---|
| Male, n (%) | 22 (55) |
| Age, median (inter quartile range) | 53 (40.5–69.5) |
| BMI, mean (±SD) | 23 ± 3.6 |
| Active smokers, n (%) | 5 (12.5) |
| Ex-smokers, n (%) | 1 (2.5) |
| Ulcerative colitis, n (%) | 24 (60) |
| Type of colitis | E1—proctosigmoiditis, n (%) 5 (20.8) E2—left colitis, n (%) 3 (12.5) E3—extensive colitis, n (%) 16 (66.7) |
| Crohn’s disease, n (%) | 16 (40) |
| Type of Crohn’s disease | L1—ileal, n (%) 5 (31.3) L2—colic, n (%) 7 (43.7) L3—ileo-colic, n (%) 4 (25) |
| Years from diagnosis, median (inter quartile range) | 16.5 (11.5–21.5) |
| 5–aminosalicylic acid, n (%) | 24 (60) (all Ulcerative colitis) |
| Topical corticosteroids, n (%) | 1 (2.5) (all Ulcerative colitis) |
| Systemic corticosteroids, n (%) | 16 (40) (10 Ulcerative colitis, 6 Crohn’s disease) |
| Topical and systemic corticosteroids, n (%) | 7 (17.5) (all Ulcerative colitis) |
| Azathioprine, n (%) | 4 (10) (all Ulcerative colitis) |
| Variables | HBI | PMS | CRP | Hemoglobin | Fecal Calprotectin | Vedolizumab Concentrations |
|---|---|---|---|---|---|---|
| T0 mean (±SD)/median (inter quartile range) | 7.5 ± 4.4 | 4.9 ± 1.6 | 16 ± 40 | 12.2 ± 1.1 | 439 (244–1062) | |
| T6 mean (±SD)/median (inter quartile range) | 3.5 ± 3.2 | 2.5 ± 2.5 | 15 ± 37.5 | 12.7 ± 1.7 | 196 (28–489) | 79.3 µg/mL ± 51.3 |
| T12 mean (±SD)/median (inter quartile range) | 3.6 ± 3.1 | 1.5 ± 2 | 12 ± 34.3 | 12.8 ± 1.5 | 109 (15–312) |
| N/TOT (%) | |
|---|---|
| Corticosteroid-free clinical response T6 | 31/40 (77.5) |
| Corticosteroid-free clinical remission T6 | 25/40 (62.5) |
| Corticosteroid-free clinical response T12 | 27/35 (77.1) |
| Corticosteroid-free clinical remission T12 | 22/35 (62.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusato, J.; Ribaldone, D.G.; Falzone, M.H.; Manca, A.; Antonucci, M.; Palermiti, A.; Saracco, G.M.; Ceccarelli, L.; Costa, F.; Bottari, A.; et al. Therapeutic Drug Monitoring as a Tool for the Clinical Outcome Prediction in Vedolizumab-Treated Patients: An Italian Pilot Study. Biomedicines 2024, 12, 824. https://doi.org/10.3390/biomedicines12040824
Cusato J, Ribaldone DG, Falzone MH, Manca A, Antonucci M, Palermiti A, Saracco GM, Ceccarelli L, Costa F, Bottari A, et al. Therapeutic Drug Monitoring as a Tool for the Clinical Outcome Prediction in Vedolizumab-Treated Patients: An Italian Pilot Study. Biomedicines. 2024; 12(4):824. https://doi.org/10.3390/biomedicines12040824
Chicago/Turabian StyleCusato, Jessica, Davide Giuseppe Ribaldone, Michela Helga Falzone, Alessandra Manca, Miriam Antonucci, Alice Palermiti, Giorgio Maria Saracco, Linda Ceccarelli, Francesco Costa, Andrea Bottari, and et al. 2024. "Therapeutic Drug Monitoring as a Tool for the Clinical Outcome Prediction in Vedolizumab-Treated Patients: An Italian Pilot Study" Biomedicines 12, no. 4: 824. https://doi.org/10.3390/biomedicines12040824
APA StyleCusato, J., Ribaldone, D. G., Falzone, M. H., Manca, A., Antonucci, M., Palermiti, A., Saracco, G. M., Ceccarelli, L., Costa, F., Bottari, A., Fornaroli, G., Caviglia, G. P., D’Avolio, A., & Bertani, L. (2024). Therapeutic Drug Monitoring as a Tool for the Clinical Outcome Prediction in Vedolizumab-Treated Patients: An Italian Pilot Study. Biomedicines, 12(4), 824. https://doi.org/10.3390/biomedicines12040824









