PD-L1 Expression in Neoplastic and Immune Cells of Thymic Epithelial Tumors: Correlations with Disease Characteristics and HDAC Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
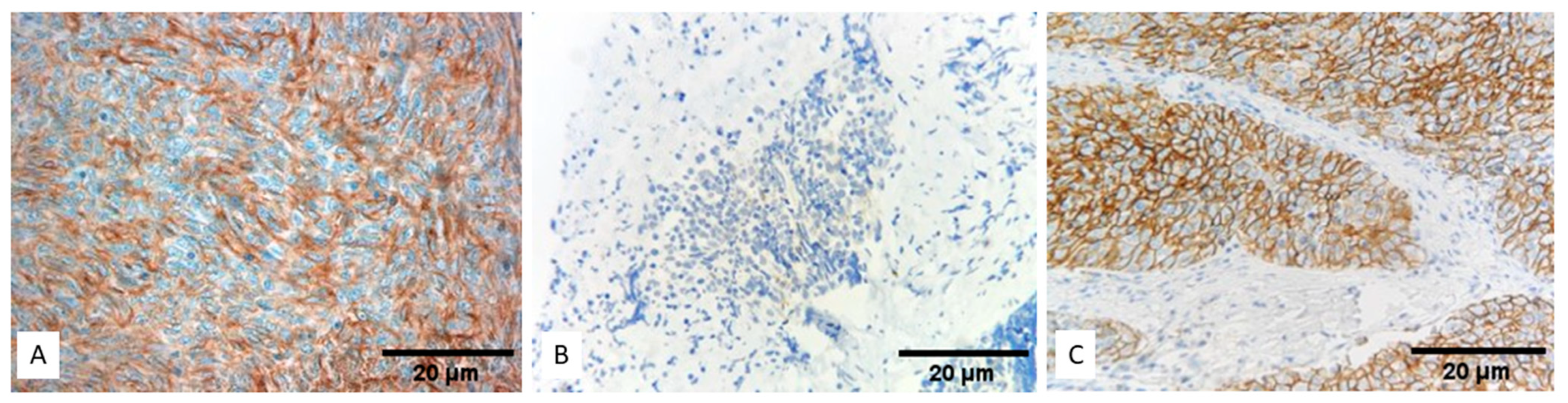
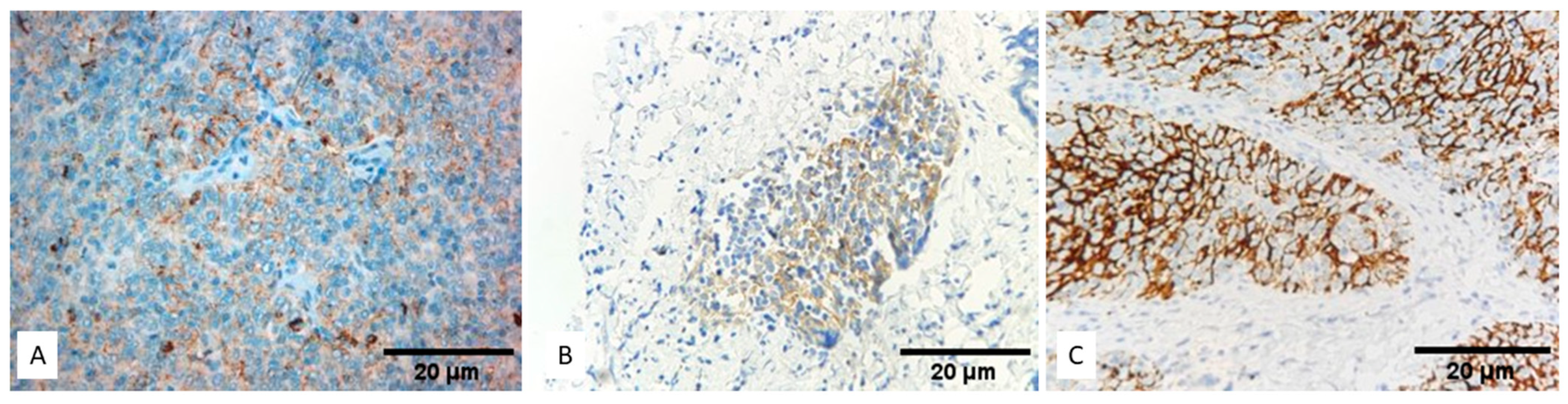
3.1. Expression of PD-L1 (SP263) in TETs
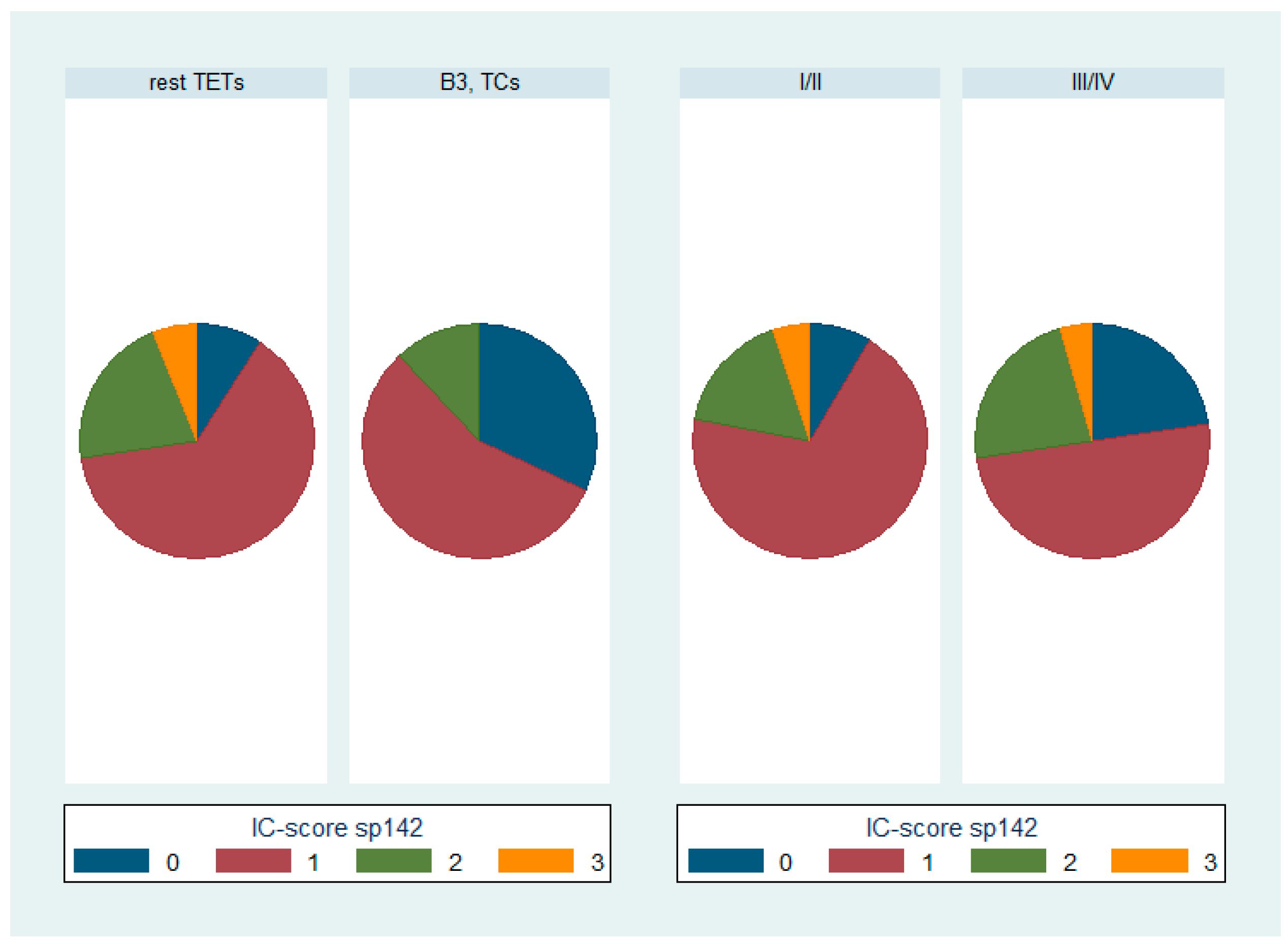
3.2. Expression of PD-L1 (SP142) in TETs
3.3. Comparison of PD-L1 (SP263) and PD-L1 (SP142)
3.4. Associations of PD-L1 Expression with HDAC-1, -2, -3, -4, -5 and -6
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, A.; Chan, J.K.C.; Chalabreysse, L.; Dacic, S.; Detterbeck, F.; French, C.A.; Hornick, J.L.; Inagaki, H.; Jain, D.; Lazar, A.J.; et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J. Thorac. Oncol. 2022, 17, 200–213. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jang, H.-J.; Shah, R.; Yoon, D.; Hamaji, M.; Wald, O.; Lee, J.-S.; Sugarbaker, D.J.; Burt, B.M. Genomic Analysis of Thymic Epithelial Tumors Identifies Novel Subtypes Associated with Distinct Clinical Features. Clin. Cancer Res. 2017, 23, 4855–4864. [Google Scholar] [CrossRef] [PubMed]
- Radovich, M.; Pickering, C.R.; Felau, I.; Ha, G.; Zhang, H.; Jo, H.; Hoadley, K.A.; Anur, P.; Zhang, J.; McLellan, M.; et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018, 33, 244–258.e210. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, T.I.; Brown, L.R.; Colby, T.V.; Harper, C.M., Jr.; Coles, D.T. Thymoma. Mayo Clin. Proc. 1993, 68, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Marchevsky, A.M.; Walts, A.E. PD-L1, PD-1, CD4, and CD8 expression in neoplastic and nonneoplastic thymus. Hum. Pathol. 2017, 60, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Honjo, T.; Minato, N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J. Exp. Med. 2000, 191, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Latchman, Y.E.; Freeman, G.J.; Sharpe, A.H. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J. Immunol. 2005, 175, 7372–7379. [Google Scholar] [CrossRef] [PubMed]
- Rouquette, I.; Taranchon-Clermont, E.; Gilhodes, J.; Bluthgen, M.V.; Perallon, R.; Chalabreysse, L.; De Muret, A.; Hofman, V.; Marx, A.; Parrens, M.; et al. Immune biomarkers in thymic epithelial tumors: Expression patterns, prognostic value and comparison of diagnostic tests for PD-L1. Biomark. Res. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Weissferdt, A.; Fujimoto, J.; Kalhor, N.; Rodriguez, J.; Bassett, R.; Wistuba, I.I.; Moran, C.A. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod. Pathol. 2017, 30, 826–833. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef]
- Salik, B.; Smyth, M.J.; Nakamura, K. Targeting immune checkpoints in hematological malignancies. J. Hematol. Oncol. 2020, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.D.; Vaibhav, G.P. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Dapergola, A.; Gomatou, G.; Trontzas, I.; Panagiotou, E.; Dimakakos, E.; Syrigos, N.; Kotteas, E. Emerging therapies in thymic epithelial tumors (Review). Oncol. Lett. 2023, 25, 84. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, A.C.; Brandão, M.; Berghmans, T.; Durieux, V.; Jungels, C. Immunotherapy and Targeted Therapies Efficacy in Thymic Epithelial Tumors: A Systematic Review. Biomedicines 2023, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Kim, C.; Thompson, J.; McGuire, C.; Kallakury, B.; Chahine, J.J.; Manning, M.; Mogg, R.; Blumenschein, W.M.; Tan, M.T.; et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol. 2018, 19, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Kim, C. Durable Response in Patients with Thymic Carcinoma Treated with Pembrolizumab After Prolonged Follow-Up. J. Thorac. Oncol. 2021, 16, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, J.-S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, V.; Coppedè, F. Epigenetics of Thymic Epithelial Tumors. Cancers 2023, 15, 360. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Huang, A. Aggresome-Autophagy Associated Gene HDAC6 Is a Potential Biomarker in Pan-Cancer, Especially in Colon Adenocarcinoma. Front. Oncol. 2021, 11, 718589. [Google Scholar] [CrossRef]
- Moran, B.; Davern, M.; Reynolds, J.V.; Donlon, N.E.; Lysaght, J. The impact of histone deacetylase inhibitors on immune cells and implications for cancer therapy. Cancer Lett. 2023, 559, 216121. [Google Scholar] [CrossRef] [PubMed]
- Knox, T.; Sahakian, E.; Banik, D.; Hadley, M.; Palmer, E.; Noonepalle, S.; Kim, J.; Powers, J.; Gracia-Hernandez, M.; Oliveira, V.; et al. Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci. Rep. 2019, 9, 6136. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.M.; Sodré, A.L.; Villagra, A.; Sarnaik, A.; Sotomayor, E.M.; Weber, J. HDAC Inhibition Upregulates PD-1 Ligands in Melanoma and Augments Immunotherapy with PD-1 Blockade. Cancer Immunol. Res. 2015, 3, 1375–1385. [Google Scholar] [CrossRef]
- Sun, T.; Li, Y.; Yang, W.; Wu, H.; Li, X.; Huang, Y.; Zhou, Y.; Du, Z. Histone deacetylase inhibition up-regulates MHC class I to facilitate cytotoxic T lymphocyte-mediated tumor cell killing in glioma cells. J. Cancer 2019, 10, 5638–5645. [Google Scholar] [CrossRef] [PubMed]
- Lienlaf, M.; Perez-Villarroel, P.; Knox, T.; Pabon, M.; Sahakian, E.; Powers, J.; Woan, K.V.; Lee, C.; Cheng, F.; Deng, S.; et al. Essential role of HDAC6 in the regulation of PD-L1 in melanoma. Mol. Oncol. 2016, 10, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Rajan, A.; Berman, A.; Kelly, R.J.; Szabo, E.; Lopez-Chavez, A.; Trepel, J.; Lee, M.J.; Cao, L.; Espinoza-Delgado, I.; et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J. Clin. Oncol. 2011, 29, 2052–2059. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Szabo, E.; Tomita, Y.; Carter, C.A.; Scepura, B.; Lopez-Chavez, A.; Lee, M.J.; Redon, C.E.; Frosch, A.; et al. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: A clinical and translational study. Clin. Cancer Res. 2014, 20, 5392–5402. [Google Scholar] [CrossRef] [PubMed]
- Palamaris, K.; Tzimou, L.-M.; Levidou, G.; Masaoutis, C.; Theochari, I.; Rontogianni, D.; Theocharis, S. Histone Deacetylases (HDACs): Promising Biomarkers and Potential Therapeutic Targets in Thymic Epithelial Tumors. Int. J. Mol. Sci. 2023, 24, 4263. [Google Scholar] [CrossRef]
- Ishibashi, H.; Suzuki, T.; Suzuki, S.; Moriya, T.; Kaneko, C.; Takizawa, T.; Sunamori, M.; Handa, M.; Kondo, T.; Sasano, H. Sex Steroid Hormone Receptors in Human Thymoma. J. Clin. Endocrinol. Metab. 2003, 88, 2309–2317. [Google Scholar] [CrossRef]
- Akhtar, M.; Rashid, S.; Al-Bozom, I.A. PD−L1 immunostaining: What pathologists need to know. Diagn. Pathol. 2021, 16, 94. [Google Scholar] [CrossRef]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zheng, P. Tumor cells versus host immune cells: Whose PD-L1 contributes to PD-1/PD-L1 blockade mediated cancer immunotherapy? Cell Biosci. 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Fundytus, A.; Booth, C.M.; Tannock, I.F. How low can you go? PD-L1 expression as a biomarker in trials of cancer immunotherapy. Ann. Oncol. 2021, 32, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Padda, S.K.; Riess, J.W.; Schwartz, E.J.; Tian, L.; Kohrt, H.E.; Neal, J.W.; West, R.B.; Wakelee, H.A. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J. Thorac. Oncol. 2015, 10, 500–508. [Google Scholar] [CrossRef]
- Arbour, K.C.; Naidoo, J.; Steele, K.E.; Ni, A.; Moreira, A.L.; Rekhtman, N.; Robbins, P.B.; Karakunnel, J.; Rimner, A.; Huang, J.; et al. Expression of PD-L1 and other immunotherapeutic targets in thymic epithelial tumors. PLoS ONE 2017, 12, e0182665. [Google Scholar] [CrossRef]
- Higuchi, R.; Goto, T.; Hirotsu, Y.; Nakagomi, T.; Yokoyama, Y.; Otake, S.; Amemiya, K.; Oyama, T.; Omata, M. PD-L1 Expression and Tumor-Infiltrating Lymphocytes in Thymic Epithelial Neoplasms. J. Clin. Med. 2019, 8, 1833. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Chai, X.; Gao, J.; Chen, G.; Zhang, W.; Zhang, Y. Correlation between the Expression of PD-L1 and Clinicopathological Features in Patients with Thymic Epithelial Tumors. Biomed. Res. Int. 2018, 2018, 5830547. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Chu, B.; Lehman, A.M.; Annamalai, L.; Yearley, J.H.; Shilo, K.; Otterson, G.A. Expression Patterns, Prognostic Value, and Intratumoral Heterogeneity of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2018, 13, 1204–1212. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, K.U.; Kim, A.; Lee, S.J.; Lee, J.H.; Suh, D.S.; Kwon, B.-S.; Hwang, C. PD-L1 expression on stromal tumor-infiltrating lymphocytes is a favorable prognostic factor in ovarian serous carcinoma. J. Ovarian Res. 2019, 12, 56. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2016, 141, 293–302. [Google Scholar] [CrossRef]
- Pulko, V.; Harris, K.J.; Liu, X.; Gibbons, R.M.; Harrington, S.M.; Krco, C.J.; Kwon, E.D.; Dong, H. B7-h1 expressed by activated CD8 T cells is essential for their survival. J. Immunol. 2011, 187, 5606–5614. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Miyoshi, H. Comparison of PD-L1 immunohistochemical assays and the significance of PD-L1 expression in thymoma. J. Thorac. Dis. 2020, 12, 7553–7560. [Google Scholar] [CrossRef]
- Terra, S.B.S.P.; Mansfield, A.S.; Vrana, J.A.; Roden, A.C. Heterogeneity of programmed death-ligand 1 expression in thymic epithelial tumours between initial specimen and synchronous or metachronous metastases or recurrences. Histopathology 2019, 74, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, Y.; Horinouchi, H.; Asao, T.; Kitahara, S.; Goto, Y.; Kanda, S.; Fujiwara, Y.; Nokihara, H.; Yamamoto, N.; Watanabe, S.-i.; et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer 2016, 99, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflamm. 2018, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, X.; Xu, L.; Li, Y.; Zeng, C. Current insight into the regulation of PD-L1 in cancer. Exp. Hematol. Oncol. 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, D.; Markovic, J.; Ceriman, V.; Peric, J.; Pavlovic, S.; Soldatovic, I. Correlation of genomic alterations and PD-L1 expression in thymoma. J. Thorac. Dis. 2020, 12, 7561–7570. [Google Scholar] [CrossRef]
- Umemura, S.; Zhu, J.; Chahine, J.J.; Kallakury, B.; Chen, V.; Kim, I.K.; Zhang, Y.W.; Goto, K.; He, Y.; Giaccone, G. Downregulation of CYLD promotes IFN-γ mediated PD-L1 expression in thymic epithelial tumors. Lung Cancer 2020, 147, 221–228. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhang, R.; Jin, T.; Qu, L.; Jin, Q.; Zheng, J.; Sun, J.; Wu, Z.; Wang, L.; et al. HDAC10 Is Positively Associated with PD-L1 Expression and Poor Prognosis in Patients with NSCLC. Front. Oncol. 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, L.; Liu, J.; Yang, L.; Xue, H.; Bai, S.; Wang, K. PD-L1 combined with HDAC9 is a useful prognostic predictor in hepatocellular carcinoma. Transl. Cancer Res. 2021, 10, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, X.; Yu, H.; Qin, G.; Pan, P.; Zhao, J.; Chen, T.; Liang, X.; Sun, Y.; Wang, B.; et al. HDAC5 modulates PD-L1 expression and cancer immunity via p65 deacetylation in pancreatic cancer. Theranostics 2022, 12, 2080–2094. [Google Scholar] [CrossRef] [PubMed]
- Antonangeli, F.; Natalini, A.; Garassino, M.C.; Sica, A.; Santoni, A.; Di Rosa, F. Regulation of PD-L1 Expression by NF-κB in Cancer. Front. Immunol. 2020, 11, 584626. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fischle, W.; Verdin, E.; Greene, W.C. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 2001, 293, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, R.; Brès, V.; Ng, R.W.; Coudart, M.P.; El Messaoudi, S.; Sardet, C.; Jin, D.Y.; Emiliani, S.; Benkirane, M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 2003, 278, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Krämer, O.H.; Knauer, S.K.; Greiner, G.; Jandt, E.; Reichardt, S.; Gührs, K.H.; Stauber, R.H.; Böhmer, F.D.; Heinzel, T. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes. Dev. 2009, 23, 223–235. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Guan, Y.J.; Chatterjee, D.; Chin, Y.E. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science 2005, 307, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, Y.; Zhang, J.; Zhang, B. PD-L1 expression increased by IFN-γ via JAK2-STAT1 signaling and predicts a poor survival in colorectal cancer. Oncol. Lett. 2020, 20, 1127–1134. [Google Scholar] [CrossRef]
- Iwasa, M.; Harada, T.; Oda, A.; Bat-Erdene, A.; Teramachi, J.; Tenshin, H.; Ashtar, M.; Oura, M.; Sogabe, K.; Udaka, K.; et al. PD-L1 upregulation in myeloma cells by panobinostat in combination with interferon-γ. Oncotarget 2019, 10, 1903–1917. [Google Scholar] [CrossRef]
- Won, H.R.; Lee, D.H.; Yeon, S.K.; Ryu, H.W.; Kim, G.W.; Kwon, S.H. HDAC6-selective inhibitor synergistically enhances the anticancer activity of immunomodulatory drugs in multiple myeloma. Int. J. Oncol. 2019, 55, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Hegedűs, L.; Rittler, D.; Garay, T.; Stockhammer, P.; Kovács, I.; Döme, B.; Theurer, S.; Hager, T.; Herold, T.; Kalbourtzis, S.; et al. HDAC Inhibition Induces PD-L1 Expression in a Novel Anaplastic Thyroid Cancer Cell Line. Pathol. Oncol. Res. 2020, 26, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Zhang, X.L.; Liu, Z.X.; Zhao, J.J.; Pan, Q.Z.; Wen, X.Z.; Xiao, W.; Xu, B.S.; Hong, D.C.; Guo, T.H.; et al. Frequent amplification of HDAC genes and efficacy of HDAC inhibitor chidamide and PD-1 blockade combination in soft tissue sarcoma. J. Immunother. Cancer 2021, 9, e001696. [Google Scholar] [CrossRef] [PubMed]
- Terranova-Barberio, M.; Thomas, S.; Ali, N.; Pawlowska, N.; Park, J.; Krings, G.; Rosenblum, M.D.; Budillon, A.; Munster, P.N. HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget 2017, 8, 114156–114172. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chang, H.; Yang, W.; Lu, Y.; Hu, J.; Jin, S. A novel IFNα-induced long noncoding RNA negatively regulates immunosuppression by interrupting H3K27 acetylation in head and neck squamous cell carcinoma. Mol. Cancer 2020, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, X.; Min, Z.; Shadia, A.S.; Yang, S.; Liu, X. MGCD0103 induces apoptosis and simultaneously increases the expression of NF-κB and PD-L1 in classical Hodgkin’s lymphoma. Exp. Ther. Med. 2018, 16, 3827–3834. [Google Scholar] [CrossRef] [PubMed]
- Briere, D.; Sudhakar, N.; Woods, D.M.; Hallin, J.; Engstrom, L.D.; Aranda, R.; Chiang, H.; Sodré, A.L.; Olson, P.; Weber, J.S.; et al. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol. Immunother. 2018, 67, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, H.; Anraku, T.; Kazama, A.; Shirono, Y.; Bilim, V.; Tomita, Y. Histone deacetylase 6 inhibition in urothelial cancer as a potential new strategy for cancer treatment. Oncol. Lett. 2021, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, K.; Powers, J.J.; Mediavilla-Varela, M.; Achille, A.; Gamal, W.; Quayle, S.; Jones, S.S.; Sahakian, E.; Pinilla-Ibarz, J. HDAC6 Inhibition Alleviates CLL-Induced T-Cell Dysfunction and Enhances Immune Checkpoint Blockade Efficacy in the Eμ-TCL1 Model. Front. Immunol. 2020, 11, 590072. [Google Scholar] [CrossRef]
- Han, R.; Ling, C.; Wang, Y.; Lu, L. Enhancing HCC Treatment: Innovatively combining HDAC2 inhibitor with PD-1/PD-L1 inhibition. Cancer Cell Int. 2023, 23, 203. [Google Scholar] [CrossRef]
- Deng, S.; Hu, Q.; Zhang, H.; Yang, F.; Peng, C.; Huang, C. HDAC3 Inhibition Upregulates PD-L1 Expression in B-Cell Lymphomas and Augments the Efficacy of Anti–PD-L1 Therapy. Mol. Cancer Ther. 2019, 18, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Xu, Q.R.; Kaul, D.; Ismail, M.; Badakhshi, H. Significance of tumor mutation burden and immune infiltration in thymic epithelial tumors. Thorac. Cancer 2021, 12, 1995–2006. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Median | Range |
|---|---|---|
| Age (years) | 62 | 27–88 |
| Number | % | |
| Gender | ||
| Male | 39/91 | 43% |
| Female | 52/91 | 57% |
| WHO subtypes | ||
| Type A | 12/91 | 13.6% |
| Type AB | 19/91 | 20.8% |
| Type B1 | 14/91 | 15.4% |
| Type B2 | 19/91 | 20.8% |
| Type B3 | 14/91 | 15.4% |
| Micronodular with lymphoid stroma | 2/91 | 2.2% |
| Type C | 11/91 | 12.1% |
| Masaoka–Koga stage | ||
| I | 15/91 | 16.5% |
| IIa | 36/91 | 39.6% |
| IIb | 16/91 | 17.6% |
| III | 18/91 | 19.8% |
| IVa | 3/91 | 3.2% |
| IVb | 3/91 | 3.2% |
| TAMG | 35/59 | 59.3% |
| Adjuvant/neoadjuvant treatment | ||
| Chemotherapy | 11/39 | 28% |
| Radiotherapy | 19/38 | 50% |
| Survival outcomes | ||
| Alive | 29/40, follow-up 5–134 months | 72.5% |
| Dead of disease | 11/40, within 7–65 months | 27.5% |
| Relapse | 4/35, within 58–65 months | 11% |
| PD-L1 (SP263) | PD-L1 (SP142) | |
|---|---|---|
| TPS < 1% | 49/91 (53.8%) | 41/91 (45%) |
| TPS ≥ 1% | 42/91 (46.2%) | 50/91 (55%) |
| IC-score = 0 | 52/91 (57.0%) | 14/91 (15.4%) |
| IC-score ≥ 1 | 39/91 (43.0%) | 77/91 (84.6%) |
| PD-L1 (SP142) TPS | PD-L1 (SP263) TPS | |
|---|---|---|
| Nuclear HDAC-1 H-score | rho = 0.01 | rho = −0.08 |
| p = 0.954 | p = 0.597 | |
| Cytoplasmic HDAC-1 H-score | rho = 0.06 | rho = −0.05 |
| p = 0.684 | p = 0.735 | |
| HDAC-2 H-score | rho = 0.24 | rho = 0.18 |
| p = 0.093 | p = 0.408 | |
| Nuclear HDAC-3 H-score | rho = −0.04 | rho = −0.07 |
| p = 0.768 | p = 0.627 | |
| Cytoplasmic HDAC-3 H-score | rho = 0.32 | rho = 0.30 |
| p = 0.091 | p = 0.034 * | |
| HDAC-4 H-score | rho = 0.36 | rho = 0.33 |
| p = 0.009 * | p = 0.016 * | |
| Nuclear HDAC-5 H-score | rho = 0.19 | rho = 0.27 |
| p = 0.170 | p = 0.0508 | |
| Cytoplasmic HDAC-5 H-score | rho = 0.31 | rho = 0.47 |
| p = 0.026 * | p < 0.001 * | |
| HDAC-6 H-score | rho = 0.02 | rho = 0.16 |
| p = 0.907 | p = 0.253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stergiou, I.E.; Palamaris, K.; Levidou, G.; Tzimou, M.; Papadakos, S.P.; Mandrakis, G.; Masaoutis, C.; Rontogianni, D.; Theocharis, S. PD-L1 Expression in Neoplastic and Immune Cells of Thymic Epithelial Tumors: Correlations with Disease Characteristics and HDAC Expression. Biomedicines 2024, 12, 772. https://doi.org/10.3390/biomedicines12040772
Stergiou IE, Palamaris K, Levidou G, Tzimou M, Papadakos SP, Mandrakis G, Masaoutis C, Rontogianni D, Theocharis S. PD-L1 Expression in Neoplastic and Immune Cells of Thymic Epithelial Tumors: Correlations with Disease Characteristics and HDAC Expression. Biomedicines. 2024; 12(4):772. https://doi.org/10.3390/biomedicines12040772
Chicago/Turabian StyleStergiou, Ioanna E., Kostas Palamaris, Georgia Levidou, Maria Tzimou, Stavros P. Papadakos, Georgios Mandrakis, Christos Masaoutis, Dimitra Rontogianni, and Stamatios Theocharis. 2024. "PD-L1 Expression in Neoplastic and Immune Cells of Thymic Epithelial Tumors: Correlations with Disease Characteristics and HDAC Expression" Biomedicines 12, no. 4: 772. https://doi.org/10.3390/biomedicines12040772
APA StyleStergiou, I. E., Palamaris, K., Levidou, G., Tzimou, M., Papadakos, S. P., Mandrakis, G., Masaoutis, C., Rontogianni, D., & Theocharis, S. (2024). PD-L1 Expression in Neoplastic and Immune Cells of Thymic Epithelial Tumors: Correlations with Disease Characteristics and HDAC Expression. Biomedicines, 12(4), 772. https://doi.org/10.3390/biomedicines12040772










