Short-Term Periodic Fasting Reduces Ischemia-Induced Necrosis in Musculocutaneous Flap Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. PF Regimen
2.3. Anesthesia
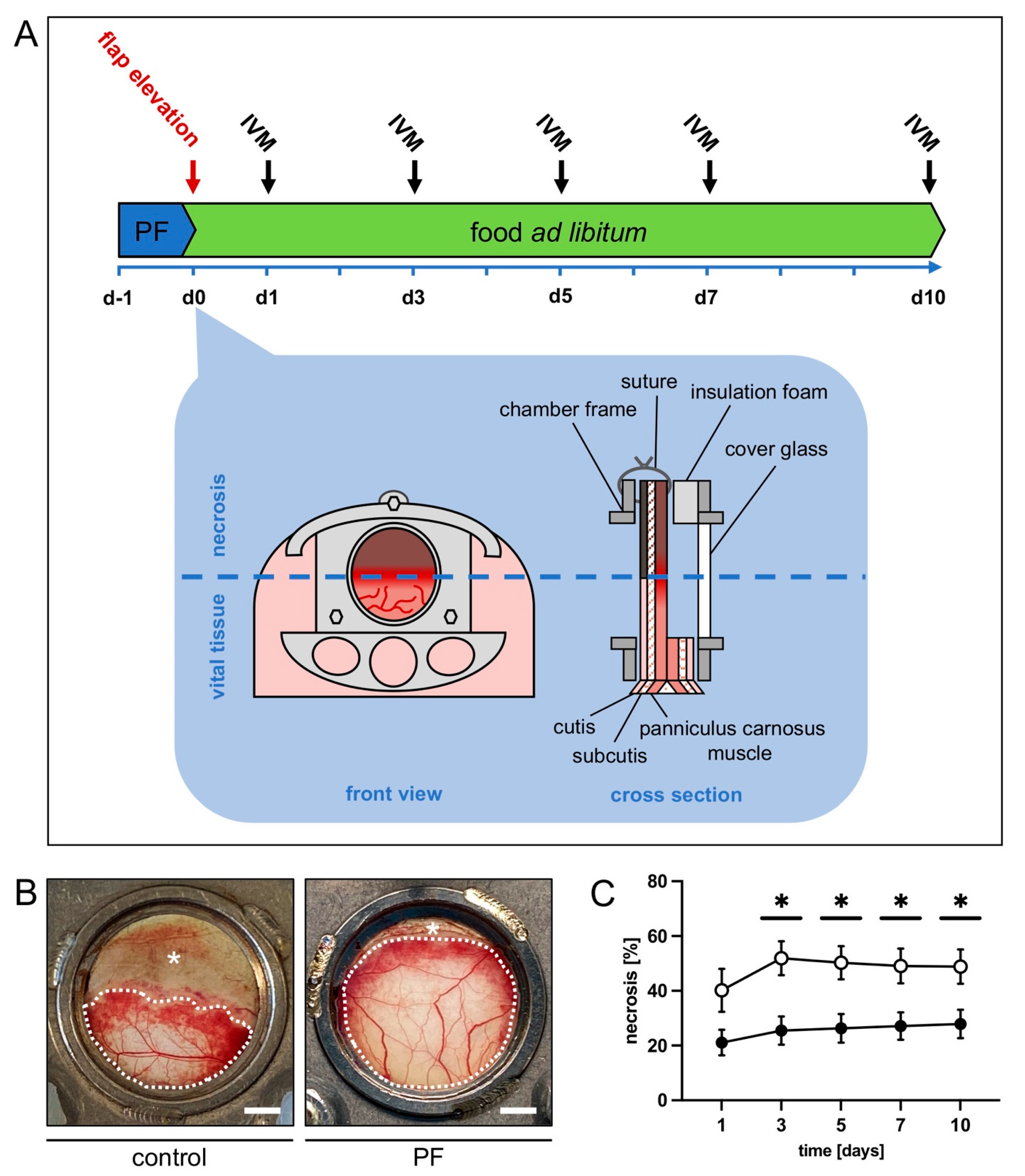
2.4. Dorsal Skinfold Chamber Flap Model
2.5. Intravital Fluorescence Microscopy
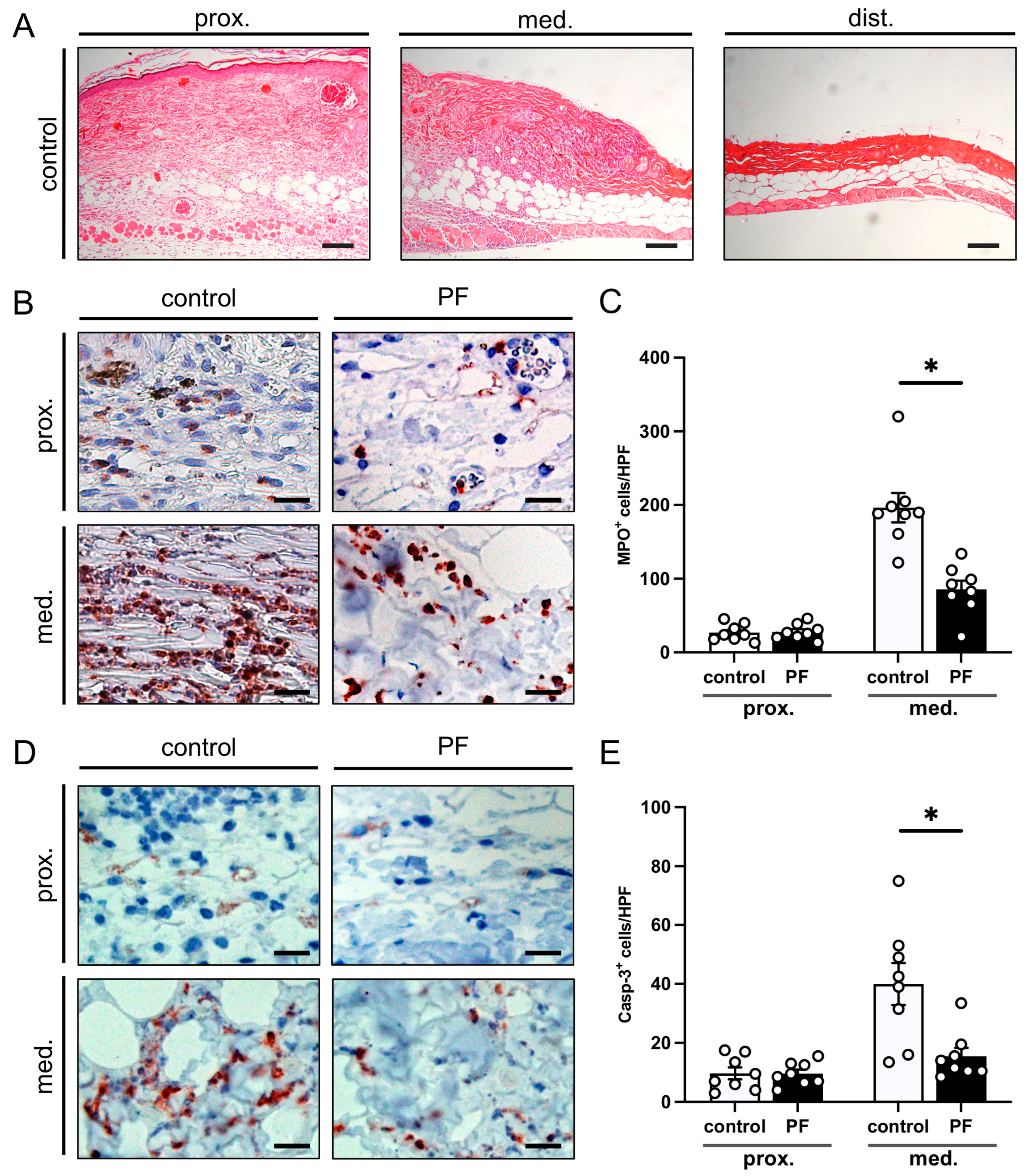
2.6. Histology and Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Intravital Fluorescence Microscopy
3.2. Histological and Immunohistochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, I.; Abe, Y.; Ishida, S.; Kashiwagi, K.; Mineda, K.; Yamashita, Y.; Yamato, R.; Toda, A.; Fukunaga, Y.; Yoshimoto, S.; et al. Development of Skin Flaps for Reconstructive Surgery: Random Pattern Flap to Perforator Flap. J. Med. Investig. 2016, 63, 159–162. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sheikh, R.; Blohmé, J.; Torbrand, C.; Malmsjö, M. Perfusion and Oxygenation of Random Advancement Skin Flaps Depend More on the Length and Thickness of the Flap Than on the Width to Length Ratio. Eplasty 2016, 16, e12. [Google Scholar]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The Perforasome Theory: Vascular Anatomy and Clinical Implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Choi, M.; Karp, N.S. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation Using Magnetic Resonance Imaging. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1439. [Google Scholar] [CrossRef]
- Schmauss, D.; Weinzierl, A.; Weiss, F.; Egaña, J.T.T.; Rezaeian, F.; Hopfner, U.; Schmauss, V.; Machens, H.G.; Harder, Y. Long-Term Pre- and Postconditioning with Low Doses of Erythropoietin Protects Critically Perfused Musculocutaneous Tissue from Necrosis. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 590–599. [Google Scholar] [CrossRef]
- Harder, Y.; Contaldo, C.; Klenk, J.; Banic, A.; Jakob, S.M.; Erni, D. Improved Skin Flap Survival after Local Heat Preconditioning in Pigs. Proc. J. Surg. Res. 2004, 119, 100–105. [Google Scholar] [CrossRef]
- Reinisch, J.F. The Pathophysiology of Skin Flap Circulation: The Delay Phenomenon. Plast. Reconstr. Surg. 1974, 54, 585–598. [Google Scholar] [CrossRef]
- Ribuffo, D.; Muratori, L.; Antoniadou, K.; Fanini, F.; Martelli, E.; Marini, M.; Messineo, D.; Trinci, M.; Scuderi, N. A Hemodynamic Approach to Clinical Results in the TRAM Flap after Selective Delay. Plast. Reconstr. Surg. 1997, 99, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Harputlugil, E.; Mitchell, J.R.; Longo, V.D. Protective Effects of Short-Term Dietary Restriction in Surgical Stress and Chemotherapy. Ageing Res. Rev. 2017, 39, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent Fasting in Type 2 Diabetes Mellitus and the Risk of Hypoglycaemia: A Randomized Controlled Trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Crupi, A.N.; Haase, J.; Brandhorst, S.; Longo, V.D. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr. Diab. Rep. 2020, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Mitchell, S.E. Caloric Restriction. Mol. Aspects Med. 2011, 32, 159–221. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Dietary Restriction, Growth Factors and Aging: From Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric Restriction and Aging: Studies in Mice and Monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef]
- Robertson, L.T.; Mitchell, J.R. Benefits of Short-Term Dietary Restriction in Mammals. Exp. Gerontol. 2013, 48, 1043–1048. [Google Scholar] [CrossRef]
- Müller, H.; De Toledo, F.W.; Resch, K.L. Fasting Followed by Vegetarian Diet in Patients with Rheumatoid Arthritis: A Systematic Review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar]
- Godar, R.J.; Ma, X.; Liu, H.; Murphy, J.T.; Weinheimer, C.J.; Kovacs, A.; Crosby, S.D.; Saftig, P.; Diwan, A. Repetitive Stimulation of Autophagy-Lysosome Machinery by Intermittent Fasting Preconditions the Myocardium to Ischemia-Reperfusion Injury. Autophagy 2015, 11, 1537–1560. [Google Scholar] [CrossRef]
- Jongbloed, F.; Saat, T.C.; Verweij, M.; Payan-Gomez, C.; Hoeijmakers, J.H.J.; Van Den Engel, S.; Van Oostrom, C.T.; Ambagtsheer, G.; Imholz, S.; Pennings, J.L.A.; et al. A Signature of Renal Stress Resistance Induced by Short-Term Dietary Restriction, Fasting, and Protein Restriction. Sci. Rep. 2017, 7, 40901. [Google Scholar] [CrossRef]
- Fann, D.Y.W.; Ng, G.Y.Q.; Poh, L.; Arumugam, T.V. Positive Effects of Intermittent Fasting in Ischemic Stroke. Exp. Gerontol. 2017, 89, 93–102. [Google Scholar] [CrossRef]
- Mauro, C.R.; Tao, M.; Yu, P.; Treviño-Villerreal, J.H.; Longchamp, A.; Kristal, B.S.; Ozaki, C.K.; Mitchell, J.R. Preoperative Dietary Restriction Reduces Intimal Hyperplasia and Protects from Ischemia-Reperfusion Injury. J. Vasc. Surg. 2016, 63, 500–509.e1. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Müller, C.; de Jong, M.; et al. Short-Term Dietary Restriction and Fasting Precondition against Ischemia Reperfusion Injury in Mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef]
- Schneider, C.A.; Taegtmeyer, H. Fasting in Vivo Delays Myocardial Cell Damage after Brief Periods of Ischemia in the Isolated Working Rat Heart. Circ. Res. 1991, 68, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.; Harder, Y.; Menger, M.; Laschke, M. Perioperative Intermittent Fasting Protects Ischemic Musculocutaneous Flap Tissue from Necrosis. Plast. Reconstr. Surg. 2023, 151, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Harder, Y.; Amon, M.; Erni, D.; Menger, M.D. Evolution of Ischemic Tissue Injury in a Random Pattern Flap: A New Mouse Model Using Intravital Microscopy. J. Surg. Res. 2004, 121, 197–205. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Verbeuren, T.J.; Vallez, M.O.; Lameire, N.H.; De Buyzere, M.; Vanhoutte, P.M. Off-Line Analysis of Red Blood Cell Velocity in Renal Arterioles. J. Vasc. Res. 2000, 37, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Wayland, H. On-Line Volume Flow Rate and Velocity Profile Measurement for Blood in Microvessels. Microvasc. Res. 1974, 7, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Menger, M.D.; Lehr, H.A. Scope and Perspectives of Intravital Microscopy—Bridge over from in Vitro to in Vivo. Immunol. Today 1993, 14, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. 2015, 70, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Shi, Y.; Van Remmen, H. The Effects of Dietary Restriction on Oxidative Stress in Rodents. Free Radic. Biol. Med. 2014, 66, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, F.; De Bruin, R.W.F.; Pennings, J.L.A.; Payán-Gómez, C.; Van Den Engel, S.; Van Oostrom, C.T.; De Bruin, A.; Hoeijmakers, J.H.J.; Van Steeg, H.; IJzermans, J.N.M.; et al. Preoperative Fasting Protects against Renal Ischemia-Reperfusion Injury in Aged and Overweight Mice. PLoS ONE 2014, 9, e100853. [Google Scholar] [CrossRef]
- Chaix, A.; Deota, S.; Bhardwaj, R.; Lin, T.; Panda, S. Sex- and Age-Dependent Outcomes of 9-Hour Time-Restricted Feeding of a Western High-Fat High-Sucrose Diet in C57BL/6J Mice. Cell Rep. 2021, 36, 109543. [Google Scholar] [CrossRef]
- Mair, W.; Goymer, P.; Pletcher, S.D.; Partridge, L. Demography of Dietary Restriction and Death in Drosophila. Science 2003, 301, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Demont, Y.; Castoldi, F.; Enot, D.; Durand, S.; Semeraro, M.; Baracco, E.E.; Pol, J.; Bravo-San Pedro, J.M.; Bordenave, C.; et al. Metabolic Effects of Fasting on Human and Mouse Blood in Vivo. Autophagy 2017, 13, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Michalsen, A.; Li, C. Fasting Therapy for Treating and Preventing Disease—Current State of Evidence. Forsch. Komplementarmed. 2013, 20, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-Dependent Differential Stress Resistance Protects Normal but Not Cancer Cells against High-Dose Chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220. [Google Scholar] [CrossRef] [PubMed]
- Tinkum, K.L.; Stemler, K.M.; White, L.S.; Loza, A.J.; Jeter-Jones, S.; Michalski, B.M.; Kuzmicki, C.; Pless, R.; Stappenbeck, T.S.; Piwnica-Worms, D.; et al. Fasting Protects Mice from Lethal DNA Damage by Promoting Small Intestinal Epithelial Stem Cell Survival. Proc. Natl. Acad. Sci. USA 2015, 112, E7148–E7154. [Google Scholar] [CrossRef] [PubMed]
- Hardiany, N.S.; Karman, A.P.; Calista, A.S.P.; Anindyanari, B.G.; Rahardjo, D.E.; Novira, P.R.; Taufiq, R.R.; Imtiyaz, S.; Antarianto, R.D. The Effect of Fasting on Oxidative Stress in the Vital Organs of New Zealand White Rabbit. Rep. Biochem. Mol. Biol. 2022, 11, 190–199. [Google Scholar] [PubMed]
- Chiba, T.; Ezaki, O. Dietary Restriction Suppresses Inflammation and Delays the Onset of Stroke in Stroke-Prone Spontaneously Hypertensive Rats. Biochem. Biophys. Res. Commun. 2010, 399, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Carden, D.L.; Smith, J.K.; Korthuis, R.J. Neutrophil-Mediated Microvascular Dysfunction in Postischemic Canine Skeletal Muscle. Role of Granulocyte Adherence. Circ. Res. 1990, 66, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Puhr-Westerheide, D.; Schink, S.J.; Fabritius, M.; Mittmann, L.; Hessenauer, M.E.T.; Pircher, J.; Zuchtriegel, G.; Uhl, B.; Holzer, M.; Massberg, S.; et al. Neutrophils Promote Venular Thrombosis by Shaping the Rheological Environment for Platelet Aggregation. Sci. Rep. 2019, 9, 15932. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Phillips, T.J. Nutrition and Cutaneous Wound Healing. Clin. Dermatol. 2022, 40, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Langkamp-Henken, B.; Kudsk, K.A.; Proctor, K.G. Fasting-Induced Reduction of Intestinal Reperfusion Injury. J. Parenter. Enter. Nutr. 1995, 19, 127–132. [Google Scholar] [CrossRef] [PubMed]


| Volumetric Blood Flow [pL/s] | d1 | d3 | d5 | d7 | d10 | |
|---|---|---|---|---|---|---|
| Arterioles | ||||||
| prox. control | 348 ± 53 | 404 ± 44 | 554 ± 91 | 767 ± 130 | 1019 ± 150 | |
| PF | 643 ± 126 * | 1165 ± 178 * | 1438 ± 282 * | 1637 ± 209 * | 1812 ± 178 * | |
| med. control | 325 ± 98 | 389 ± 104 | 530 ± 124 | 770 ± 203 | 1087 ± 247 | |
| PF | 742 ± 149 * | 1367 ± 230 * | 1523 ± 289 * | 1907 ± 301 * | 2000 ± 269 * | |
| dist. control | 271 ± 45 | 341 ± 193 | 603 ± 4 | 478 ± 203 | 640 ± 219 | |
| PF | 70 ± 157 | 931 ± 344 | 1496 ± 342 | 1375 ± 399 | 1610 ± 469 | |
| Capillaries | ||||||
| prox. control | 1 ± 0 | 2 ± 0 | 3 ± 0 | 4 ± 1 | 6 ± 1 | |
| PF | 2 ± 0 * | 4 ± 1 * | 6 ± 1 * | 9 ± 1 * | 13 ± 2 * | |
| med. control | 1 ± 0 | 2 ± 0 | 3 ± 0 | 4 ± 1 | 5 ± 1 | |
| PF | 2 ± 0 | 4 ± 0 * | 6 ± 1 * | 10 ± 1 * | 12 ± 2 | |
| dist. control | 1 ± 0 | 2 ± 1 | 2 ± 0 | 4 ± 1 | 5 ± 0 | |
| PF | 1 ± 0 | 3 ± 1 | 5 ± 1 * | 9 ± 3 | 11 ± 2 * | |
| Venules | ||||||
| prox. control | 441 ± 92 | 679 ± 161 | 1304 ± 307 | 1872 ± 452 | 2154 ± 491 | |
| PF | 881 ± 287 | 1377 ± 261 * | 1500 ± 256 | 2568 ± 508 | 3060 ± 567 | |
| med. control | 424 ± 116 | 537 ± 115 | 1020 ± 166 | 1675 ± 280 | 2713 ± 409 | |
| PF | 539 ± 157 | 1443 ± 446 * | 1692 ± 262 | 3125 ± 533 * | 3767 ± 558 | |
| dist. control | 197 ± 107 | 577 ± 131 | 637 ± 203 | 1184 ± 155 | 1565 ± 137 | |
| PF | 514 ± 153 | 1088 ± 310 | 1768 ± 460 | 2650 ± 884 | 2484 ± 514 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinzierl, A.; Coerper, M.; Harder, Y.; Menger, M.D.; Laschke, M.W. Short-Term Periodic Fasting Reduces Ischemia-Induced Necrosis in Musculocutaneous Flap Tissue. Biomedicines 2024, 12, 690. https://doi.org/10.3390/biomedicines12030690
Weinzierl A, Coerper M, Harder Y, Menger MD, Laschke MW. Short-Term Periodic Fasting Reduces Ischemia-Induced Necrosis in Musculocutaneous Flap Tissue. Biomedicines. 2024; 12(3):690. https://doi.org/10.3390/biomedicines12030690
Chicago/Turabian StyleWeinzierl, Andrea, Maximilian Coerper, Yves Harder, Michael D. Menger, and Matthias W. Laschke. 2024. "Short-Term Periodic Fasting Reduces Ischemia-Induced Necrosis in Musculocutaneous Flap Tissue" Biomedicines 12, no. 3: 690. https://doi.org/10.3390/biomedicines12030690
APA StyleWeinzierl, A., Coerper, M., Harder, Y., Menger, M. D., & Laschke, M. W. (2024). Short-Term Periodic Fasting Reduces Ischemia-Induced Necrosis in Musculocutaneous Flap Tissue. Biomedicines, 12(3), 690. https://doi.org/10.3390/biomedicines12030690







