Age-Dependent Effects of Oxytocin and Oxytocin Receptor Antagonists on Bladder Contractions: Implications for the Treatment of Overactive Bladder Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Tissue Collection
2.3. Reagent Preparation
2.4. Organ Bath Studies
2.5. Spontaneous Contractions
2.6. Contractile Responses to Oxytocin
2.7. Immunohistochemistry and Tissue Stains
2.8. Analysis of IHC Experiments
3. Results
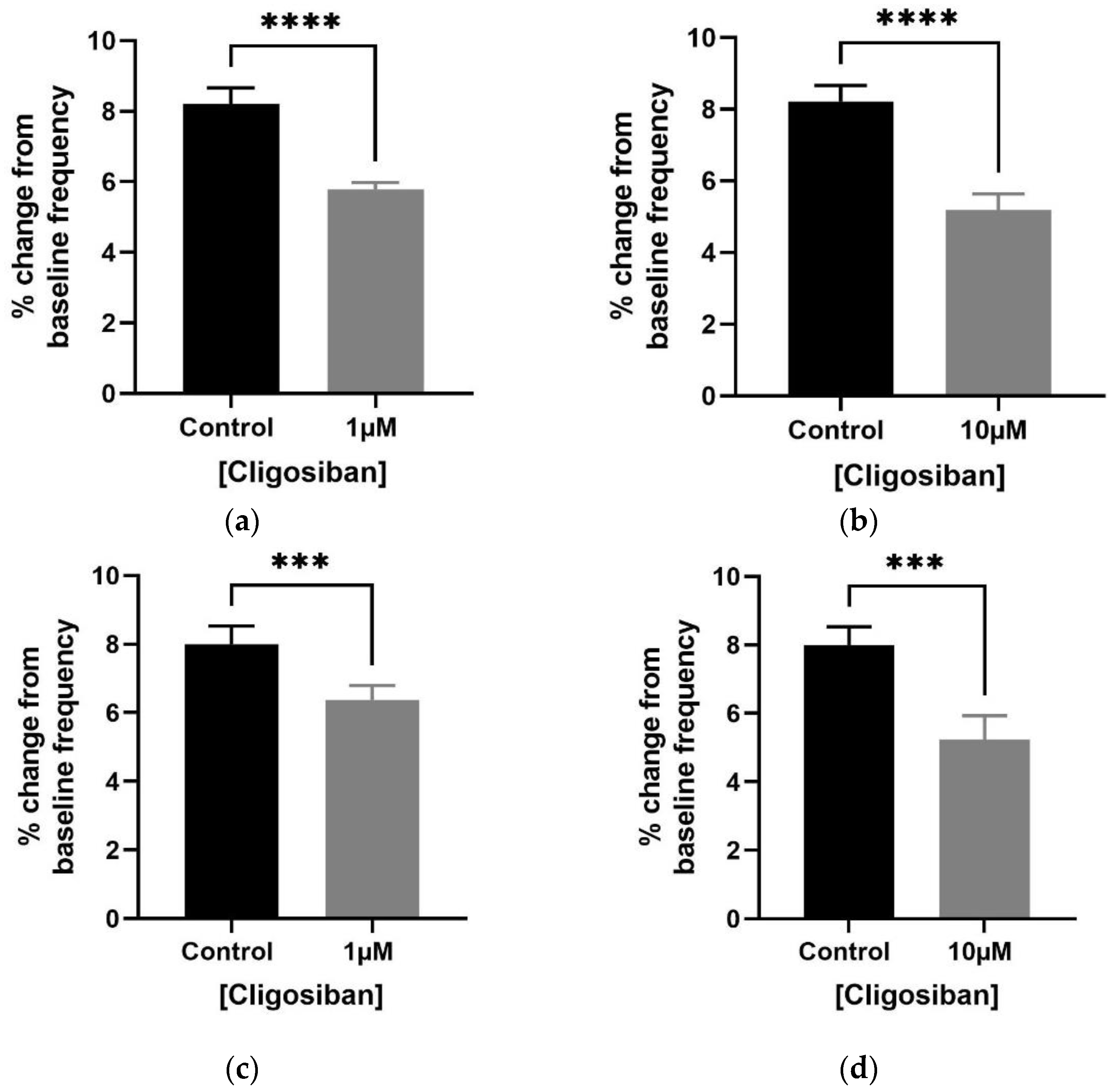
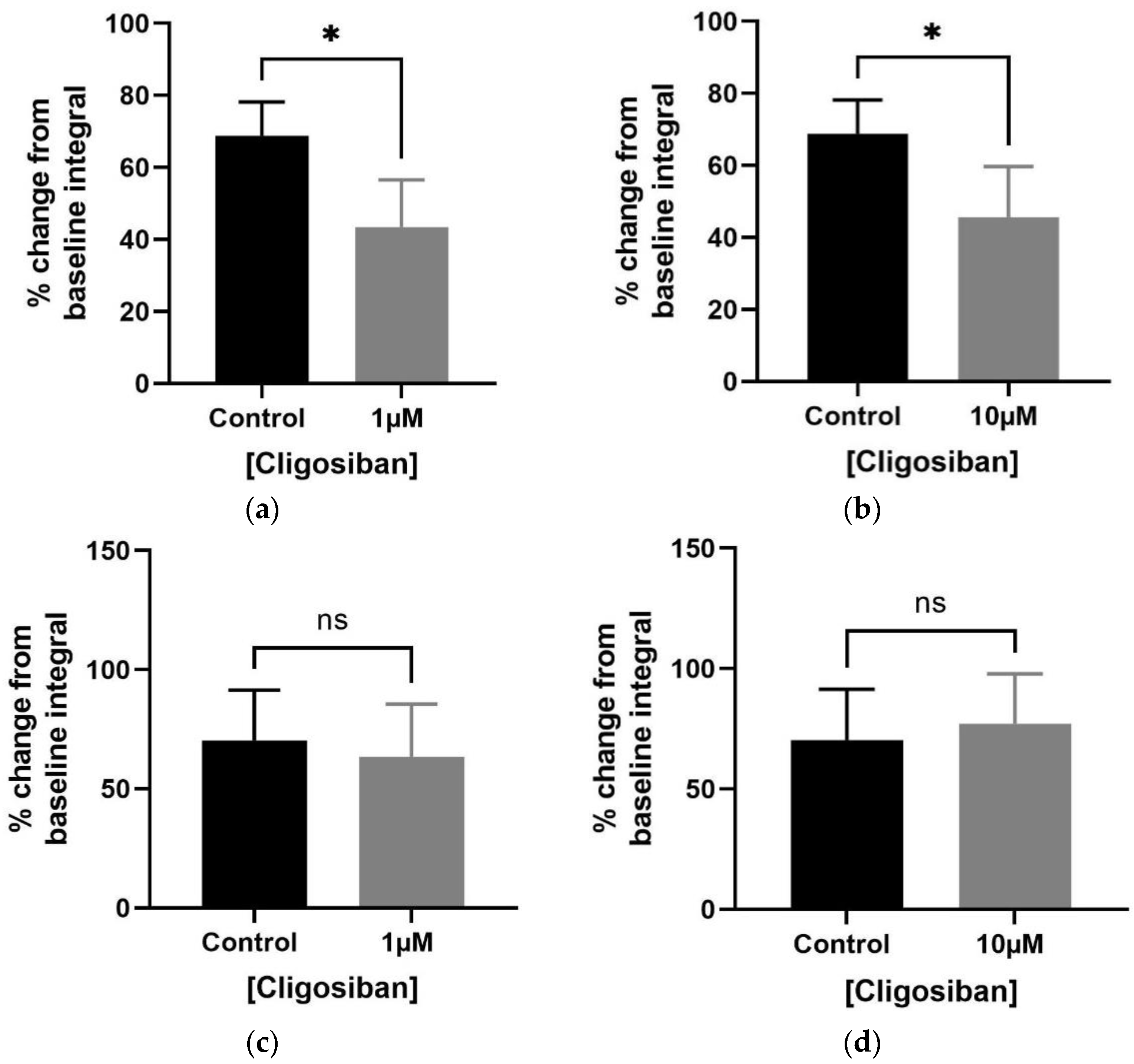
3.1. Organ Bath Findings
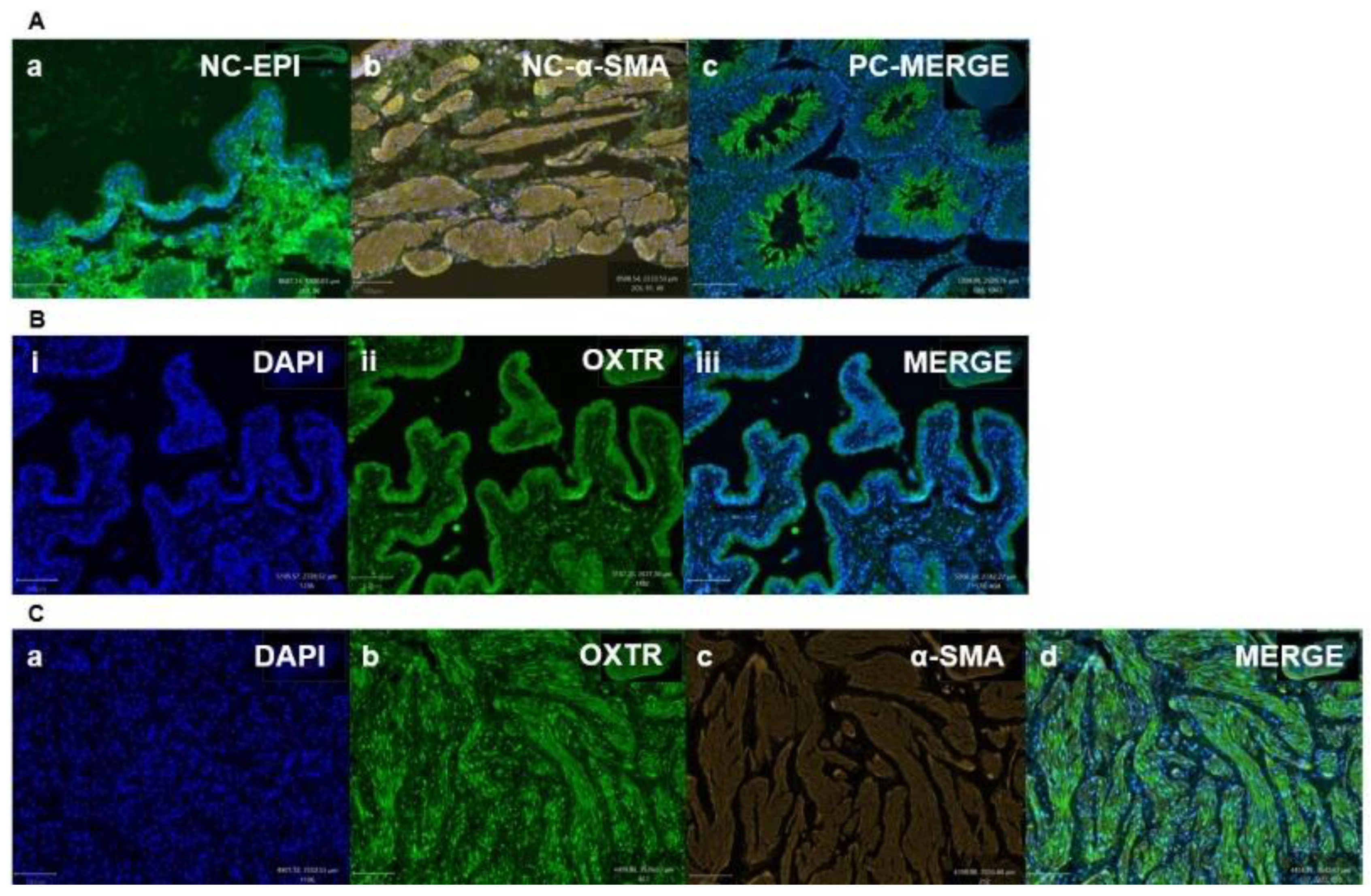
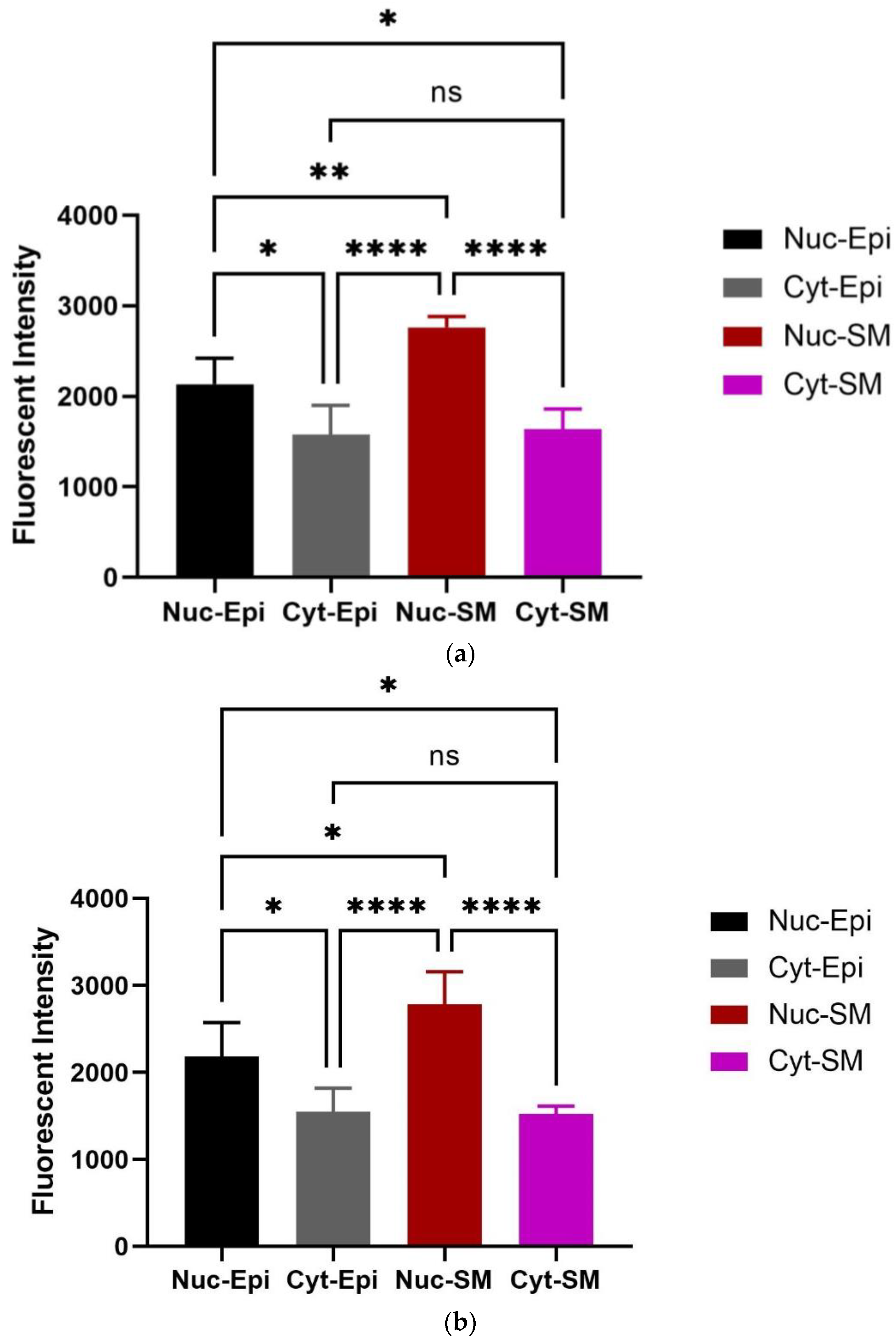
3.2. Immunohistochemistry Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardization of terminology in lower urinary tract function: Report from 310 the standaridsation sub-committee of the Internationl Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 2010, 29, 4–20. [Google Scholar] [CrossRef]
- Milsom, I.; Abrams, P.; Cardozo, L.; Roberts, R.; Thüroff, J.; Wein, A.J. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001, 87, 760–766. [Google Scholar] [CrossRef]
- Ameda, K.; Koyanagi, T.; Nantani, M.; Taniguchi, K.; Matsuno, T. The relevance of preoperative cystometrography in patients with benign prostatic hyperplasia: Correlating the findings with clinical features and outcome after prostatectomy. J. Urol. 1994, 152, 443–447. [Google Scholar] [CrossRef]
- Ameda, K.; Sullivan, M.P.; BAE, R.J.; YALLA, S.V. Urodynamic characterization of nonobstructive voiding dysfunction in symptomatic elderly men. J. Urol. 1999, 162, 142–146. [Google Scholar] [CrossRef]
- Hampel, C.; Gillitzer, R.; Pahernik, S.; Hohenfellner, M.; Thüroff, J. Epidemiologie und Ätiologie der instabilen Blase. Der Urol. Ausg. A 2003, 42, 776–786. [Google Scholar] [CrossRef]
- Willis-Gray, M.G.; Dieter, A.A.; Geller, E.J. Evaluation and management of overactive bladder: Strategies for optimizing care. Res. Rep. Urol. 2016, 8, 113–122. [Google Scholar]
- Vasdev, N.; Biles, B.D.; Sandher, R.; Hasan, T.S. The surgical management of the refractory overactive bladder. Indian J. Urol. IJU J. Urol. Soc. India 2010, 26, 263. [Google Scholar] [CrossRef]
- Jayarajan, J.; Radomski, S.B. Pharmacotherapy of overactive bladder in adults: A review of efficacy, tolerability, and quality of life. Res. Rep. Urol. 2013, 6, 1–16. [Google Scholar]
- Hegde, S.; Choppin, A.; Bonhaus, D.; Briaud, S.; Loeb, M.; Moy, T.; Loury, D.; Eglen, R. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997, 120, 1409–1418. [Google Scholar] [CrossRef]
- Hegde, S.S.; Eglen, R.M. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999, 64, 419–428. [Google Scholar] [CrossRef]
- Kuteesa, W.; Moore, K.H. Anticholinergic drugs for overactive bladder. Aust. Prescr. 2006, 29, 22–24. [Google Scholar] [CrossRef]
- Khullar, V.; Amarenco, G.; Angulo, J.C.; Cambronero, J.; Høye, K.; Milsom, I.; Radziszewski, P.; Rechberger, T.; Boerrigter, P.; Drogendijk, T. Efficacy and tolerability of mirabegron, a β3-adrenoceptor agonist, in patients with overactive bladder: Results from a randomised European–Australian phase 3 trial. Eur. Urol. 2013, 63, 283–295. [Google Scholar] [CrossRef]
- Leron, E.; Weintraub, A.Y.; Mastrolia, S.A.; Schwarzman, P. Overactive bladder syndrome: Evaluation and management. Curr. Urol. 2018, 11, 117–125. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Hedlund, P. Pharmacologic perspective on the physiology of the lower urinary tract. Urology 2002, 60, 13–20. [Google Scholar] [CrossRef]
- Ouslander, J.G. Management of overactive bladder. N. Engl. J. Med. 2004, 350, 786–799. [Google Scholar] [CrossRef]
- Keam, S.J. Vibegron: First global approval. Drugs 2018, 78, 1835–1839. [Google Scholar] [CrossRef]
- Edmondson, S.D.; Zhu, C.; Kar, N.F.; Di Salvo, J.; Nagabukuro, H.; Sacre-Salem, B.; Dingley, K.; Berger, R.; Goble, S.D.; Morriello, G. Discovery of vibegron: A potent and selective β3 adrenergic receptor agonist for the treatment of overactive bladder. J. Med. Chem. 2016, 59, 609–623. [Google Scholar] [CrossRef]
- Cardozo, L.; Thorpe, A.; Warner, J.; Sidhu, M. The cost-effectiveness of solifenacin vs fesoterodine, oxybutynin immediate-release, propiverine, tolterodine extended-release and tolterodine immediate-release in the treatment of patients with overactive bladder in the UK National Health Service. BJU Int. 2010, 106, 506–514. [Google Scholar] [CrossRef]
- Sand, P.K.; Rovner, E.S.; Watanabe, J.H.; Oefelein, M.G. Once-daily trospium chloride 60 mg extended release in subjects with overactive bladder syndrome who use multiple concomitant medications: Post hoc analysis of pooled data from two randomized, placebo-controlled trials. Drugs Aging 2011, 28, 151–160. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.-E.; Birder, L.; Brubaker, L.; Cardozo, L.; Chapple, C.; Cottenden, A.; Davila, W.; De Ridder, D.; Dmochowski, R. Fourth International Consultation on Incontinence Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 2010, 29, 213–240. [Google Scholar] [CrossRef]
- Brown, C.H. Magnocellular neurons and posterior pituitary function. Compr. Physiol. 2011, 6, 1701–1741. [Google Scholar]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Patel, H.; Jessu, R.; Tiwari, V. Physiology, Posterior Pituitary; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Arrowsmith, S. Oxytocin and vasopressin signalling and myometrial contraction. Curr. Opin. Physiol. 2020, 13, 62–70. [Google Scholar] [CrossRef]
- Filippi, S.; Vannelli, G.; Granchi, S.; Luconi, M.; Crescioli, C.; Mancina, R.; Natali, A.; Brocchi, S.; Vignozzi, L.; Bencini, E. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol. Cell. Endocrinol. 2002, 193, 89–100. [Google Scholar] [CrossRef]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Mewe, M.; Wulfsen, I.; Middendorff, R.; Bauer, C.K. Differential modulation of bovine epididymal activity by oxytocin and noradrenaline. Reproduction 2007, 134, 493–501. [Google Scholar] [CrossRef]
- Whittington, K.; Assinder, S.; Parkinson, T.; Lapwood, K.; Nicholson, H. Function and localization of oxytocin receptors in the reproductive tissue of rams. Reproduction 2001, 122, 317–325. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Dey, A.; Lam, M.; Ventura, S.; Exintaris, B. Tamsulosin modulates, but does not abolish the spontaneous activity in the guinea pig prostate gland. Neurourol. Urodyn. 2015, 34, 482–488. [Google Scholar] [CrossRef]
- Cafarchio, E.M.; Da Silva, L.A.; Auresco, L.C.; Rodart, I.F.; De Souza, J.S.; Antonio, B.B.; Venancio, D.P.; Maifrino, L.B.; Maciel, R.M.; Giannocco, G. Oxytocin reduces intravesical pressure in anesthetized female rats: Action on oxytocin receptors of the urinary bladder. Front. Physiol. 2020, 11, 382. [Google Scholar] [CrossRef]
- Bodanszky, M.; Sharaf, H.; Roy, J.B.; Said, S.I. Contractile activity of vasotocin, oxytocin, and vasopressin on mammalian prostate. Eur. J. Pharmacol. 1992, 216, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, H.; Foda, H.; Said, S.; Bodanszky, M. Oxytocin and related peptides elicit contractions of prostate and seminal vesicle. Ann. N. Y. Acad. Sci. 1992, 652, 474–477. [Google Scholar] [CrossRef]
- Badshah, M.; Ibrahim, J.; Su, N.; Whiley, P.; Whittaker, M.; Exintaris, B. The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists. Biomedicines 2023, 11, 2956. [Google Scholar] [CrossRef]
- Canguven, O.; Talib, R. Are We missing out the role of oxytocin in overactive bladder syndrome? Aging Male 2019, 23, 700–704. [Google Scholar] [CrossRef]
- Lee, S.N.; Kraska, J.; Papargiris, M.; Teng, L.; Niranjan, B.; Hammar, J.; Ryan, A.; Frydenberg, M.; Lawrentschuk, N.; Middendorff, R. Oxytocin receptor antagonists as a novel pharmacological agent for reducing smooth muscle tone in the human prostate. Sci. Rep. 2021, 11, 6352. [Google Scholar] [CrossRef]
- Cook, A.A.; Leung, T.C.S.; Rice, M.; Nachman, M.; Zadigue-Dube, É.; Watt, A.J. Endosomal dysfunction contributes to cerebellar deficits in spinocerebellar ataxia type 6. eLife 2023, 12, RP90510. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef]
- Breton, C.; Chellil, H.; Kabbaj-Benmansour, M.; Carnazzi, E.; Seyer, R.; Phalipou, S.; Morin, D.; Durroux, T.; Zingg, H.; Barberis, C. Direct identification of human oxytocin receptor-binding domains using a photoactivatable cyclic peptide antagonist: Comparison with the human V1a vasopressin receptor. J. Biol. Chem. 2001, 276, 26931–26941. [Google Scholar] [CrossRef]
- Chini, B.; Fanelli, F. Molecular basis of ligand binding and receptor activation in the oxytocin and vasopressin receptor family. Exp. Physiol. 2000, 85, 59s–66s. [Google Scholar] [CrossRef]
- Gupta, J.; Russell, R.; Wayman, C.; Hurley, D.; Jackson, V. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br. J. Pharmacol. 2008, 155, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Stadler, B.; Whittaker, M.R.; Exintaris, B.; Middendorff, R. Oxytocin in the male reproductive tract; the therapeutic potential of oxytocin-agonists and-antagonists. Front. Endocrinol. 2020, 11, 565731. [Google Scholar] [CrossRef]
- Wing, D.A.; Goharkhay, N.; Felix, J.C.; Rostamkhani, M.; Naidu, Y.M.; Kovacs, B.W. Expression of the oxytocin and V1a vasopressin receptors in human myometrium in differing physiologic states and following misoprostol administration. Gynecol. Obstet. Investig. 2006, 62, 181–185. [Google Scholar] [CrossRef]
- Barberis, C.; Mouillac, B.; Durroux, T. Structural bases of vasopressin/oxytocin receptor function. J. Endocrinol. 1998, 156, 223–229. [Google Scholar] [CrossRef]
- Åkerlund, M.; Bossmar, T.; Brouard, R.; Kostrzewska, A.; Laudanski, T.; Lemancewicz, A.; Gal, C.S.L.; Steinwall, M. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. BJOG Int. J. Obstet. Gynaecol. 1999, 106, 1047–1053. [Google Scholar] [CrossRef]
- Hicks, C.; Ramos, L.; Reekie, T.; Misagh, G.; Narlawar, R.; Kassiou, M.; McGregor, I. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: A biotelemetry study in rats. Br. J. Pharmacol. 2014, 171, 2868–2887. [Google Scholar] [CrossRef]
- Chacko, S.; Cortes, E.; Drake, M.J.; Fry, C.H. Does altered myogenic activity contribute to OAB symptoms from detrusor overactivity? ICI-RS 2013. Neurourol. Urodyn. 2014, 33, 577–580. [Google Scholar] [CrossRef]
- Brading, A.F. A myogenic basis for the overactive bladder. Urology 1997, 50, 57–67. [Google Scholar] [CrossRef]
- Romine, M.T.; Anderson, G.F. Evidence for oxytocin receptors in the urinary bladder of the rabbit. Can. J. Physiol. Pharmacol. 1985, 63, 287–291. [Google Scholar] [CrossRef]
- Pandita, R.; Nylen, A.; Andersson, K.-E. Oxytocin-induced stimulation and inhibition of bladder activity in normal, conscious rats—Influence of nitric oxide synthase inhibition. Neuroscience 1998, 85, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, F.; Çağlayan Duman, N.; Özkula, S.; Karaalp, A.; Cangüven, Ö. In vitro contractile responses of human detrusor smooth muscle to oxytocin: Does it really have effect? Aging Male 2020, 23, 1141–1145. [Google Scholar] [CrossRef]
- Cafarchio, E.M.; Auresco, L.C.; da Silva, L.A.; Rodart, I.F.; do Vale, B.; de Souza, J.S.; Antonio, B.B.; Venancio, D.P.; Giannocco, G.; Aronsson, P. Unravelling the intravenous and in situ vasopressin effects on the urinary bladder in anesthetized female rats: More than one vasopressin receptor subtype involved? Eur. J. Pharmacol. 2018, 834, 109–117. [Google Scholar] [CrossRef]
- Crankshaw, D. [Arg8] vasopressin-induced contractions of rabbit urinary bladder smooth muscle. Eur. J. Pharmacol. 1989, 173, 183–188. [Google Scholar] [CrossRef]
- Holmquist, F.; Lundin, S.; Larsson, B.; Hedlund, H.; Andersson, K. Studies on binding sites, contents, and effects of AVP in isolated bladder and urethra from rabbits and humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1991, 261, R865–R874. [Google Scholar] [CrossRef]
- Dehpour, A.R.; Kivaj, G.; Delfan, A.; Shahrokhi, M. The effects of lithium, indomethacin, and neomycin on vasopressin-induced contractions in rat urinary bladder. Gen. Pharmacol. 1997, 28, 777–780. [Google Scholar] [CrossRef]
- Birder, L.A.; Wolf-Johnston, A.S.; Jackson, E.K.; Wein, A.J.; Dmochowski, R. Aging increases the expression of vasopressin receptors in both the kidney and urinary bladder. Neurourol. Urodyn. 2019, 38, 393–397. [Google Scholar] [CrossRef]
- Chess-Williams, R. Muscarinic receptors of the urinary bladder: Detrusor, urothelial and prejunctional. Auton. Autacoid Pharmacol. 2002, 22, 133–145. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badshah, M.; Ibrahim, J.; Su, N.; Whiley, P.; Middendorff, R.; Whittaker, M.; Exintaris, B. Age-Dependent Effects of Oxytocin and Oxytocin Receptor Antagonists on Bladder Contractions: Implications for the Treatment of Overactive Bladder Syndrome. Biomedicines 2024, 12, 674. https://doi.org/10.3390/biomedicines12030674
Badshah M, Ibrahim J, Su N, Whiley P, Middendorff R, Whittaker M, Exintaris B. Age-Dependent Effects of Oxytocin and Oxytocin Receptor Antagonists on Bladder Contractions: Implications for the Treatment of Overactive Bladder Syndrome. Biomedicines. 2024; 12(3):674. https://doi.org/10.3390/biomedicines12030674
Chicago/Turabian StyleBadshah, Masroor, Jibriil Ibrahim, Nguok Su, Penny Whiley, Ralf Middendorff, Michael Whittaker, and Betty Exintaris. 2024. "Age-Dependent Effects of Oxytocin and Oxytocin Receptor Antagonists on Bladder Contractions: Implications for the Treatment of Overactive Bladder Syndrome" Biomedicines 12, no. 3: 674. https://doi.org/10.3390/biomedicines12030674
APA StyleBadshah, M., Ibrahim, J., Su, N., Whiley, P., Middendorff, R., Whittaker, M., & Exintaris, B. (2024). Age-Dependent Effects of Oxytocin and Oxytocin Receptor Antagonists on Bladder Contractions: Implications for the Treatment of Overactive Bladder Syndrome. Biomedicines, 12(3), 674. https://doi.org/10.3390/biomedicines12030674





