The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sample Collection and Processing
2.3. Imaging Data and Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Whiteley, W. Identifying blood biomarkers to improve the diagnosis of stroke. J. R. Coll. Physicians Edinb. 2011, 41, 152–154. [Google Scholar] [CrossRef]
- Mouhieddine, T.H.; Itani, M.M.; Nokkari, A.; Ren, C.; Daoud, G.; Zeidan, A.; Mondello, S.; Kobeissy, F.H. Nanotheragnostic applications for ischemic and hemorrhagic strokes: Improved delivery for a better prognosis. Curr. Neurol. Neurosci. Rep. 2015, 15, 505. [Google Scholar] [CrossRef]
- Chalela, J.A.; Kidwell, C.S.; Nentwich, L.M.; Luby, M.; Butman, J.A.; Demchuk, A.M.; Hill, M.D.; Patronas, N.; Latour, L.; Warach, S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet 2007, 369, 293–298. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Foerch, C.; Montaner, J.; Furie, K.L.; Ning, M.M.; Lo, E.H. Invited article: Searching for oracles? Blood biomarkers in acute stroke. Neurology 2009, 73, 393–399. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Authorizes Marketing of First Blood Test to Aid in the Evaluation of Concussion in Adults. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-blood-test-aid-evaluation-concussion-adults (accessed on 17 January 2024).
- Brunkhorst, R.; Pfeilschifter, W.; Foerch, C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Transl. Stroke Res. 2010, 1, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Foerch, C.; Niessner, M.; Back, T.; Bauerle, M.; De Marchis, G.M.; Ferbert, A.; Grehl, H.; Hamann, G.F.; Jacobs, A.; Kastrup, A.; et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin. Chem. 2012, 58, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.H.; Makris, K.; Stefani, D.; Koniari, K.; Gialouri, E.; Lelekis, M.; Chondrogianni, M.; Zompola, C.; Dardiotis, E.; Rizos, I.; et al. Plasma Glial Fibrillary Acidic Protein in the Differential Diagnosis of Intracerebral Hemorrhage. Stroke 2017, 48, 2586–2588. [Google Scholar] [CrossRef]
- Luger, S.; Witsch, J.; Dietz, A.; Hamann, G.F.; Minnerup, J.; Schneider, H.; Sitzer, M.; Wartenberg, K.E.; Niessner, M.; Foerch, C.; et al. Glial Fibrillary Acidic Protein Serum Levels Distinguish between Intracerebral Hemorrhage and Cerebral Ischemia in the Early Phase of Stroke. Clin. Chem. 2017, 63, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.A.; Lucarelli, T.; Penny-Dimri, J.C.; McInnes, M.D.; Mondello, S.; Bustamante, A.; Montaner, J.; Foerch, C.; Kwan, P.; Davis, S.; et al. Glial fibrillary acidic protein for the early diagnosis of intracerebral hemorrhage: Systematic review and meta-analysis of diagnostic test accuracy. Int. J. Stroke 2019, 14, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zoltewicz, S.; Guingab-Cagmat, J.; Anagli, J.; Gao, M.; Hafeez, A.; Li, N.; Cao, J.; Geng, X.; Kobeissy, F.; et al. Different expression of ubiquitin C-terminal hydrolase-L1 and αII-spectrin in ischemic and hemorrhagic stroke: Potential biomarkers in diagnosis. Brain Res. 2013, 1540, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kobeissy, F.; Alawieh, A.; Li, N.; Li, N.; Zibara, K.; Zoltewicz, S.; Guingab-Cagmat, J.; Larner, S.F.; Ding, Y.; et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Sci. Rep. 2016, 6, 24588. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Ansari, S.A.; McTaggart, R.A.; Bulsara, K.R.; Goyal, M.; Chen, M.; Fraser, J.F.; Society of NeuroInterventional Surgery. Indications for thrombectomy in acute ischemic stroke from emergent large vessel occlusion (ELVO): Report of the SNIS Standards and Guidelines Committee. J. Neurointerv. Surg. 2019, 11, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, P.D.; Köhrmann, M. 4.5-hour time window for intravenous thrombolysis with recombinant tissue-type plasminogen activator is established firmly. Stroke 2014, 45, 912–913. [Google Scholar] [CrossRef]
- Lakomkin, N.; Dhamoon, M.; Carroll, K.; Singh, I.P.; Tuhrim, S.; Lee, J.; Fifi, J.T.; Mocco, J. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: A 10-year systematic review of the literature. J. Neurointerv. Surg. 2019, 11, 241–245. [Google Scholar] [CrossRef]
- Ismail, M.; Armoiry, X.; Tau, N.; Zhu, F.; Sadeh-Gonik, U.; Piotin, M.; Blanc, R.; Mazighi, M.; Bracard, S.; Anxionnat, R.; et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: A systematic review and meta-analysis. J. Neurointerv. Surg. 2019, 11, 14–19. [Google Scholar] [CrossRef]
- Morotti, A.; Paciaroni, M.; Zini, A.; Silvestrelli, G.; Del Zotto, E.; Caso, V.; Dell’Acqua, M.L.; Simone, A.M.; Lanari, A.; Costa, P.; et al. Risk Profile of Symptomatic Lacunar Stroke Versus Nonlobar Intracerebral Hemorrhage. Stroke 2016, 47, 2141–2143. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke (U.S.). NIH Stroke Scale. Available online: https://www.ninds.nih.gov/health-information/public-education/know-stroke/health-professionals/nih-stroke-scale (accessed on 17 January 2024).
- Clinical and Laboratory Standards Institute (CLSI). Establishing and Verifying an Extended Measuring Interval through Specimen Dilution and Spiking; CLSI Guideline EP34; CLSI: Berwyn, PA, USA, 2018; Volume 1, pp. 1–11. [Google Scholar]
- Gong, B.; Leznik, E. The role of ubiquitin C-terminal hydrolase L1 in neurodegenerative disorders. Drug News Perspect. 2007, 20, 365–370. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Diaz-Arrastia, R.; Wang, K.K.; Papa, L.; Sorani, M.D.; Yue, J.K.; Puccio, A.M.; McMahon, P.J.; Inoue, T.; Yuh, E.L.; Lingsma, H.F.; et al. Acute biomarkers of traumatic brain injury: Relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 2014, 31, 19–25. [Google Scholar] [CrossRef]
- Yigit, I.; Atescelik, M.; Yilmaz, M.; Goktekin, M.C.; Gurger, M.; Ilhan, N. Investigation of UCH-L1 levels in ischemic stroke, intracranial hemorrhage and metabolic disorder induced impaired consciousness. Am. J. Emerg. Med. 2017, 35, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Ebner, F.; Moseby-Knappe, M.; Mattsson-Carlgren, N.; Lilja, G.; Dragancea, I.; Undén, J.; Friberg, H.; Erlinge, D.; Kjaergaard, J.; Hassager, C.; et al. Serum GFAP and UCH-L1 for the prediction of neurological outcome in comatose cardiac arrest patients. Resuscitation 2020, 154, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ai, M.L.; Feng, Q.; Deng, S.; Liu, Z.Y.; Zhang, L.N.; Ai, Y.H. Serum glial fibrillary acidic protein and ubiquitin C-terminal hydrolase-L1 for diagnosis of sepsis-associated encephalopathy and outcome prognostication. J. Crit. Care 2019, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Lewis, L.M.; Silvestri, S.; Falk, J.L.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Liu, M.C.; Mo, J.; Akinyi, L.; et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma. Acute Care Surg. 2012, 72, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Lewis, L.M.; Falk, J.L.; Zhang, Z.; Silvestri, S.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Dixit, N.K.; Ferguson, I.; et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 2012, 59, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Misra, S.; Yadav, A.K.; Sagar, R.; Verma, B.; Grover, A.; Prasad, K. Role of glial fibrillary acidic protein as a biomarker in differentiating intracerebral haemorrhage from ischaemic stroke and stroke mimics: A meta-analysis. Biomarkers 2020, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aurell, A.; Rosengren, L.E.; Karlsson, B.; Olsson, J.E.; Zbornikova, V.; Haglid, K.G. Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke 1991, 22, 1254–1258. [Google Scholar] [CrossRef]
- Herrmann, M.; Vos, P.; Wunderlich, M.T.; de Bruijn, C.H.; Lamers, K.J. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 2000, 31, 2670–2677. [Google Scholar] [CrossRef]
- Liu, M.C.; Akinyi, L.; Scharf, D.; Mo, J.; Larner, S.F.; Muller, U.; Oli, M.W.; Zheng, W.; Kobeissy, F.; Papa, L.; et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 2010, 31, 722–732. [Google Scholar] [CrossRef]
- Hu, C.; Yang, X.; Mao, D.; Lou, S.; Dai, Q.; Chen, J.; Cheng, X.; Wang, S. Expression levels of ubiquitin C-terminal hydrolase-L1 and serum glial fibrillary acidic protein and its clinical significance in patients with acute cerebral infarction. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017, 42, 284–290. [Google Scholar] [CrossRef]
- Fransen, P.S.; Berkhemer, O.A.; Lingsma, H.F.; Beumer, D.; van den Berg, L.A.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J. Time to Reperfusion and Treatment Effect for Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2016, 73, 190–196. [Google Scholar] [CrossRef]
- Barakzie, A.; Jansen, A.J.G.; Ten Cate, H.; de Maat, M.P.M. Coagulation biomarkers for ischemic stroke. Res. Pract. Thromb. Haemost. 2023, 7, 100160. [Google Scholar] [CrossRef]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Pritchett, J.W. C-reactive protein levels determine the severity of soft-tissue injuries. Am. J. Orthop. 1996, 25, 759–761. [Google Scholar]
- Somford, D.M.; Nederkoorn, P.J.; Rutgers, D.R.; Kappelle, L.J.; Mali, W.P.; van der Grond, J. Proximal and distal hyperattenuating middle cerebral artery signs at CT: Different prognostic implications. Radiology 2002, 223, 667–671. [Google Scholar] [CrossRef]
- Amalia, L. Glial Fibrillary Acidic Protein (GFAP): Neuroinflammation Biomarker in Acute Ischemic Stroke. J. Inflamm. Res. 2021, 14, 7501–7506. [Google Scholar] [CrossRef]
- Surjawan, Y.; As’ad, S.; Ranakusuma, T.A.S.; Wijaya, A. GFAP and S100B Protein Are Associated with Discharged NIHSS of Anterior Circulation Ischemic Stroke. Indones. Biomed. J. 2012, 4, 7–112. [Google Scholar] [CrossRef]
- Di Donna, A.; Muto, G.; Giordano, F.; Muto, M.; Guarnieri, G.; Servillo, G.; De Mase, A.; Spina, E.; Leone, G. Diagnosis and management of tandem occlusion in acute ischemic stroke. Eur. J. Radiol. Open. 2023, 11, 100513. [Google Scholar] [CrossRef]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef]
- Papa, L.; Brophy, G.M.; Alvarez, W.; Hirschl, R.; Cress, M.; Weber, K.; Giordano, P. Sex differences in time course and diagnostic accuracy of GFAP and UCH-L1 in trauma patients with mild traumatic brain injury. Sci. Rep. 2023, 13, 11833. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef]
| Control (n = 22) | SVO (n = 29) | LVO (n = 40) | p Value | |
|---|---|---|---|---|
| Age, years (GM, GSDF) | 66.77, 1.09 | 72.03, 1.16 | 77.6, 1.15 | <0.0001 * |
| Women (n, %) | 10 (45.45%) | 9 (31.03%) | 29 (72.5%) | 0.002 # |
| Arterial hypertension (n, %) | 18 (81.82%) | 21 (72.41%) | 29 (72.5%) | 0.722 # |
| Diabetes (n, %) | 5 (22.73%) | 5 (17.24%) | 7 (17.5%) | 0.867 # |
| Atrial fibrillation (n, %) | 9 (40.91%) | 10 (34.48%) | 15 (37.5%) | 0.89 # |
| SVO (n = 29) | LVO (n = 40) | p Value * | R2 Value * | |||
|---|---|---|---|---|---|---|
| GM | GSDF | GM | GSDF | |||
| Leukocytes (number × 109) | 8.243 | 1.42 | 8.944 | 1.387 | 0.332 | 1.45% |
| Neutrophils (%) | 67.53 | 1.193 | 72.54 | 1.197 | 0.079 | 4.68% |
| Lymphocytes (%) | 18.39 | 1.834 | 15.61 | 1.665 | 0.154 | 3.1% |
| Na+ (mmol/L) | 140.7 | 1.02 | 140.8 | 1.028 | 0.852 | 0.05% |
| K+ (mmol/L) | 4.117 | 1.102 | 3.94 | 1.14 | 0.142 | 3.4% |
| LDH (mmol/L) | 178.8 | 1.66 | 211.8 | 1.292 | 0.212 | 3.16% |
| CRP (mg/L) | 3.335 | 3.507 | 5.347 | 3.914 | 0.154 | 3.1% |
| PT (s) | 1.141 | 1.178 | 0.927 | 1.408 | 0.0019 | 15.13% |
| Control (n = 22) | SVO (n = 29) | LVO (n = 40) | p Value * | R2 Value * | ||||
|---|---|---|---|---|---|---|---|---|
| GM | GSDF | GM | GSDF | GM | GSDF | |||
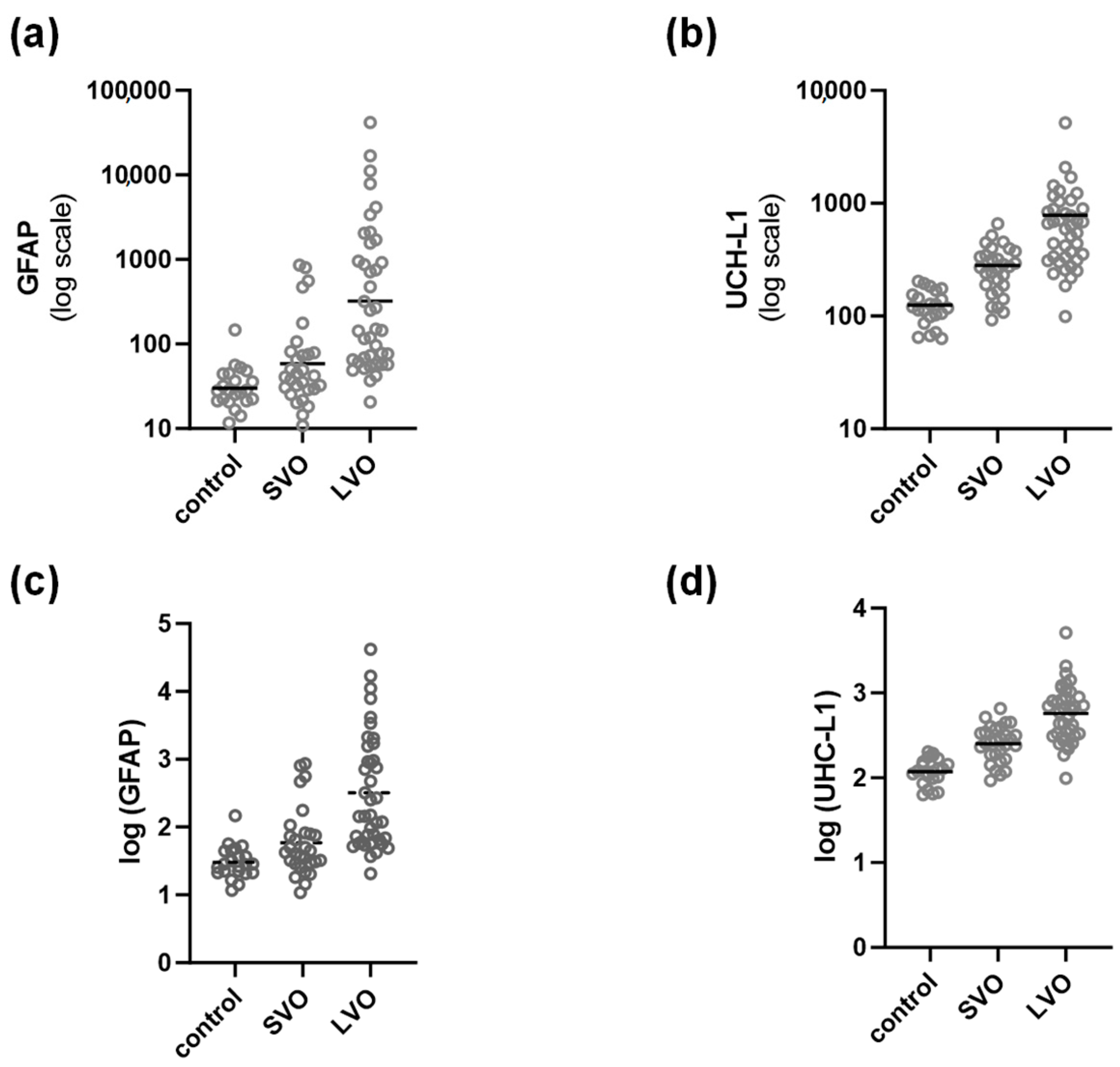
| GFAP (pg/mL) | 30.19 | 1.72 | 58.6 | 3.172 | 321.3 | 6.93 | p < 0.0001 | 31.32% |
| UCH-L1 (pg/mL) | 117.7 | 1.429 | 251.8 | 1.637 | 573.1 | 2.129 | 53.74% | |
| GFAP | UCH-L1 | |||
|---|---|---|---|---|
| Outcome | LVO vs. SVO * | LVO vs. Control # | LVO vs. SVO † | LVO vs. Control ‡ |
| AUC | 0.7996 | 0.9403 | 0.8159 | 0.9795 |
| specificity (%) | 62.07 | 81.82 | 62.07 | 95.45 |
| sensitivity (%) | 85.5 | 92.5 | 75 | 95 |
| accuracy (%) | 76.81 | 88.71 | 69.57 | 95.16 |
| cut-off (pg/mL) | 200.53 | 63.74 | 498.89 | 288.09 |
| GFAP (pg/mL) | UCH-L1 (pg/mL) | ||
|---|---|---|---|
| Favorable NIHSS (n = 16) | GM | 300.9 | 497.8 |
| GSDF | 7.82 | 2.51 | |
| Unfavorable NIHSS (n = 13) | GM | 298.2 | 565.7 |
| GSDF | 4.595 | 1.997 | |
| p Value * | 0.395 | 0.682 | |
| R2 Value * | 2.7% | 0.63% | |
| Positive “hyperdense sign” (n = 23) | GM | 286.8 | 525.4 |
| GSDF | 4.9 | 1.73 | |
| Negative “hyperdense sign” (n = 17) | GM | 480.4 | 644.6 |
| GSDF | 10 | 2.655 | |
| p Value * | 0.934 | 0.405 | |
| R2 Value * | 0.02% | 1.8% | |
| Multiple vessel occlusion (n = 13) | GM | 242.6 | 524.3 |
| GSDF | 8.81 | 2.21 | |
| Single vessel occlusion (n = 25) | GM | 369.6 | 608.4 |
| GSDF | 6.758 | 2.1 | |
| p Value * | 0.526 | 0.57 | |
| R2 Value * | 1.1% | 0.9% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraljević, I.; Sablić, S.; Marinović Guić, M.; Budimir Mršić, D.; Štula, I.; Dolić, K.; Benzon, B.; Košta, V.; Čaljkušić, K.; Marčić, M.; et al. The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke. Biomedicines 2024, 12, 608. https://doi.org/10.3390/biomedicines12030608
Kraljević I, Sablić S, Marinović Guić M, Budimir Mršić D, Štula I, Dolić K, Benzon B, Košta V, Čaljkušić K, Marčić M, et al. The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke. Biomedicines. 2024; 12(3):608. https://doi.org/10.3390/biomedicines12030608
Chicago/Turabian StyleKraljević, Ivan, Sara Sablić, Maja Marinović Guić, Danijela Budimir Mršić, Ivana Štula, Krešimir Dolić, Benjamin Benzon, Vana Košta, Krešimir Čaljkušić, Marino Marčić, and et al. 2024. "The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke" Biomedicines 12, no. 3: 608. https://doi.org/10.3390/biomedicines12030608
APA StyleKraljević, I., Sablić, S., Marinović Guić, M., Budimir Mršić, D., Štula, I., Dolić, K., Benzon, B., Košta, V., Čaljkušić, K., Marčić, M., Šupe Domić, D., & Lovrić Kojundžić, S. (2024). The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke. Biomedicines, 12(3), 608. https://doi.org/10.3390/biomedicines12030608









