1. Introduction
According to the WHO, the prevalence of
SARS-CoV-2 reached approximately 662 million cases on the day of 16 January 2023, leading to 6 million deaths due to the development of the COVID-19 syndrome [
1]. Although the pandemic has already passed, some people, especially those who suffered from full-blown COVID-19, may experience acute consequences of COVID-19, the so-called long COVID or post-COVID-19 syndrome (PCS), recognized by the WHO as the next epidemic of the 21st century [
2]. This term refers to symptoms persisting for more than 3 months after COVID-19, causing long-term changes in single organs or multi-organ changes [
1]. It is predicted that PCS may affect millions of people worldwide [
1], mainly people with comorbidities [
3], e.g., type 2 diabetes mellitus (2TDM). The symptoms of PCS are difficulty with concentration, cognitive dysfunction, amnesia, depression, fatigue and anxiety [
3], and the risk factors for the persistence of neuropsychiatric symptoms in PCS are older age, female sex and the severity of comorbidities, e.g., diabetes [
4], which is often associated with tachycardia, sarcopenia, microcirculatory dysfunction or organ damage [
5]. PCS is therefore a phenomenon that affects life expectancy.
The study reported in this manuscript focuses on diabetes, which was shown to develop de novo in patients suffering from COVID-19 [
4]. Diabetes is estimated to be associated with approximately 15% of patients suffering from severe COVID-19. The mortality rate in COVID-19-positive diabetic patients was reported to be 2- to 3-fold higher compared to COVID-19-negative diabetic patients [
5]. The literature (studies and meta-analyses) shows higher mortality in the COVID-19-positive diabetic population compared to the COVID-19-positive non-diabetic population [
6,
7,
8,
9]. Moreover, optimal diabetic control (in the case of T1DM/T2DM) was associated with better outcomes and fewer comorbidities among COVID-19-positive patients [
10]. Such statistics account for the urge to partake in therapeutic intervention [
5]. However, the information on the COVID-19-associated diabetic cases and their responses to drug treatment are scarce. Research into new strategies in its treatment and prevention may improve the quality of medical decisions in terms of mortality, exacerbation and optimal response to drugs [
11], and shed some light on the metabolic alterations in these patients.
A handful of clinical studies aimed to combine the classic anti-viral and anti-inflammatory drugs in one treatment scheme in order to combat COVID-19 [
11]. However, since the molecular action of agents used in antidiabetic treatment is multidimensional and may possibly modulate the course of COVID-19 and its related oxidative stress and cytokine storm [
12,
13,
14,
15], interactions with antidiabetic treatments ought to be taken into account when analyzing multidimensional models used in more complex studies. To our knowledge, no studies analyzed the difference in the odds of mortality associated with antidiabetic treatment in the context of the simultaneous effects of the coexisting covariates (comorbidities, patient characteristics and demography, and biochemical parameters). The lack of such an investigation renders the one-dimensional studies prone to generate false assumptions, owing to the aforementioned multifaceted action of antidiabetic drugs and the
SARS-CoV-2 affinity towards cell membrane proteins [
5,
13,
15,
16,
17,
18], often acting as intrinsic cell-to-cell messengers.
The aim of this preliminary study was to explore the mortality-wise effect of insulin and metformin administration in the European (Polish) model of the COVID-19-positive diabetic population sample composed of all patients admitted to the Temporary COVID-19 Hospital in Wroclaw, Poland. Typically, patients transferred to this specific hospital were characterized by an increased risk of in-hospital mortality due to increased COVID-19 severity. Along with the estimated effect of insulin and metformin on survival, their interactions with other factors (including other agents) were studied (in the form of second- or third-degree interactions) to check for synergies in modulating the mortality rate. Only significant interactions were reported and further analyzed, as opposed to the less optimal process of adjusting the findings with a pre-assumed set of patient features. This study design was chosen so as to give a foundation for future, more patient- and treatment-oriented risk assessment models that would be validated on bigger, stratified, diabetic population samples. These tools would enable the identification of patients with a higher risk of death from COVID-19. This targets those for whom analysis of the values of the selected laboratory and demographic parameters, and taking into account the treatment methods for both T2DM and COVID-19, would prove to be at-risk in the context of assessment of the outcome of the disease.
4. Discussion
COVID-19, as many other inflammation-driving diseases/syndromes, induces the excessive production of inflammatory cytokines (‘cytokine storm’) leading to activation of CD4- and CD8-positive lymphocytes. This dysregulation leads to development of various aforementioned comorbidities, often leading to increased mortality [
4,
21]. In this study, non-survivors were characterized by male sex; higher age; the following traits upon their admission: SpO
2 ≤ 94%, undergoing oxygen therapy (in many cases due to the severity of the disease) and hemorrhage; and the following traits associated with their medical history: heart failure, heart infarction and chronic kidney disease. These findings were in line with the literature, which links higher mortality in patients suffering from COVID-19 and T2DM with male sex [
22] heart failure, chronic kidney disease [
23], hyperglycemia (poor diabetic control) [
24], lower oxygen saturation [
25] or heart infarction in the past [
26]. Another study lists cardiovascular diseases (such as coronary artery disease) and stroke as other death-promoting factors [
27]. Moreover, one of the aforementioned studies [
21] also showed that death during hospitalization was more frequent among the patients who had developed the following during the hospitalization: hypovolemic shock, cardiogenic shock, heart infarction, sepsis, chronic obstructive pulmonary disease (COPD) or other inflammatory pulmonary syndromes [
22]. However, to our knowledge, the literature does not sufficiently cover the topic of risk modeling in these patients (COVID-19 and T2DM), as it focuses on analyzing the effects of different factors on mortality with use of the models. Albeit the models correct the estimated values (odds, risk etc.) for characteristics such as sex, age and comorbidity, they do not utilize nor enable exploring the interactions between these factors, let alone exploring the interactions between the drugs administered during the hospitalization. An example of such a study carried out on the COVID-19- and T2DM-positive stratum [
28] showed that COPD increased the odds of death. However, this effect was not further explored. In this situation, one would be left to their own interpretation, not knowing whether the COPD-associated increase in mortality would be further modulated by any comorbidity, requiring the use of a different set of patient characteristics depending on these comorbidities. Explorative interaction analysis could prove an answer to this question, possibly revealing the population strata that does not show an association between COPD and higher mortality. This and similar musings led to the conceptualization of our preliminary study [
29,
30,
31,
32].
The aim of this study was dichotomic. Univariate analysis and multivariate AI-assisted extraction of the key factors associated with the odds of death in the COVID-19- and T2DM-positive patient stratum acted as a prelude to exploring whether the pro-fatal effect of antidiabetic and anti-viral drugs administered during hospitalization was affected by any patient-specific characteristic upon admission.
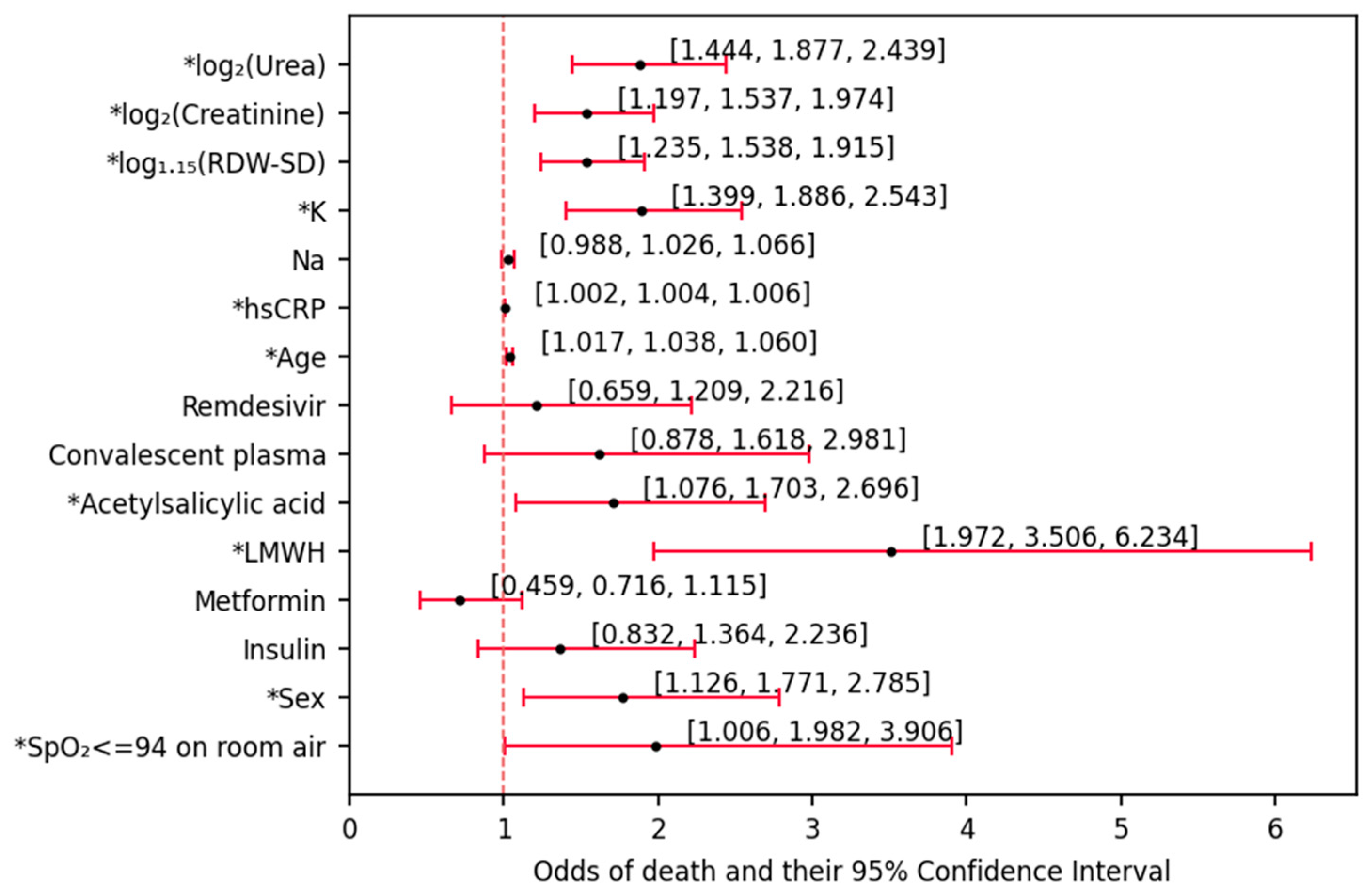
Nearly all of the available parameters were associated with the odds of death based on univariate (not adjusted by any other variables) logistic regression analysis. The analysis revealed that older age and male sex (unsurprisingly) were positive factors for estimating mortality odds. Having an oxygen saturation under the physiological values (95%–100%), likewise, was positively associated with a fatal outcome. LMWH and acetylsalicylic acid were iatrogenic factors promoting in-hospital death. Moreover, among the laboratory parameters, hsCRP, K, creatinine, urea and RDW-SD were positive indicators of higher odds of death. While it is logical that the inflammation- and diuresis- related parameters would be associated with higher severity of the disease [
29,
30,
31], the anisocytosis parameter (RDW-SD) may not be a first-pick candidate for a poor outcome predictor. In clinical practice, RDW is a predictor of outcome in critically ill and septic patients (an increase in RDW is caused by an increase in the number of old red blood cells, which have a lower volume). Several meta-analyses have demonstrated the association between RDW and the risk of mortality in patients with COVID-19 [
33] and proved the important role of RDW in predicting prognosis [
34]. RDW-SD has also been shown to be a strong independent predictor of infection severity and death in COVID-19 patients: an RDW-SD ≤ 43 showed no risk of death, while RDW-SD > 47 indicated severe disease and a high risk of mortality [
35]. If the RDW-SD value would fall in the range of 43 < RDW-SD ≤ 47, the course of the disease would be severe, but the risk of death was low. Therefore, it seems likely that determining the value of RDW may prove important in undertaking early intervention to reduce mortality in COVID-19 patients, especially in the case of limited resources. However, so far, the role of RDW has not been previously demonstrated in patients with COVID-19 and type 2 diabetes. Therefore, univariate logistic regression analysis showed that age, male sex and RDW-SD are positive factors in estimating the odds of death. Moreover, RDW-SD is a strong independent predictor of infection severity and death in COVID-19 patients. An RDW-SD value > 47 indicated severe disease and a high risk of death.
It should be emphasized that during the first (univariate) step of our analysis, insulin and metformin administration were revealed to be insignificant in terms of mortality odds modulation. While this does not necessarily mean that insulin and metformin play no role in such predictions (as will be shown later in the discussion), it could be rightfully pointed out that insulin and metformin could not be used, on their own, in the process of estimating the risk of death in these patients.
In the next step of the analysis (deriving a multivariate model,
Figure 3), the factors that were used in the previous step were all included in the initial pool of candidates for predictors of death. Subsequently, they were discarded one by one in a stepwise manner based on how much information they brought to the classifying (death/survival) model. As the pool of rejected factors began to increase, all of these variables were re-checked whether they should be again included in the model classifying mortality status. The multivariate model derived in the process enables estimation of the odds of death based on five factors: LMWH treatment, age, RDW-SD, sex and K. Calculating the odds of death of a patient admitted to the ward could be performed by multiplying the baseline odds (denoted by the intercept) based on the aforementioned characteristics. The value of the intercept and OR associated with sex show that survival would be the most frequent outcome (odds << 1) in a typical patient (described in the figure description) suffering from both COVID-19 and diabetes, regardless of sex (women: odds = 0.009, men: odds = 0.009 ∙ 2.798 ≈ 0.025). Had two patients of the same outcome been compared, each one-year gap between them would render the older patient 6.9% (odds = 1.069) more likely to die during the hospitalization. Each increase in potassium (by 1 mmol/L) and RDW-SD (by 15%) would, likewise promote death (by 98% and 87.7%, respectively). The association of these factors with death may be explained with cellular damage due to oxidative stress caused by inflammation. While, in this state, K would be simply liberated from the cells, the RDW-SD increase would be associated with the increase in anisocytosis in the state of constant/transient anemia—not only caused by their lysis per se but also correlated with kidney damage due to the lack of erythropoietin secretion. An over 10-fold increase in the odds of death upon LMWH treatment does not indicate that LMWH promotes death—it should rather hint that the decision of its administration was made in case of patients of higher disease severity emerging from possible increase of D-dimers, which account for the increased fibrinolysis. The mentioned D-dimers have been posed as mortality predictors in COVID-19 patients [
36]. Moreover, increase plasmatic concentration of D-dimers among T2DM patients was shown to be associated with increased risk of cardiovascular disease events, regardless of conventional risk factors or the treatment-wise factors [
36]. However, to our knowledge, D-dimers have not been proven (due to not having been studied) to pose as direct predictors of death in patients with T2DM nor patients with both T2DM and COVID-19. The last observation, already used while analyzing the baseline odds, is that men were of markedly higher odds of dying (in this model, OR = 2.798). While, as mentioned before, the odds in both sexes would be favoring survival in typical patients (64-year-old, 45.78% RDW-SD, 4.10 mmol/L K, no LMWH treatment), this sex-related difference would, epidemiologically, play an important role among the patients of higher age and disease severity. While this risk assessment model needs to undergo validation and comparison to different models in future studies to be taken more seriously, one is certain that the multivariate models, likewise to univariate, provide a hint that the administration of insulin and metformin is not a factor informative enough to be used in risk assessment of the entire COVID-19- and T2DM-positive population. Potential possibilities stemming from this information were revealed upon the last part of the analysis. The developed multivariate model allowed for the estimation of the chance of death based on LMWH treatment, age, RDW-SD, gender and K. Each increase in the patient’s age by one year increased the chance of death by 6.9%, each increase in potassium concentration by 1 mmol/L increased the chance of death by 98% and an increase in RDW-SD by 15% increased the chance of death by 87%, which was caused by cell damage by oxidative stress. LMWH was used in patients with advanced COVID-19, in whom an increase in D-dimer levels (increased fibrinolysis) was observed. The 10-fold increase in the risk of death after LMWH treatment was not due to the treatment itself, but to the stage of advancement of COVID-19. The association of gender with a higher risk of death concerned older patients with advanced disease. There was no gender effect observed for younger patients, aged 64.
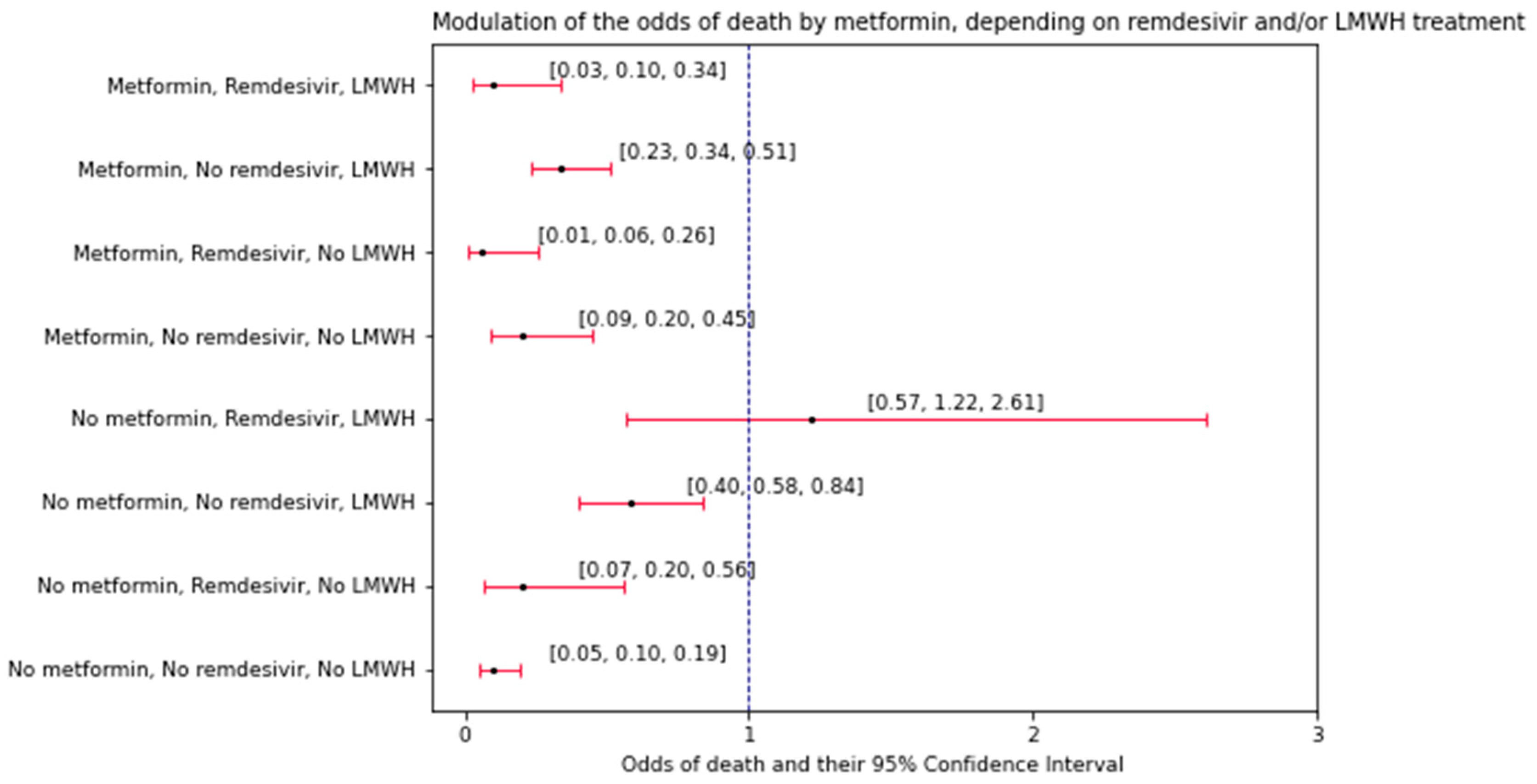
The last part of the analysis explored the interactions between the variables (factors) in terms of changing the odds of death. What makes these interactions different to the convention used in the previous part of the study is that they explore whether any pair of factors (patient characteristics) has a multiplicative effect on the modulation of the odds of death by the third factor—a drug used during the hospitalization. LMWH and remdesivir were independent on each other in how they modulated the effect of metformin on the odds of death. Conversely, age and hsCRP interacted with each other, having a multiplicative effect on the difference in the odds of mortality between individuals who took insulin vs. the ones who did not. In the first observed interaction, remdesivir and LMWH treatments showed different patterns in affecting the odds of death, between patients administered with metformin and those under no such treatment. Upon analyzing the odds of death (N deaths/N survivals,
Figure 4) it could be observed that the odds of death were different upon administration of remdesivir and LMWH, depending on whether the patient was under treatment with metformin. While metformin-administered patients showed an increasing pattern in the odds of death (LMWH and remdesivir > LMWH only > remdesivir only > neither LMWH nor remdesivir), such a pattern was not observed among the patients under no metformin treatment. One may argue about the novelty of this observation due to the fact that the treatment with remdesivir and LMWH blatantly shows the severity of the disease, thus implicating higher odds of death. However, to our knowledge, our study is the first one to show that this rationale was not universal for all of the COVID-19 T2DM patients. To gather more precise information on the odds of in-hospital death, before risk modeling, the entire population may need to be stratified in regards to metformin treatment and, perhaps, treatment with other antidiabetic agents as well. Another observation from this study was that metformin intake was associated with higher odds of death (0.20 vs. 0.10,
Figure 4) compared to no intake among patients under no LMWH and remdesivir treatment. There is no possible way to discuss this matter referring to the literature since other studies did not report the odds of death in the same context as our study (three-way interaction). First and foremost, it is stated that metformin has both in vivo and in vitro effects on
SARS-CoV-2, letting one assume there might be possibly different outcomes (and sets of its predictors) depending on metformin treatment [
32]. Some studies on COVID-19 patients indicate lower likelihood of death upon metformin treatment [
37,
38,
39]. However, DeFronzo et al. reported a lack of this association, but observed a markedly lower likelihood of heart failure among patients administered with metformin [
38,
40,
41,
42]. Analyzing interactions with inhibitors of dipeptydylpeptidase 4 (DPP-4i) may be a good choice for future analyses similar to ours, since this agent appears to have both direct and indirect effects on
SARS-CoV-2 infection. DPP-4i, being a gliptin [
41] drug representative, owing to its anti-inflammatory action, could be hypothesized to indirectly (through lowering the CRP concentration) affect the severity of COVID-19 [
38]. Another hypothesized indirect action of DPP-4i is combating the ‘cytokine storm’ through inhibiting the activation of TLR4 in the lung alveoli [
5]. As
SARS-CoV-2 binds with these receptors, DPP-4i could help combat the pulmonary ‘cytokine storm’, leading to a decrease in lung injuries and collateral damage to other organs that could induce the state of multi-organ failure over the duration of COVID-19 [
11,
43]. Moreover, DPP-4i acts as a receptor for the
SARS-CoV-2 [
13]; likewise, the drug binds with
MERS-CoV [
44]. This occurrence could have been associated with the observation of a lower concentration of soluble (in serum/plasma) DPP-4i among the diabetic COVID-19 patients [
45]. So far, the idea behind using DPP-4i as a predictor of death/severity of the disease in the mentioned population could not be taken for granted due to the inconsistencies in the literature [
46,
47,
48,
49]. Likewise, remdesivir treatment had positive effects on the clinical improvement associated with the reduced risk of severe acute respiratory distress syndrome in need of intubation but it seemed not to affect mortality among COVID-19 patients [
50]. In the presented publication, the analysis of interactions between variables (factors) in terms of the change in the probability of death showed that LMWH and remdesivir independently modulated the effect of metformin on the risk of death, while age and hsCRP interacted in their effect on the difference in the risk of death between people taking insulin and those not taking it. Metformin increased the risk of death the most in the group of people taking both LMWH and remdesivir. This observation was not demonstrated in the same group of people who did not take metformin. Additionally, age and hsCRP modulated the chance of death only in people who did not receive insulin.
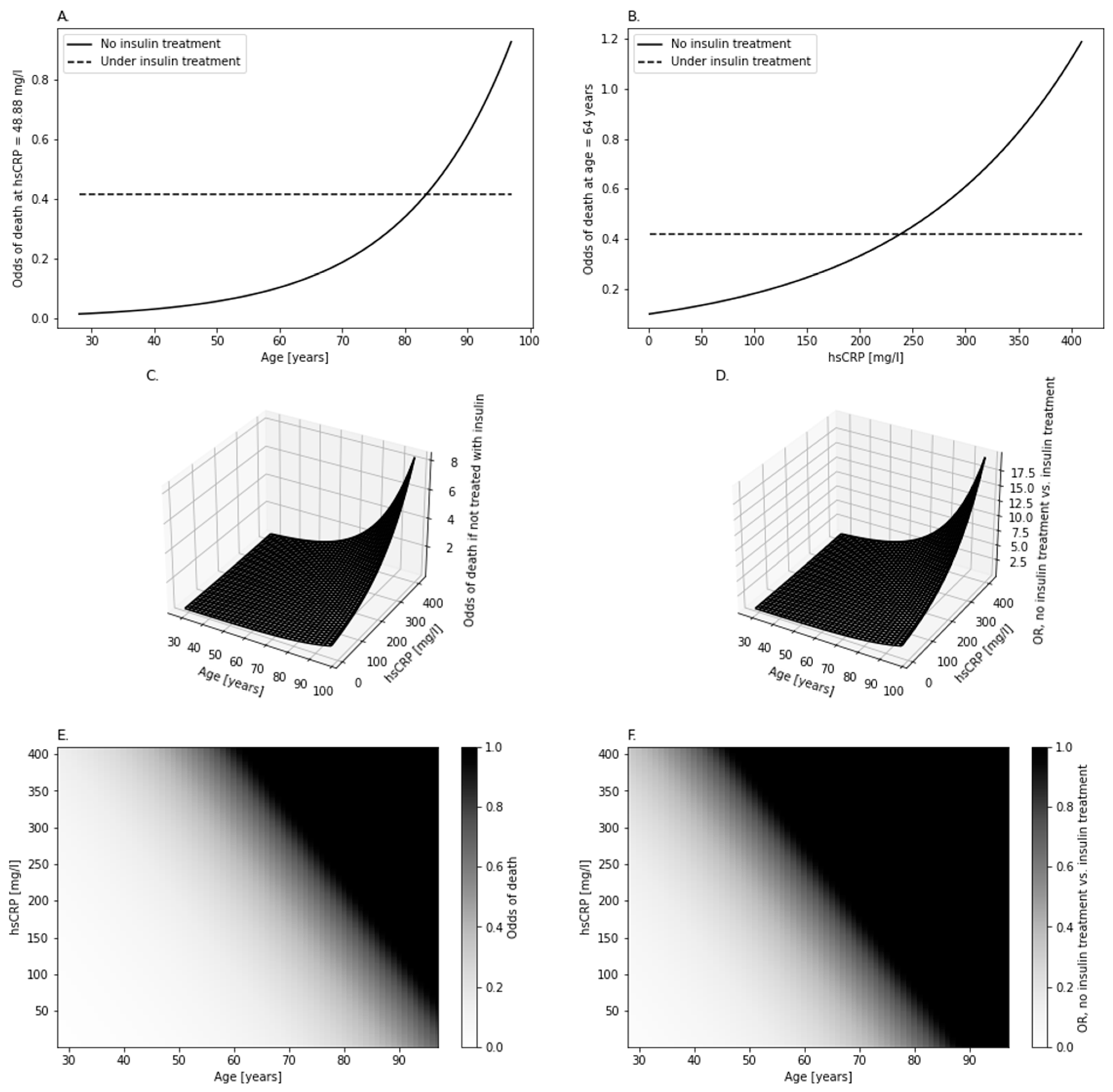
However, DPP-4i could take part in interactions on which the information is scarce. Significant interaction of insulin with hsCRP and age hinted at different modulation of the odds of death by insulin, depending on these two other variables. This occurrence was due to fact that age and hsCRP did not significantly change the odds of death among patients administered with insulin (
Table 3,
Figure 5A,B), while the patients not administered with it showed a positive association between these odds and either hsCRP or age (
Table 3,
Figure 5A–C). This insulin-related difference between patients deepened the difference in odds between them when given more advanced age and/or higher hsCRP (
Figure 5D,F). However, there is no universal answer to whether any of these groups would be more prone to showing a fatal outcome—it all is a matter of age and hsCRP (
Figure 5F). Moreover, upon reaching a specific threshold of age and hsCRP, the odds would become of favor of death (e.g., more patients would die compared to the count of survivors) among the patients not administered with insulin. This interaction is not as complex as it could be, since age and hsCRP, although both simultaneously affecting the odds of death, had an isolated effect on it—hsCRP and age did not modulate the effect of each other on the odds (‘hsCRP*Age’ in
Table 3) regardless of administration with insulin (‘Insulin*hsCRP*Age’ in
Table 3). The cause of such a phenomenon, not discussed or mentioned in other studies, remains a mystery until validated and further analyzed on a bigger population, with possibly more factors brought into the model. A study [
12] showed insulin treatment to be positively associated with the likelihood of death. However, the said study did not explore the possible effect of age on the insulin–mortality association. Perhaps a future model could employ both insulin–age and metformin–remdesivir–LMWH interactions. Hopefully, new research would investigate this matter on bigger retrospective data and/or a diabetic population not suffering from COVID-19 (assuming no COVID-19 outbreak in the future).
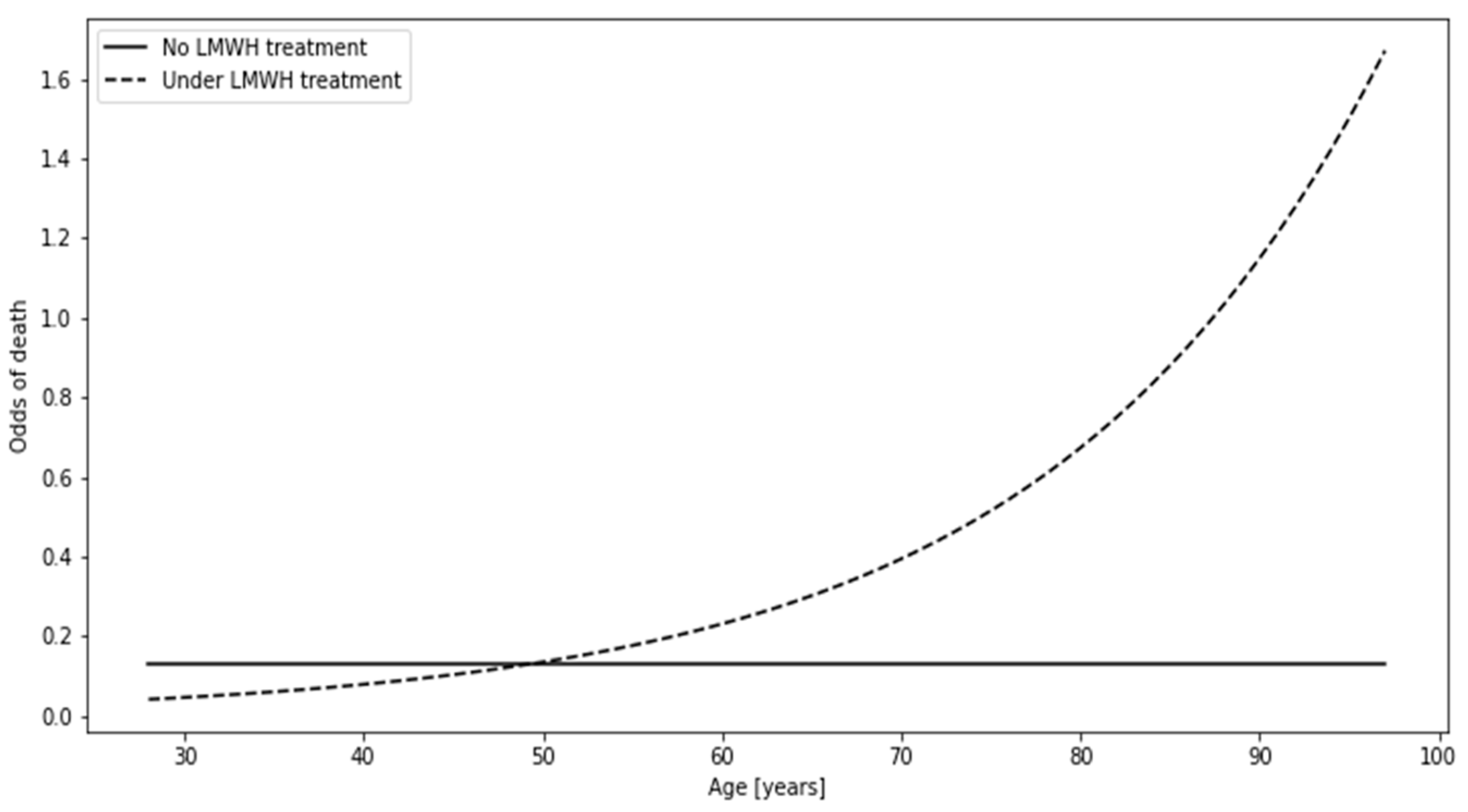
The third interaction was featured in this manuscript since it is associated with LMWH treatment, which is featured in both the multivariate model (
Figure 3) and the aforementioned interaction (
Figure 4). If one was to divide patients in the context of LMWH administration, the individuals under no such treatment would show constant odds of death equal to 0.202 regardless of age, meaning that the number of deceased patients would constitute about 1/5 of the survivors. Patients under LMWH treatment showed an increase in the odds with age, reaching the threshold which favors death at the age of approximately 85 years (odds > 1, thus N deaths > N survivors).
Before concluding the findings, study limitations need to be introduced. The first limitation comes from the rather low sample size (
Figure 1). It should be emphasized that the data of all hospitalized T2DM patients from the Temporary COVID Hospital were employed for carrying out this study. Thus, we assumed the data to be randomly collected (in spite of the sample size), since all patients participated in the process. However, low sample size restricted us to study only up to two-way interactions and forming three-way interactions to be analyzed so as to avoid redundancy. Moreover, all of the observed interactions were not added to the multivariate model (
Figure 3), having in mind that such a model would be highly prone to overfitting, thus would be biased with an increased false discovery rate. The sample size for such a model would need to be more than 1000 individuals (50–100 for every variable/interaction in the model), which exceeded the possibilities of our cooperation with this one hospital. The lack of comorbidities (seen in
Table 1) in the initial set of variables was intentional so as to remove factors that could be so strongly associated with mortality that they would render other factors too weak to be spotted upon being analyzed in a population sample of such size. This choice was made upon assessing the comorbidity-associated frequencies and their statistics in regards to mortality (
Table 1). Moreover, some patient features that could have had an impact on the observed were not registered upon creating this database in the times of COVID-19 onset. These features include BMI, diabetes duration, glycemic control, overall frailty, the stage of T2DM, and the severity of COVID-19. Since the decision on treatment with LMWH and remdesivir was made upon the admission of the study participants, there was no need for adjusting the models based on length of treatment with these agents. Our future study plans to gather the information from the patients regarding whether the treatment strategy for them changed after ending the hospitalization in the Temporary COVID-19 Hospital. Moreover, information on the post-hospitalization mortality in these individuals will be based on analyzing the national registry. Lastly, some may argue that the study shows neither goodness-of-fit metrics nor the classification quality of the model. While showing these properties of the model would be vital in a study that strived to determine the best death likelihood assessment model, our study focused on analyzing the models and interactions related to treatment. We explored the factors that may not even modulate the odds of risk per se, without their interactions with other patient characteristics (in this study: age, hsCRP and treatment with remdesivir or LMWH). Having these drawbacks in mind, we would like to encourage the readers to view this study as preliminary.
The insights from this study unfold to be rather peculiar, bringing some skepticism in the case of analyzing mortality risk with models based solely on logistic regression or (presumably) other regression methods. This study hints at possible caveats that could be encountered by simply using multivariate models without previously investigating whether the patterns of mortality changes associated with the predictors were affected by treatment. In this study, although insulin and/or metformin were not informative enough to be included in the multivariate assessment of the likelihood of death, the information about their administration revealed a contrast in how remdesivir and LMWH (in the case of metformin) compared to hsCRP and age (in the case of insulin) affected the odds of death in hospitalized T2DM patients suffering from COVID-19. Moreover, the association of LMWH treatment (one of the predictors in the multivariate model) with death was shown to be dependent on age. These observations not only show an importance of taking treatment into account when assessing death likelihood in the specific COVID-19 T2DM population, but hopefully may prove as grounds for future research into mortality modeling among T2DM patients. Although society has liberated itself from the grasp of the COVID-19 pandemic, the deleterious impact of the SARS-CoV-2 infection may come with time in the form of newly studied post-COVID syndrome, leading to a sheer increase in the frequency of various comorbidities. If this time were to come, the analysis of interactions stemming from varying intakes of drugs may pose as a key to successful risk assessment, possibly saving thousands of lives and broadening our knowledge of other threats yet to come. In the further part of this research, we plan to analyze the mortality of patients included in the presented study in the second follow-up (after two years). We will also examine levels of early markers of kidney damage, neurological disorders and intravascular damage in patients who have had symptomatic COVID-19.
5. Conclusions
In a multivariate model, along with other multivariate-adjusted significant features (LMWH treatment, age, sex, K concentration), RDW-SD was associated with mortality among the patients suffering from COVID-19 and type 2 diabetes. For every 15% increase in RDW-SD, the odds of death increased by 87.7%.
Stratification by insulin administration revealed that age and hsCRP increased the odds of death exclusively among the patients who were not administered with insulin. Metformin intake was positively associated with death among those of low age and low hsCRP. Upon increase in both age and/or hsCRP above the threshold (mapped in
Figure 5F), metformin intake started to be negatively associated with death. The impact of this effect kept rising with age and hsCRP.
Administration of remdesivir and/or LMWH changed the association between metformin and the odds of death from positive (if neither remdesivir nor LMWH were administered) to negative (if any of these drugs was administered). Moreover, remdesivir and LMWH had an additive effect on the magnitude of the pro-survival impact of metformin intake among the patients.
The association between LMWH administration and the odds of death changed from negative to positive with the increase in age.
The above findings ought to be taken with a pinch of salt until they have been validated with more sophisticated models (with these and other interactions), in a bigger population sample. Future research will, likewise, need to test these associations in a diabetic population not suffering from COVID-19.
Although metformin and insulin may not, per se, act as universal indicators of death in diabetic patients with COVID-19, their role could vary within the higher personalization of the risk assessment model (through adding and exploring their interactions with various patient characteristics). Such a practice, when utilized in large models, could provide a definite answer, cutting down the discussion of whether these agents are associated with death, when facing corroborating results from the literature. This conclusion applies regardless of whether the diabetic patients would be suffering from COVID-19 or not, since the studies into metformin and insulin in context of mortality lack the exploration of their interactions.