Genistein and Sex Hormone Supplementation Modulated Hepatic PPARα, δ, and γ Subtypes and STAT1 Expressions in a NASH Rat Model with Bilateral Orchidectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedures
2.2. Histopathological Analysis
2.3. Hepatic Lipid Deposition by Oil Red O Staining
2.4. Total RNA Preparation, cDNA Synthesis and Quantitative RT-PCR Technique
2.5. Hepatic Immunohistochemistry Processes and Analysis
2.6. Hepatic Peroxisome Proliferator-Activated Receptor Alpha, Delta, and Gamma Subtypes (PPAR α, δ, and γ) and Phospho-STAT1 Protein Expression by Immunoblot Analysis
2.7. Statistical Analysis
3. Results
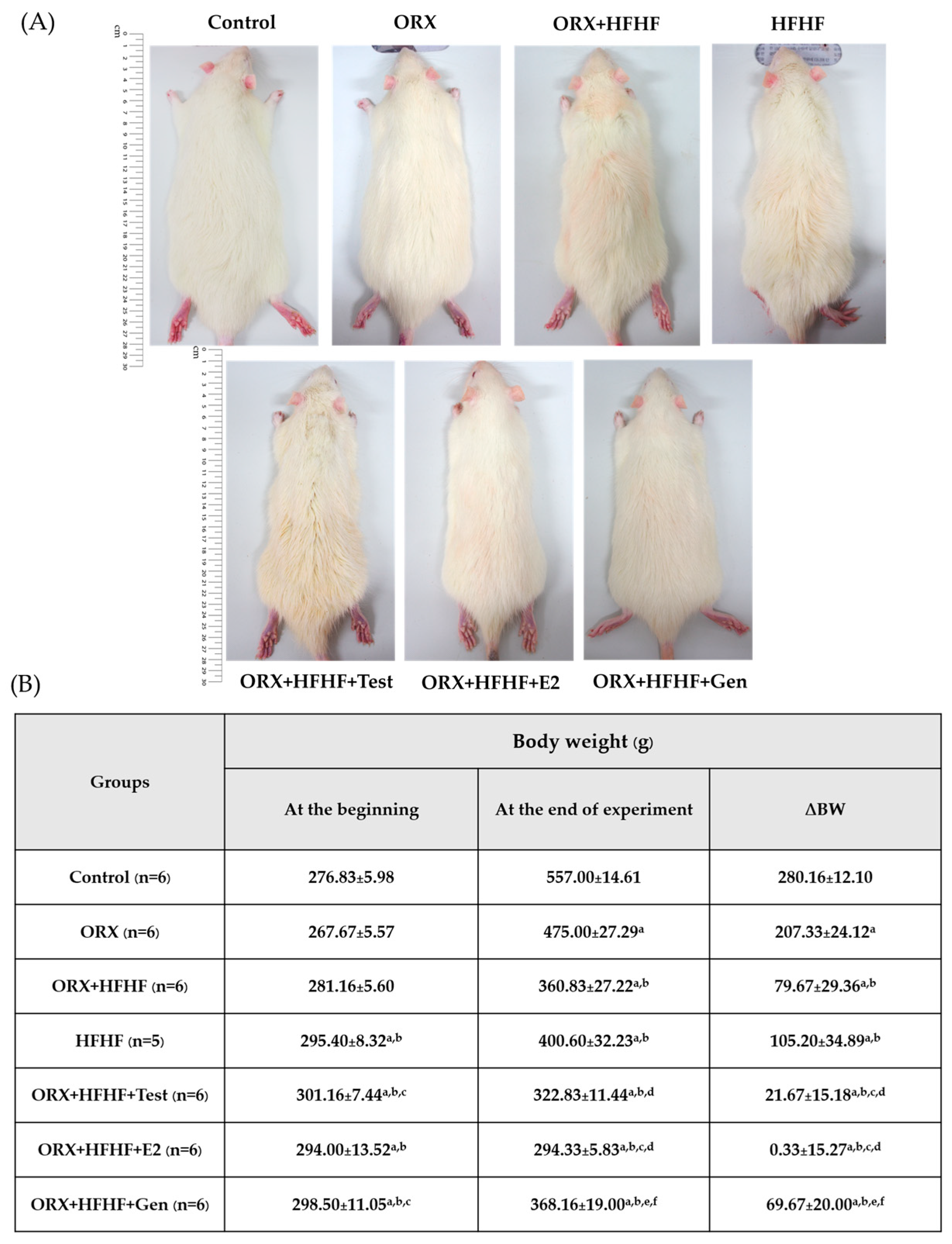
3.1. Rats’ General Appearance and Delta Body Weight Changes
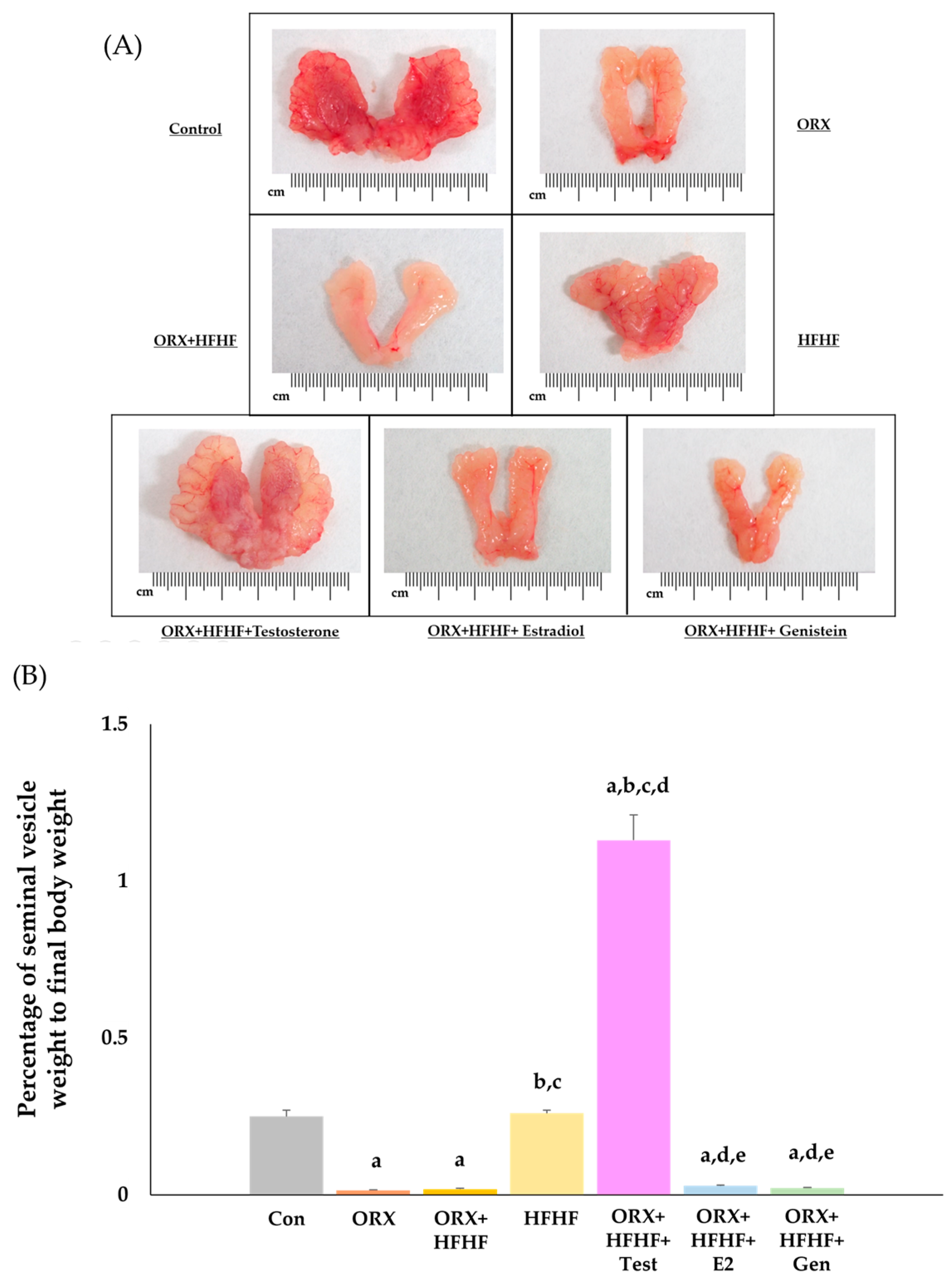
3.2. The Confirmation of Complete Bilateral Orchidectomy
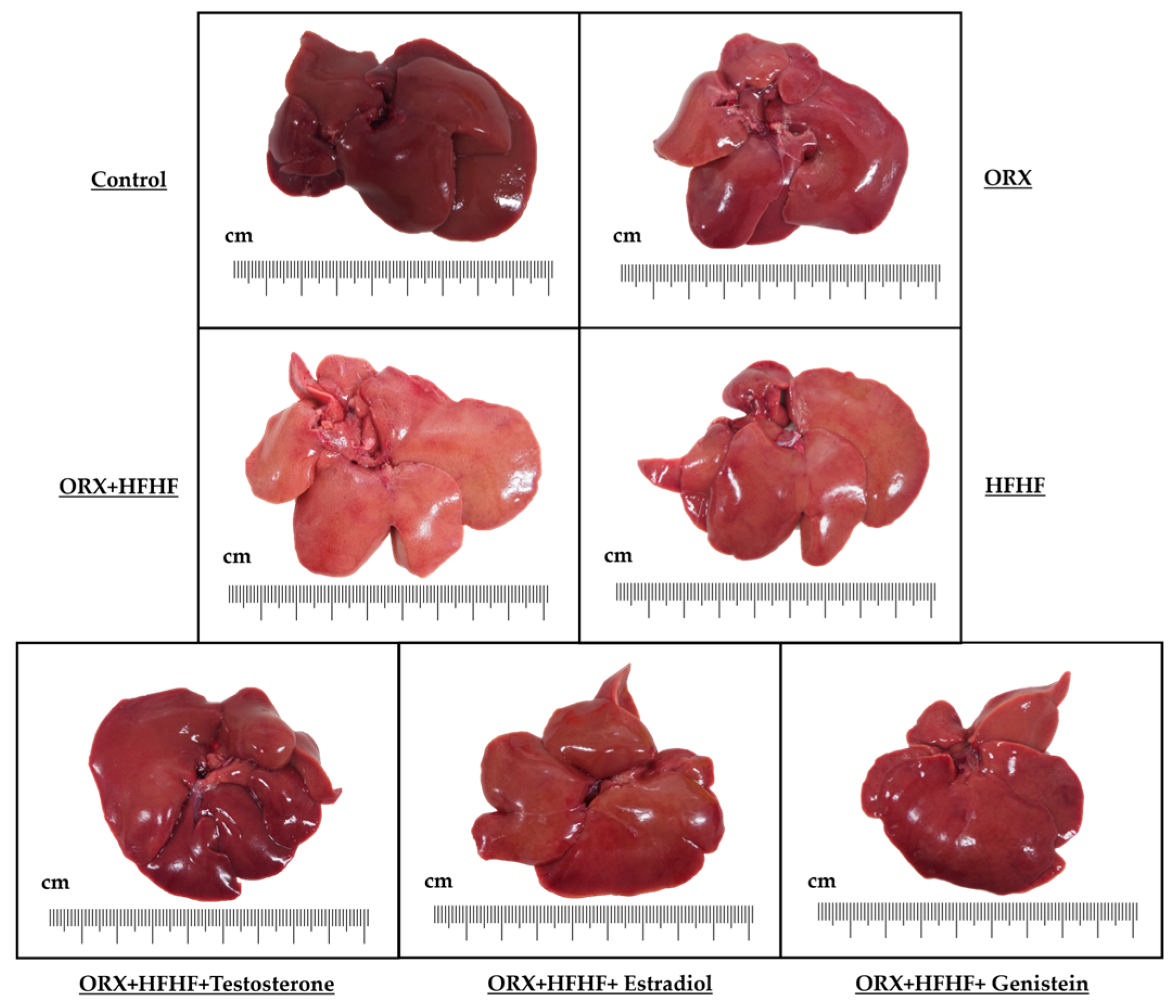
3.3. Liver General Appearance and Histopathology
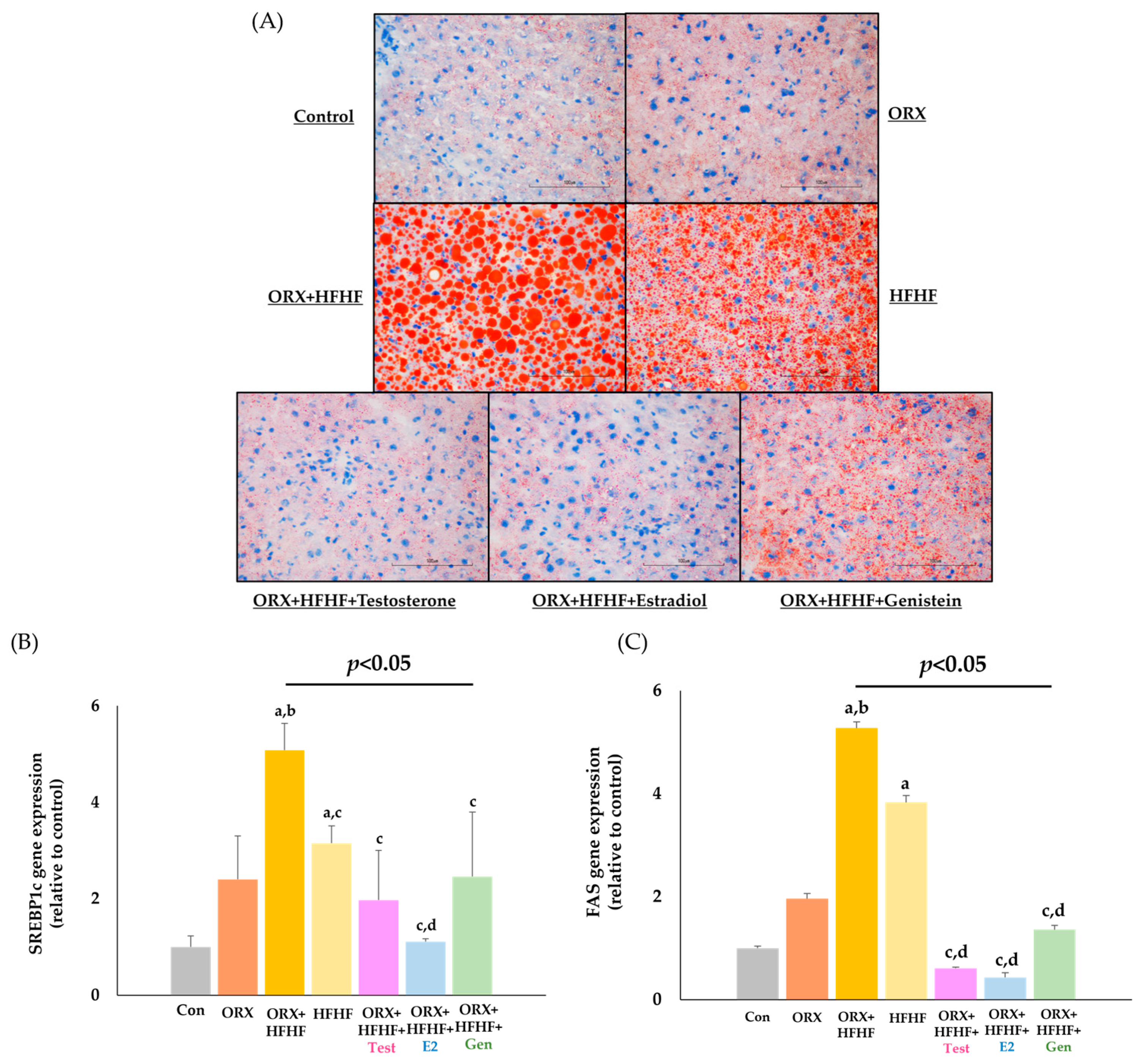
3.4. Hepatic Lipid Deposition and Lipogenic Genes Expression
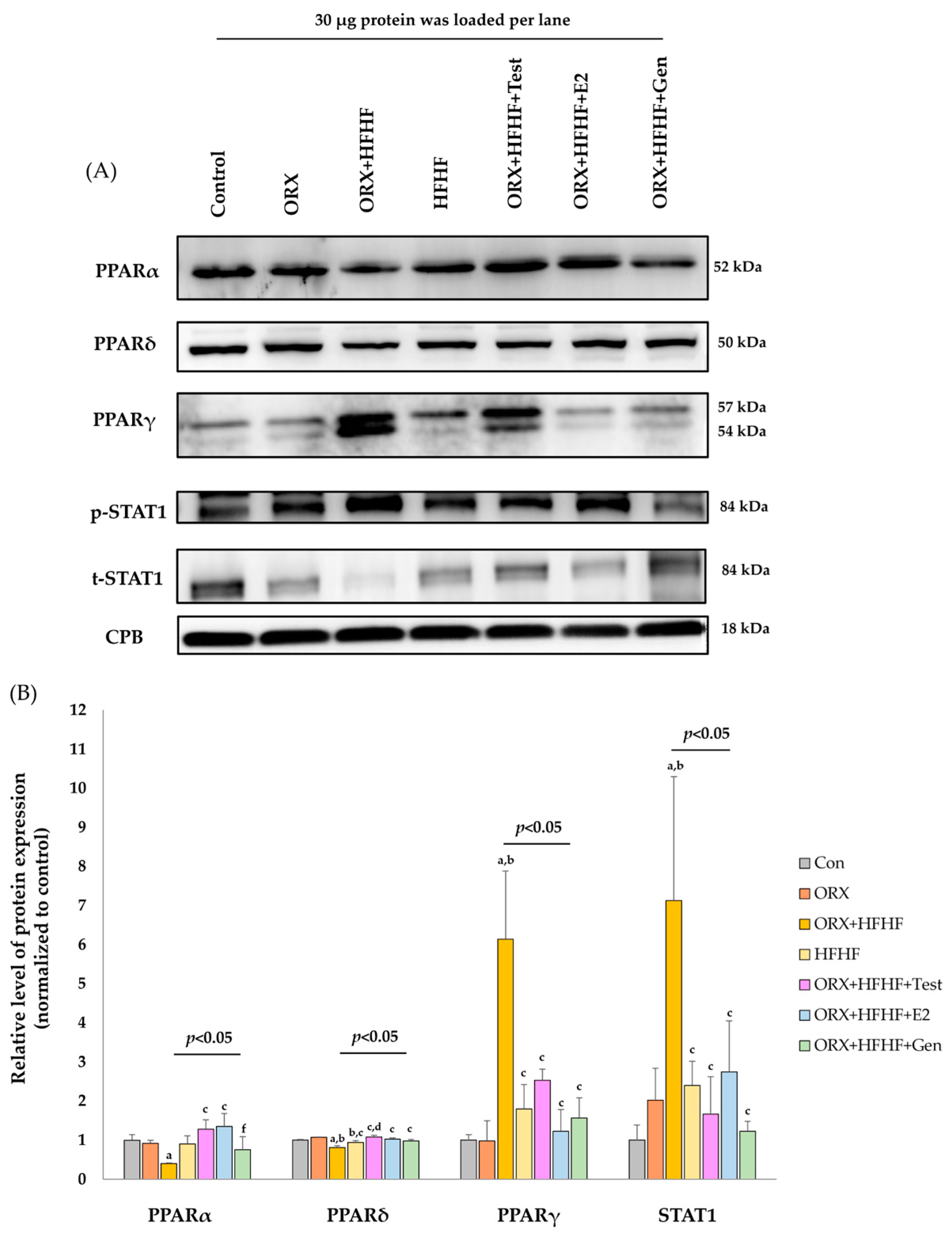
3.5. Hepatic PPARα, δ, γ and STAT1 Expression by Immonoblotting
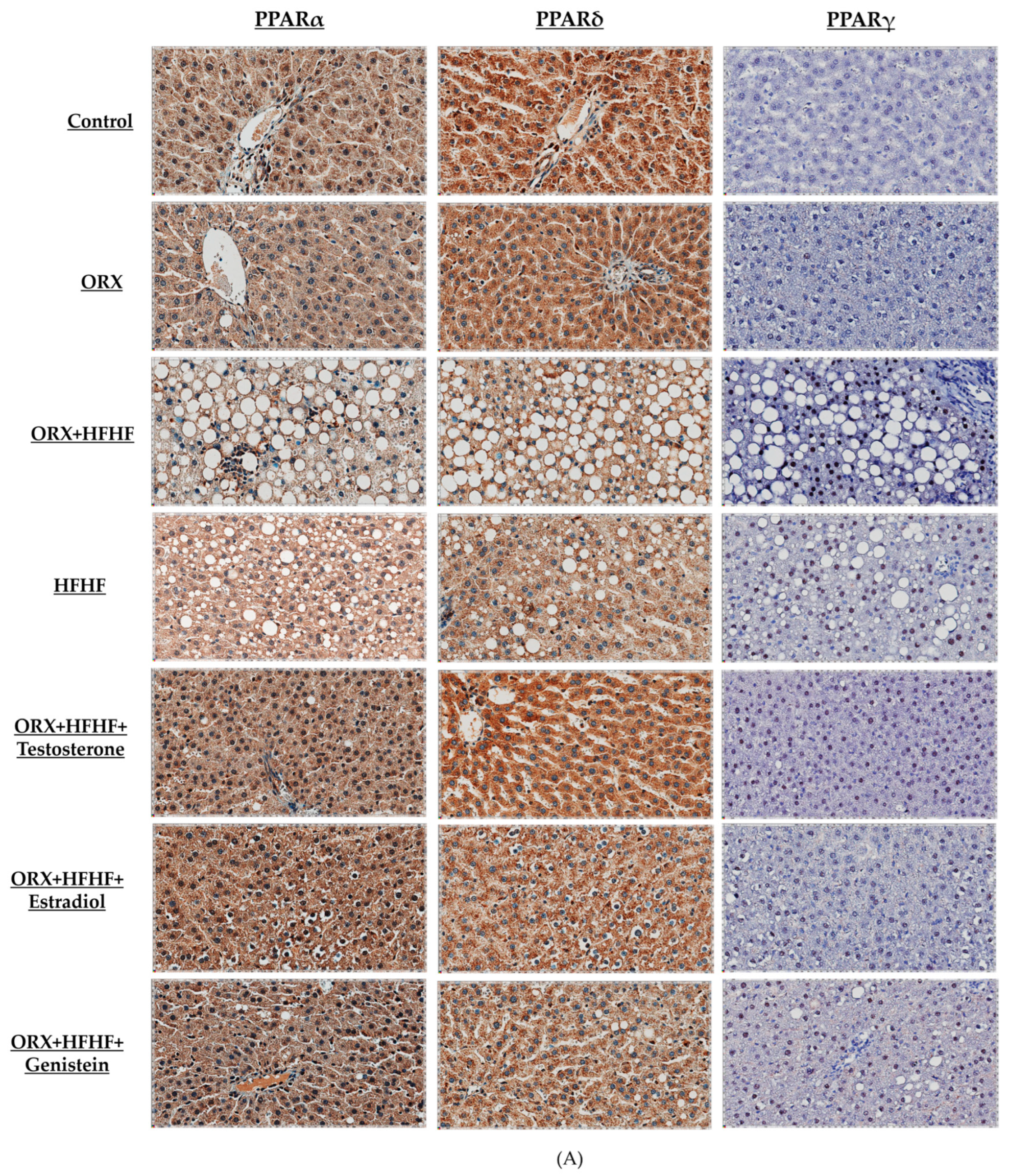
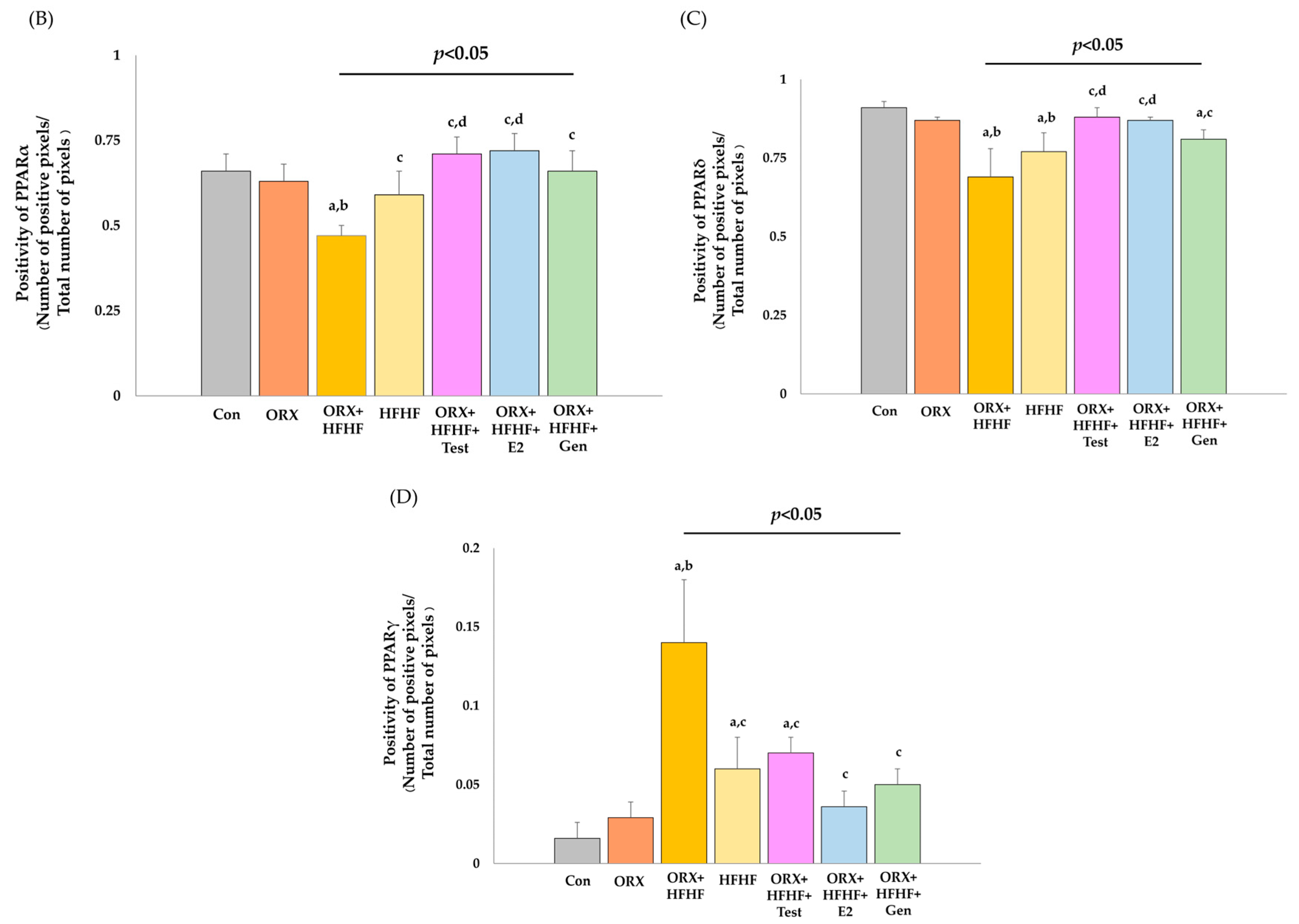
3.6. Hepatic PPARα, δ, and γ Expression by Immunohistochemistry Detection
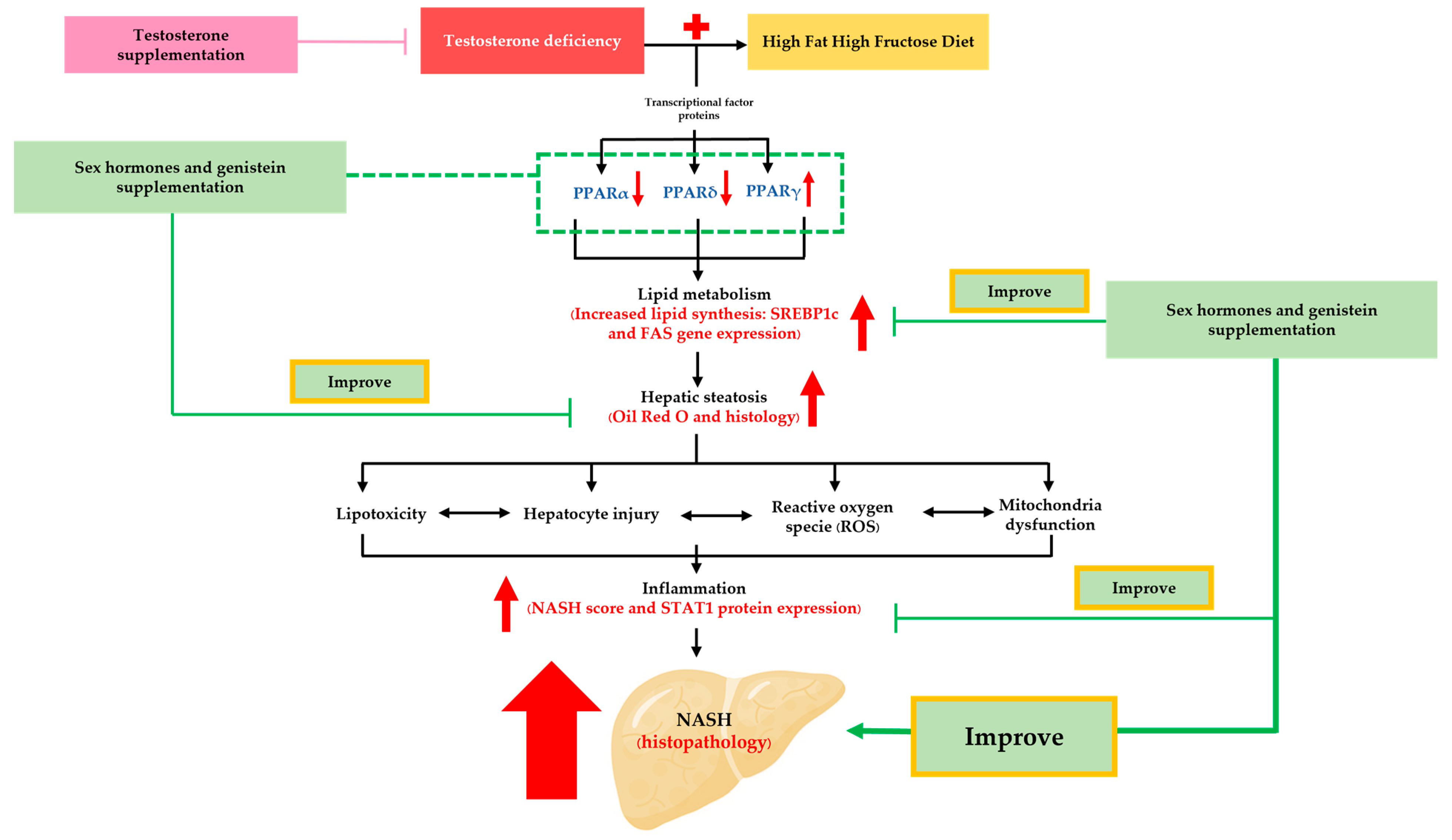
4. Discussion
4.1. Testosterone Deficiency and NASH Pathogenesis
4.2. High-Fat High-Fructose Diet Exacerbated NASH Development in Bilateral ORX Rats
4.3. Sex Hormone Supplementation Attenuated Fat Accumulation and Inflammation, and Improved NASH Pathophysiology through the Modification of Hepatic PPARα,δ,γ and STAT1 in ORX + HFHF Rats
4.4. Genistein Ameliorated Fat Accumulation and Inflammation, and Improved NASH Pathophysiology through the Modification of Hepatic PPARα,δ,γ and STAT1 in ORX + HFHF Rats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Toshimitsu, K.; Matsuura, B.; Ohkubo, I.; Niiya, T.; Furukawa, S.; Hiasa, Y.; Kawamura, M.; Ebihara, K.; Onji, M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 2007, 23, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Rosato, V.; Masarone, M.; Torre, P.; Dallio, M.; Romeo, M.; Persico, M. The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights. Nutrients 2021, 13, 1314. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Pummoung, S.; Werawatganon, D.; Klaikeaw, N.; Siriviriyakul, P. Genistein-attenuated hepatic steatosis and inflammation in nonalcoholic steatohepatitis with bilateral ovariectomized rats. Phamacognosy Mag. 2018, 14, 20–24. [Google Scholar]
- Sarkar, M.; Yates, K.; Suzuki, A.; Lavine, J.; Gill, R.; Ziegler, T.; Terrault, N.; Dhindsa, S. Low testosterone is associated with nonalcoholic steatohepatitis (NASH) and severity of NASH fibrosis in men with NAFLD. Clin. Gastroenterol. Hepatol. 2019, 19, 400–402.e2. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Jiang, X.; Pan, Y.; Chen, L.; Zhang, L.; Zhu, K.; Cai, Y.; Ling, Y.; Chen, F.; Xu, X.; et al. Transcriptomic analysis of hepatic responses to testosterone deficiency in miniature pigs fed a high-cholesterol diet. BMC Genom. 2015, 16, 59. [Google Scholar] [CrossRef]
- Chokrungvaranont, P.; Selvaggi, G.; Jindarak, S.; Angspatt, A.; Pungrasmi, P.; Suwajo, P.; Tiewtranon, P. The development of sex reassignment surgery in Thailand: A social perspective. Sci. World J. 2014, 2014, 182981. [Google Scholar] [CrossRef]
- Hehemann, M.C.; Walsh, T.J. Orchiectomy as bridge or alternative to vaginoplasty. Urol. Clin. N. Am. 2019, 46, 505–510. [Google Scholar] [CrossRef]
- Nelson, M.; Szczepaniak, L.; Wei, J.; Szczepaniak, E.; Sánchez, F.; Vilain, E.; Stern, J.; Bergman, R.; Merz, C.; Clegg, D. Transwomen and the metabolic syndrome: Is orchiectomy protective? Transgender Health 2016, 1, 165–171. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wang, L.; Li, Z.; Zhang, H.; Wu, J.; Rahman, N.; Guo, Y.; Li, D.; Li, N.; et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J. Lipid Res. 2013, 54, 345–357. [Google Scholar] [CrossRef]
- de Blok, C.J.M.; Wiepjes, C.M.; Nota, N.M.; van Engelen, K.; Adank, M.A.; Dreijerink, K.M.A.; Barbé, E.; Konings, I.R.H.M.; den Heijer, M. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in the Netherlands. Br. Med. J. 2019, 365, l1652. [Google Scholar] [CrossRef]
- Susutlertpanya, W.; Werawatganon, D.; Siriviriyakul, P.; Klaikeaw, N. Genistein attenuates nonalcoholic steatohepatitis and increases hepatic PPARγ in a rat model. Evid. Based Complement. Altern. Med. 2015, 2015, 509057. [Google Scholar] [CrossRef] [PubMed]
- Pummoung, S.; Werawatganon, D.; Chayanupatkul, M.; Klaikeaw, N.; Siriviriyakul, P. Genistein modulated lipid metabolism, hepatic PPARγ, and adiponectin expression in bilateral ovariectomized rats with nonalcoholic steatohepatitis (NASH). Antioxidants 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Xing, K.; Li, G.; Liu, D.; Guo, Y. Dietary genistein alleviates lipid metabolism disorder and inflammatory response in laying hens with fatty liver syndrome. Front. Physiol. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Felsher, D.W. A tale of two complications of obesity: NASH and hepatocellular carcinoma. Hepatology 2019, 70, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tang, W.J.; Yin, J.J.; Zhou, B.J. Signal transductions and nonalcoholic fatty liver: A mini-review. Int. J. Clin. Exp. Med. 2014, 7, 1624–1631. [Google Scholar] [PubMed]
- Grohmann, M.; Wiede, F.; Dodd, G.T.; Gurzov, E.N.; Ooi, G.J.; Butt, T.; Rasmiena, A.A.; Kaur, S.; Gulati, T.; Goh, P.K.; et al. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell 2018, 175, 1289–1306.e20. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ma, Y.; Zhang, H.; Yan, P.; Huo, L.; Hu, Y.; Chen, X.; Li, T.; Zhang, M.; Liu, Z. Differential regulation of morphology and estrogen receptor-alpha expression in the vagina of ovariectomized adult virgin rats by estrogen replacement: A histological study. Int. J. Endocrinol. 2016, 2016, 1093512. [Google Scholar] [CrossRef]
- Witayavanitkul, N.; Werawatganon, D.; Chayanupatkul, M.; Klaikeaw, N.; Siriviriyakul, P. Genistein and exercise treatment reduced NASH related HDAC3, IL-13 and MMP-12 expressions in ovariectomized rats fed with high fat high fructose diet. J. Tradit. Complement. Med. 2021, 11, 503–512. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Zuo, G.; Xu, W.; Gao, H.; Yang, Y.; Yamahara, J.; Wang, J.; Li, Y. Oleanolic acid diminishes liquid fructose-induced fatty liver in rats: Role of modulation of hepatic sterol regulatory element-binding protein-1c-mediated expression of genes responsible for de novo fatty acid dynthesis. Evid.-Based Complement. Altern. Med. 2013, 2013, 534084. [Google Scholar] [CrossRef] [PubMed]
- Battifora, H. The multitumor (sausage) tissue block: Novel method for immunohistochemical antibody testing. Lab. Investig. 1986, 55, 244–248. [Google Scholar] [PubMed]
- Vodo, S.; Bechi, N.; Petroni, A.; Muscoli, C.; Aloisi, A.M. Testosterone-induced effects on lipids and inflammation. Mediat. Inflamm. 2013, 2013, 183041. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Laurent, M.R.; Jardi, F.; Antonio, L.; Lemaire, K.; Goyvaerts, L.; Deldicque, L.; Carmeliet, G.; Decallonne, B.; Vanderschueren, D.; et al. Androgen deficiency exacerbates high-fat diet-induced metabolic alterations in male mice. Endocrinology 2016, 157, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The effects of testosterone deficiency and its replacement on inflammatory markers in rats: A pilot study. Int. J. Endocrinol. Metab. 2017, 15, e43053. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Yi, J.; Lan, X.; Lu, K.; Du, X.; Guo, Z.; Guo, Y.; Geng, M.; Li, D.; et al. IFN-γ contributes to the hepatic inflammation in HFD-induced nonalcoholic steatohepatitis by STAT1β/TLR2 signaling pathway. Mol. Immunol. 2021, 134, 118–128. [Google Scholar] [CrossRef]
- Meraz, M.A.; White, J.M.; Sheehan, K.C.F.; Bach, E.A.; Rodig, S.J.; Dighe, A.S.; Kaplan, D.H.; Riley, J.K.; Greenlund, A.C.; Campbell, D.; et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 1996, 84, 431–442. [Google Scholar] [CrossRef]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.-M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef]
- Sellmann, C.; Priebs, J.; Landmann, M.; Degen, C.; Engstler, A.J.; Jin, C.J.; Gärttner, S.; Spruss, A.; Huber, O.; Bergheim, I. Diets rich in fructose, fat or fructose and fat alter intestinal barrier function and lead to the development of nonalcoholic fatty liver disease over time. J. Nutr. Biochem. 2015, 26, 1183–1192. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Schupp, M.; Lazar, M.A. Endogenous ligands for nuclear receptors: Digging deeper. J. Biol. Chem. 2010, 285, 40409–40415. [Google Scholar] [CrossRef] [PubMed]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Francque, S.; Szabo, G.; Abdelmalek, M.F.; Byrne, C.D.; Cusi, K.; Dufour, J.-F.; Roden, M.; Sacks, F.; Tacke, F. Nonalcoholic steatohepatitis: The role of peroxisome proliferator-activated receptors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 24–39. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Klaunig, J.E. Modulation of xenobiotic nuclear receptors in high-fat diet induced non-alcoholic fatty liver disease. Toxicology 2018, 410, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Steensels, S.; Qiao, J.; Ersoy, B.A. Transcriptional regulation in non-alcoholic fatty liver disease. Metabolites 2020, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; Lalarme, E.; Maire, M.-A.; Febvret, V.; Grégoire, S.; Gambert, S.; Acar, N.; Bretillon, L. Early impairments in the retina of rats fed with high fructose/high fat diet are associated with glucose metabolism deregulation but not dyslipidaemia. Sci. Rep. 2019, 9, 5997. [Google Scholar] [CrossRef]
- Charlton, M.; Krishnan, A.; Viker, K.; Sanderson, S.; Cazanave, S.; McConico, A.; Masuoko, H.; Gores, G. Fast food diet mouse: Novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G825–G834. [Google Scholar] [CrossRef]
- Barrera, J.; Chambliss, K.L.; Ahmed, M.; Tanigaki, K.; Thompson, B.; McDonald, J.G.; Mineo, C.; Shaul, P.W. Bazedoxifene and conjugated estrogen prevent diet-induced obesity, hepatic steatosis, and type 2 diabetes in mice without impacting the reproductive tract. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E345–E354. [Google Scholar] [CrossRef]
- Mohammadi-Farani, A.; Haghighi, A.; Ghazvineh, M. Effects of long term administration of testosterone and estradiol on spatial memory in rats. Res. Pharm. Sci. 2015, 10, 407–418. [Google Scholar] [PubMed]
- Fernández-Pérez, L.; Santana-Farré, R.; de Mirecki-Garrido, M.; García, I.; Guerra, B.; Mateo-Díaz, C.; Iglesias-Gato, D.; Díaz-Chico, J.C.; Flores-Morales, A.; Díaz, M. Lipid profiling and transcriptomic analysis reveals a functional interplay between estradiol and growth hormone in liver. PLoS ONE 2014, 9, e96305. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Nettleship, J.E.; Akhtar, S.; Muraleedharan, V.; Sellers, D.J.; Brooke, J.C.; McLaren, D.S.; Channer, K.S.; Jones, T.H. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci. 2014, 109, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Fan, C.-K.; Yu, H.-J.; Wu, C.-C.; Chen, K.-C.; Liu, S.-P.; Cheng, P.-C. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. PLoS ONE 2017, 12, e0180244. [Google Scholar]
- Hou, Y.; Li, X.; Li, Q.; Xu, J.; Yang, H.; Xue, M.; Niu, G.; Zhuo, S.; Mu, K.; Wu, G.; et al. STAT1 facilitates oestrogen receptor α transcription and stimulates breast cancer cell proliferation. J. Cell. Mol. Med. 2018, 22, 6077–6086. [Google Scholar] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-A.k. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Polkowski, K.; Mazurek, A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Pol. Pharm. 2000, 57, 135–155. [Google Scholar]
- Jiang, Z.; Yang, Z.; Zhang, H.; Yao, Y.; Ma, H. Genistein activated adenosine 5’-monophosphate-activated protein kinase-sirtuin1/peroxisome proliferator-activated receptor γ coactivator-1α pathway potentially through adiponectin and estrogen receptor β signaling to suppress fat deposition in broiler chickens. Poult. Sci. 2021, 100, 246–255. [Google Scholar]
- Huang, C.; Qiao, X.; Dong, B. Neonatal exposure to genistein ameliorates high-fat diet-induced non-alcoholic steatohepatitis in rats. Br. J. Nutr. 2011, 106, 105–113. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.-R.; Van Hul, W.; Mertens, I.; et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Gao, B.; Wang, H.; Lafdil, F.; Feng, D. STAT proteins—Key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J. Hepatol. 2012, 57, 430–441. [Google Scholar] [CrossRef]
- Jantaratnotai, N.; Utaisincharoen, P.; Sanvarinda, P.; Thampithak, A.; Sanvarinda, Y. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2013, 17, 483–488. [Google Scholar] [CrossRef]

| Group | n | Steatosis | Lobular Inflammation | Hepatocyte Ballooning | NASH Activity Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | |||
| Control | 6 | 6 | - | - | - | 4 | 2 | - | - | 3 | 3 | - | 0.83 ± 0.30 |
| ORX | 6 | 6 | - | - | - | - | 5 | 1 | - | 2 | 3 | 1 | 2.00 ± 0.44 |
| ORX + HFHF | 6 | - | 1 | 2 | 3 | - | 2 | 4 | - | - | 1 | 5 | 5.50 ± 0.42 a,b |
| HFHF | 5 | 1 | 2 | 2 | - | - | 3 | 2 | - | - | 2 | 3 | 4.20 ± 0.20 a,b |
| ORX + HFHF + Testosterone | 6 | 4 | 2 | - | - | 5 | 1 | - | - | 1 | 3 | 2 | 1.83 ± 0.30 c,d |
| ORX + HFHF + Estradiol | 6 | 5 | 1 | - | - | 6 | - | - | - | - | 1 | 5 | 2.00 ± 0.00 c,d |
| ORX + HFHF + Genistein | 6 | 1 | 3 | 1 | 1 | 2 | 4 | - | - | - | 3 | 3 | 3.50 ± 0.34 a,b,c,e,f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrit, F.; Chayanupatkul, M.; Siriviriyakul, P.; Wanpiyarat, N.; Werawatganon, D. Genistein and Sex Hormone Supplementation Modulated Hepatic PPARα, δ, and γ Subtypes and STAT1 Expressions in a NASH Rat Model with Bilateral Orchidectomy. Biomedicines 2024, 12, 483. https://doi.org/10.3390/biomedicines12030483
Okrit F, Chayanupatkul M, Siriviriyakul P, Wanpiyarat N, Werawatganon D. Genistein and Sex Hormone Supplementation Modulated Hepatic PPARα, δ, and γ Subtypes and STAT1 Expressions in a NASH Rat Model with Bilateral Orchidectomy. Biomedicines. 2024; 12(3):483. https://doi.org/10.3390/biomedicines12030483
Chicago/Turabian StyleOkrit, Fatist, Maneerat Chayanupatkul, Prasong Siriviriyakul, Natcha Wanpiyarat, and Duangporn Werawatganon. 2024. "Genistein and Sex Hormone Supplementation Modulated Hepatic PPARα, δ, and γ Subtypes and STAT1 Expressions in a NASH Rat Model with Bilateral Orchidectomy" Biomedicines 12, no. 3: 483. https://doi.org/10.3390/biomedicines12030483
APA StyleOkrit, F., Chayanupatkul, M., Siriviriyakul, P., Wanpiyarat, N., & Werawatganon, D. (2024). Genistein and Sex Hormone Supplementation Modulated Hepatic PPARα, δ, and γ Subtypes and STAT1 Expressions in a NASH Rat Model with Bilateral Orchidectomy. Biomedicines, 12(3), 483. https://doi.org/10.3390/biomedicines12030483







