The Hemorrhagic Side of Primary Angiitis of the Central Nervous System (PACNS)
Abstract
1. Introduction
2. Diagnostic Challenges in PACNS
3. Intracerebral Bleeding in PACNS
3.1. Clinical Issues
3.2. Neuroimaging Issues
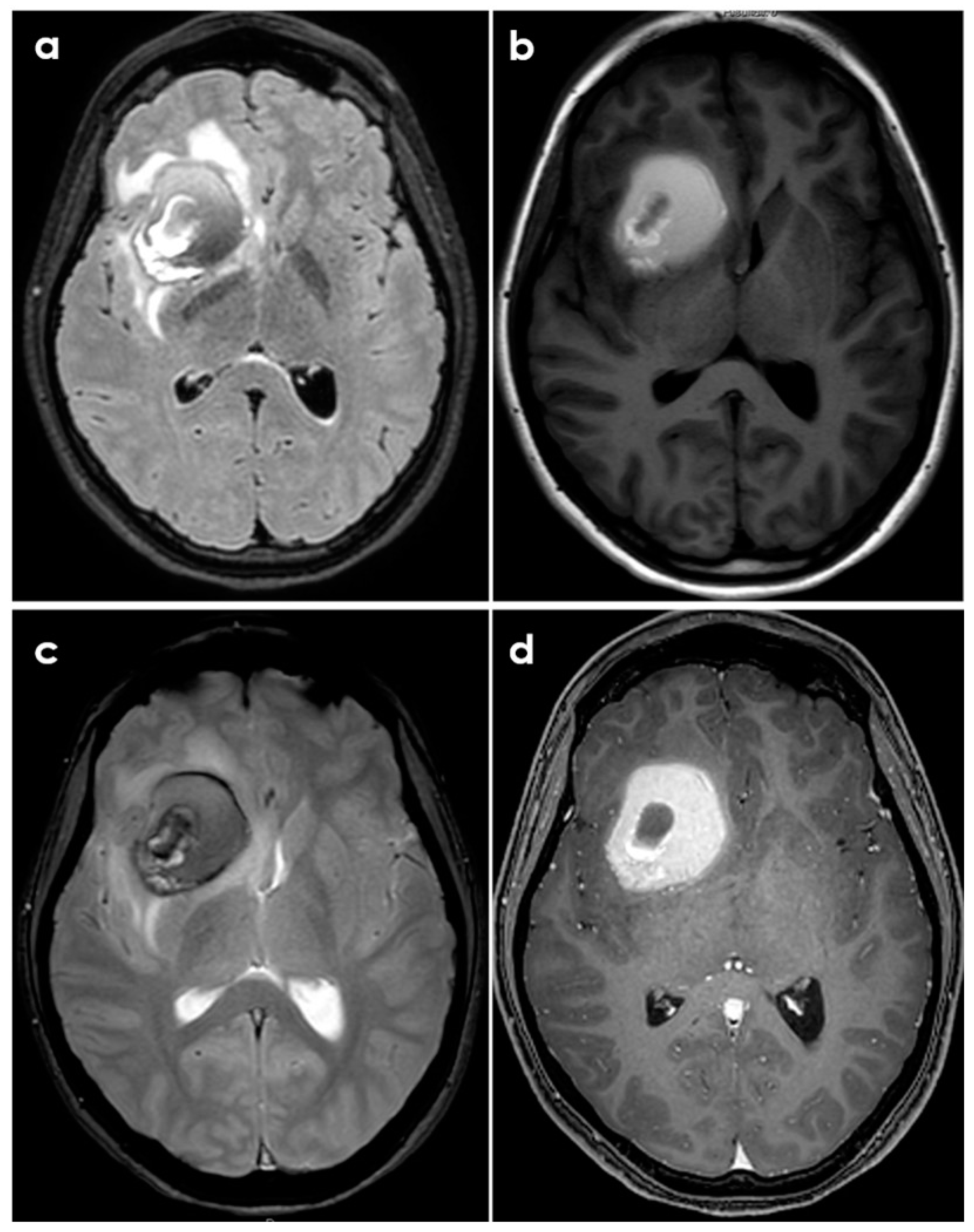
- Case A had a small right frontal cortical hemorrhage with subarachnoid extension and leptomeningeal enhancement over the right hemisphere on post-gadolinium T1-weighted sequences, persisting over the following 3 months together with accumulation of small acute infarcts and persistent leptomeningeal enhancement; CTA was normal, and the diagnosis was achieved by brain biopsy.
- Case B had a right cerebellar hemorrhage on anticoagulant therapy and underwent emergent posterior fossa decompression. In the early follow-up, a new left cerebellar hemorrhage and an acute left pontine infarction occurred; the diagnosis was provided by histopathologic examination of the surgically evacuated tissue.
- Case C had a right parietal lobar hemorrhage with subarachnoid extension, multiple small disseminated acute and subacute infarcts and small rounded sulcal hyperintensities on a fluid-attenuated inversion recovery sequence (dot sign); the diagnosis was provided by the angiographic pattern involving distal branch arteries.
- Case D had a right temporal lobe hemorrhage with small disseminated acute infarcts and chronic microbleeds; diagnosis was provided by angiography, and biopsy was negative.
3.3. Histopathological Issues
4. Management Issues
5. Critical Appraisal and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calabrese, L.H.; Mallek, J.A. Primary angiitis of the central nervous system: Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine 1988, 67, 20–39. [Google Scholar] [CrossRef]
- Birnbaum, J.; Hellmann, D.B. Primary Angiitis of the Central Nervous System. Arch. Neurol. 2009, 66, 704–709. [Google Scholar] [CrossRef]
- Hajj-Ali, R.A.; Furlan, A.; Abou-Chebel, A.; Calabrese, L.H. Benign angiopathy of the central nervous system: Cohort of 16 patients with clinical course and long-term followup. Arthritis Rheum. 2002, 47, 662–669. [Google Scholar] [CrossRef]
- Miller, T.R.; Shivashankar, R.; Mossa-Basha, M.; Gandhi, D. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. AJNR Am. J. Neuroradiol. 2015, 36, 1392–1399. [Google Scholar] [CrossRef]
- Pascarella, R.; Antonenko, K.; Boulouis, G.; De Boysson, H.; Giannini, C.; Heldner, M.R.; Kargiotis, O.; Nguyen, T.N.; Rice, C.M.; Salvarani, C.; et al. European Stroke Organisation (ESO) guidelines on Primary Angiitis of the Central Nervous System (PACNS). Eur. Stroke J. 2023, 8, 842–879. [Google Scholar] [CrossRef]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Brown, R.D.; Christianson, T.; Miller, D.V.; Giannini, C.; Huston, J.; Hunder, G.G. An update of the Mayo Clinic cohort of patients with adult primary central nervous system vasculitis: Description of 163 patients. Medicine 2015, 94, e738. [Google Scholar] [CrossRef]
- Ferro, J.M. Vasculitis of the central nervous system. J. Neurol. 1998, 245, 766–776. [Google Scholar] [CrossRef]
- Nonaka, H.; Akiba, M.; Hatori, T.; Nagayama, T.; Zhang, Z.; Ihara, F. Microvasculature of the human cerebral white matter: Arteries of the deep white matter. Neuropathology 2003, 23, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Duna, G.F.; Calabrese, L.H. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J. Rheumatol. 1995, 22, 662–667. [Google Scholar] [PubMed]
- Singhal, A.B.; Hajj-Ali, R.A.; Topcuoglu, M.A.; Fok, J.; Bena, J.; Yang, D.; Calabrese, L.H. Reversible cerebral vasoconstriction syndromes: Analysis of 139 cases. Arch. Neurol. 2011, 68, 1005–1012. [Google Scholar] [CrossRef]
- Singhal, A.B.; Topcuoglu, M.A.; Fok, J.W.; Kursun, O.; Nogueira, R.G.; Frosch, M.P.; Caviness, V.S. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: Clinical, imaging, and angiographic comparison. Ann. Neurol. 2016, 79, 882–894. [Google Scholar] [CrossRef]
- Ducros, A.; Fiedler, U.; Porcher, R.; Boukobza, M.; Stapf, C.; Bousser, M.G. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: Frequency, features, and risk factors. Stroke 2010, 41, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Brown, R.D., Jr.; Calamia, K.T.; Christianson, T.J.H.; Huston, J.; Meschia, J.F.; Giannini, C.; Miller, D.V.; Hunder, G.G. Primary central nervous system vasculitis presenting with intracranial hemorrhage. Arthritis Rheum. 2011, 63, 3598–3606. [Google Scholar] [CrossRef]
- Topcuoglu, M.A.; Singhal, A.B. Hemorrhagic reversible cerebral vasoconstriction syndrome: Features and mechanisms. Stroke 2016, 47, 1742–1747. [Google Scholar] [CrossRef]
- Topcuoglu, M.A.; Jha, R.M.; George, J.; Frosch, M.P.; Singhal, A.B. Hemorrhagic primary CNS angiitis and vasoconstrictive drug exposure. Neurol. Clin. Pract. 2017, 7, 26–34. [Google Scholar] [CrossRef] [PubMed]
- de Boysson, H.; Zuber, M.; Naggara, O.; Neau, J.; Gray, F.; Bousser, M.; Crassard, I.; Touzé, E.; Couraud, P.; Kerschen, P.; et al. Primary angiitis of the central nervous system: Description of the first fifty-two adults enrolled in the French cohort of patients with primary vasculitis of the central nervous system. Arthritis Rheumatol. 2014, 66, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Barra, L.J.; Sposato, L.A.; Mandzia, J.L. Primary CNS vasculitis: A systematic review on clinical characteristics associated with abnormal biopsy and angiography. Autoimmun. Rev. 2021, 20, 102714. [Google Scholar] [CrossRef]
- McVerry, F.; McCluskey, G.; McCarron, P.; Muir, K.W.; McCarron, M.O. Diagnostic test results in primary CNS vasculitis: A systematic review of published cases. Neurol. Clin. Pract. 2017, 7, 256–265. [Google Scholar] [CrossRef]
- Beuker, C.; Strunk, D.; Rawal, R.; Schmidt-Pogoda, A.; Werring, N.; Milles, L.; Minnerup, J. Primary angiitis of the CNS: A systematic review and meta-analysis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1093. [Google Scholar] [CrossRef]
- Sarti, C.; Picchioni, A.; Telese, R.; Pasi, M.; Failli, Y.; Pracucci, G.; Cammelli, D.; Inzitari, D. “When should primary angiitis of the central nervous system (PACNS) be suspected?”: Literature review and proposal of a preliminary screening algorithm. Neurol. Sci. 2020, 41, 3135–3148. [Google Scholar] [CrossRef]
- Thaler, C.; Kaufmann-Bühler, A.-K.; Gansukh, T.; Gansukh, A.; Schuster, S.; Bachmann, H.; Thomalla, G.; Magnus, T.; Matschke, J.; Fiehler, J.; et al. Neuroradiologic Characteristics of Primary Angiitis of the Central Nervous System According to the Affected Vessel Size. Clin. Neuroradiol. 2019, 29, 37–44. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023, 22, 602–618. [Google Scholar] [CrossRef]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Raposo, N.; Zanon Zotin, M.C.; Seiffge, D.J.; Li, Q.; Goeldlin, M.B.; Charidimou, A.; Shoamanesh, A.; Jäger, H.R.; Cordonnier, C.; Klijn, C.J.; et al. A Causal Classification System for Intracerebral Hemorrhage Subtypes. Ann Neurol. 2023, 93, 16–28. [Google Scholar] [CrossRef]
- Scolding, N.J.; Joseph, F.; Kirby, P.A.; Mazanti, I.; Gray, F.; Mikol, J.; Ellison, D.; Hilton, D.A.; Williams, T.L.; MacKenzie, J.M.; et al. Abeta-related angiitis: Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005, 128 Pt 3, 500–515. [Google Scholar] [CrossRef]
- Auriel, E.; Charidimou, A.; Gurol, M.E.; Ni, J.; Van Etten, E.S.; Martinez-Ramirez, S.; Boulouis, G.; Piazza, F.; DiFrancesco, J.C.; Frosch, M.P.; et al. Validation of Clinicoradiological Criteria for the Diagnosis of Cerebral Amyloid Angiopathy-Related Inflammation. JAMA Neurol. 2016, 73, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Antolini, L.; DiFrancesco, J.C.; Zedde, M.; Basso, G.; Arighi, A.; Shima, A.; Cagnin, A.; Caulo, M.; Carare, R.O.; Charidimou, A.; et al. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology 2021, 97, e1809–e1822. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Brown, R.D., Jr.; Christianson, T.J.; Huston, J.; Giannini, C.; Hunder, G.G. Long-term remission, relapses and maintenance therapy in adult primary central nervous system vasculitis: A single-center 35-year experience. Autoimmun. Rev. 2020, 19, 102497. [Google Scholar] [CrossRef]
- Lieber, A.C.; McNeill, I.T.; Scaggiante, J.; Nistal, D.A.; Fowkes, M.; Umphlett, M.; Pan, J.; Roussos, P.; Mobbs, C.V.; Mocco, J.; et al. Biopsy During Minimally Invasive Intracerebral Hemorrhage Clot Evacuation. World Neurosurg. 2019, 124, e169–e175. [Google Scholar] [CrossRef] [PubMed]


| Year of Publication | Criteria |
|---|---|
| 1988 [1] |
|
| 2009 [2] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Napoli, M.; Moratti, C.; Pezzella, F.R.; Seiffge, D.J.; Tsivgoulis, G.; Caputi, L.; Salvarani, C.; Toni, D.; Valzania, F.; et al. The Hemorrhagic Side of Primary Angiitis of the Central Nervous System (PACNS). Biomedicines 2024, 12, 459. https://doi.org/10.3390/biomedicines12020459
Zedde M, Napoli M, Moratti C, Pezzella FR, Seiffge DJ, Tsivgoulis G, Caputi L, Salvarani C, Toni D, Valzania F, et al. The Hemorrhagic Side of Primary Angiitis of the Central Nervous System (PACNS). Biomedicines. 2024; 12(2):459. https://doi.org/10.3390/biomedicines12020459
Chicago/Turabian StyleZedde, Marialuisa, Manuela Napoli, Claudio Moratti, Francesca Romana Pezzella, David Julian Seiffge, Georgios Tsivgoulis, Luigi Caputi, Carlo Salvarani, Danilo Toni, Franco Valzania, and et al. 2024. "The Hemorrhagic Side of Primary Angiitis of the Central Nervous System (PACNS)" Biomedicines 12, no. 2: 459. https://doi.org/10.3390/biomedicines12020459
APA StyleZedde, M., Napoli, M., Moratti, C., Pezzella, F. R., Seiffge, D. J., Tsivgoulis, G., Caputi, L., Salvarani, C., Toni, D., Valzania, F., & Pascarella, R. (2024). The Hemorrhagic Side of Primary Angiitis of the Central Nervous System (PACNS). Biomedicines, 12(2), 459. https://doi.org/10.3390/biomedicines12020459








