Glycine Transporter 1 Inhibitors: Predictions on Their Possible Mechanisms in the Development of Opioid Analgesic Tolerance
Abstract
1. Introduction
2. N-Methyl-D-aspartate Acid Glutamate Receptors as Key Players in the Development of Opioid Analgesic Tolerance
2.1. Metabotropic Glutamate and NMDA-Type Glutamate Receptors
2.2. Glutamatergic Ionotropic NMDA Receptors and Opioid Analgesic Tolerance
| Glutamate Receptor Subunits | Area (Region) | Distribution Pattern | Subject | References |
|---|---|---|---|---|
| GluN1 (NMDAR1) | GluN1-2: widely distributed in the CNS GluN1-1: in rostral regions (e.g., cortex) GluN1-4: in caudal regions (e.g., brainstem) Dorsal horn of spinal cord Spinal cord Spinal cord: laminae I–III | +++ +++ +++ +++ +++ +++ | Rat | [64,65,66,67,68] |
| GluN2A (NMDAR2A) | Cerebral cortex Substantia gelatinosa neurons (synaptic localization) Spinal cord | +++ +++ | Rat | [67,69,70] |
| GluN2B (NMDAR2B) | Telencephalon and thalamus DRG neurons (primary afferent neurons) Substantia gelatinosa neurons (extrasynaptic localization) Spinal cord: laminae I–III Spinal cord: lamina II (and IX) | +++ +++ * ++/+++ +/++ | Rat | [67,68,69,70,71] |
| GluN2C (NMDAR2C) | All regions except cerebellar cortex Spinal cord: laminae I–III Spinal cord | + 0 0 | Rat | [67,68,69] |
| GluN2D (NMDAR2D) | Brainstem, cortex Substantia gelatinosa neurons Spinal cord | + * 0 | Rat | [67,69,70] |
| GluN3A (NR3A) | Thalamus (VPL) Cervical spinal cord: in proximity to the dorsal horn | ++ +++ | Rat (postnatal day 16) | [72] |
| GluN3B (NR3B) | Cerebral cortex Spinal cord: laminae I–II (and VIII-IX) (**) Spinal cord: laminae III–VI | +++ ++/+++ | Rat | [73] |
| Glutamate Re-ceptor Subunits | Endogenous Agonists | Exogenous Agonists | Exogenous Antagonists | Channel Blocker | |||
|---|---|---|---|---|---|---|---|
| Glutamate Site | Glycine Site | Glutamate Site | Glycine Site | Glutamate Site | Glycine Site | ||
| GluN1 (NMDAR1) | L and D-Asp | Glycine D-serine | NMDA HQA * | (+)-HA966 * | - | 5,7-DCKA | |
| GluN2A (NMDAR2A) | L and D-Asp | Glycine D-serine | NMDA HQA * | (+)-HA966 * | D-AP5 | 5,7-DCKA | Mg2+, MK-801, ketamine, phencyclidine, amantadine. |
| GluN2B (NMDAR2B) | L and D-Asp | Glycine D-serine | NMDA HQA * | (+)-HA966 * | D-AP5 | 5,7-DCKA | Mg2+, MK-801, ketamine, Phencyclidine, amantadine. |
| GluN2C (NMDAR2C) | L and D-Asp | Glycine D-serine | NMDA HQA * | - | D-AP5 | 5,7-DCKA | Phencyclidine, ketamine, amantadine, Mg2+, MK-801. |
| GluN2D (NMDAR2D) | L and D-Asp | Glycine D-serine | NMDA HQA * | - | D-AP5 | 5,7-DCKA | Mg2+, MK-801, amantadine, ketamine, phencyclidine. |
| GluN3A (NR3A) | |||||||
| GluN3B (NR3B) | |||||||
3. Glycine Transporter Type 1 and N-Methyl-D-aspartate Acid Glutamate Receptors: Their Locations and Functions in the Glial–Neural Tripartite Synapse
3.1. Classification of Glycine Transporter Inhibitors
3.2. Compounds Acting as Ligands for Glycine Transporter Type 1 and NMDA Receptor Interactions: Some Operational Characteristics
4. Interplay between Glycine Transporter Type 1 and N-Methyl-D-aspartate Acid Glutamate Receptors in Reversal of Opioid Tolerance: A Hypothesis
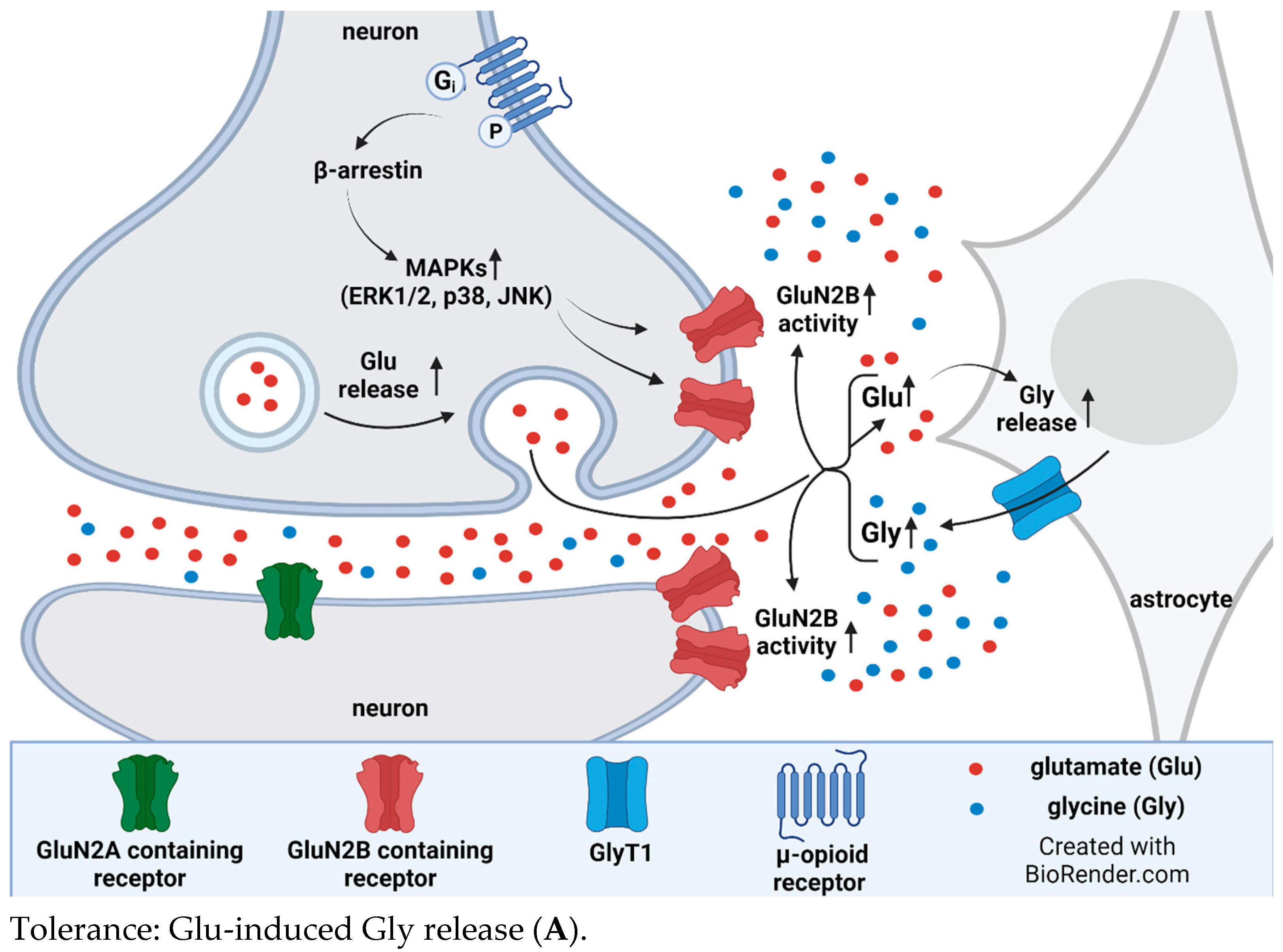
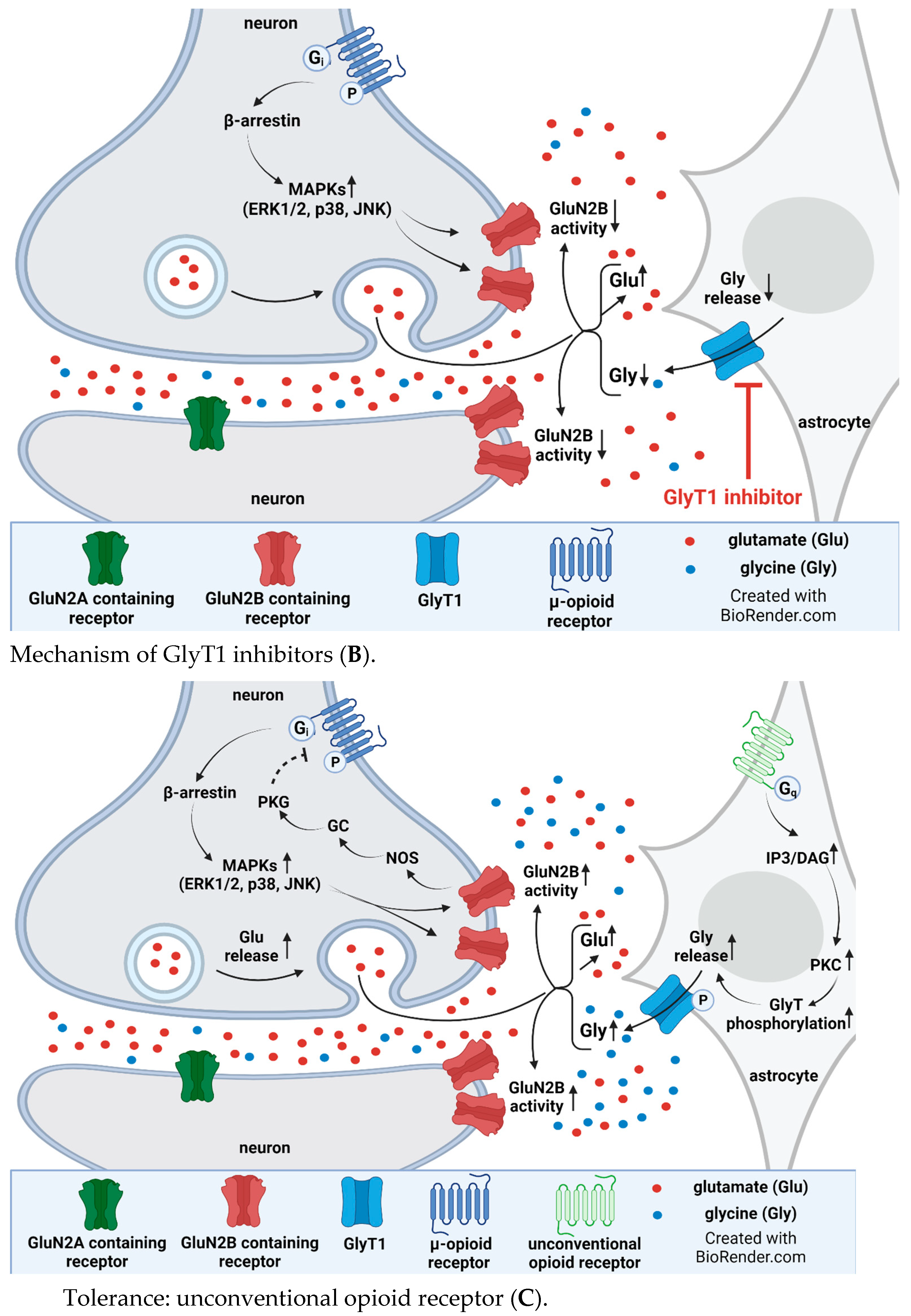
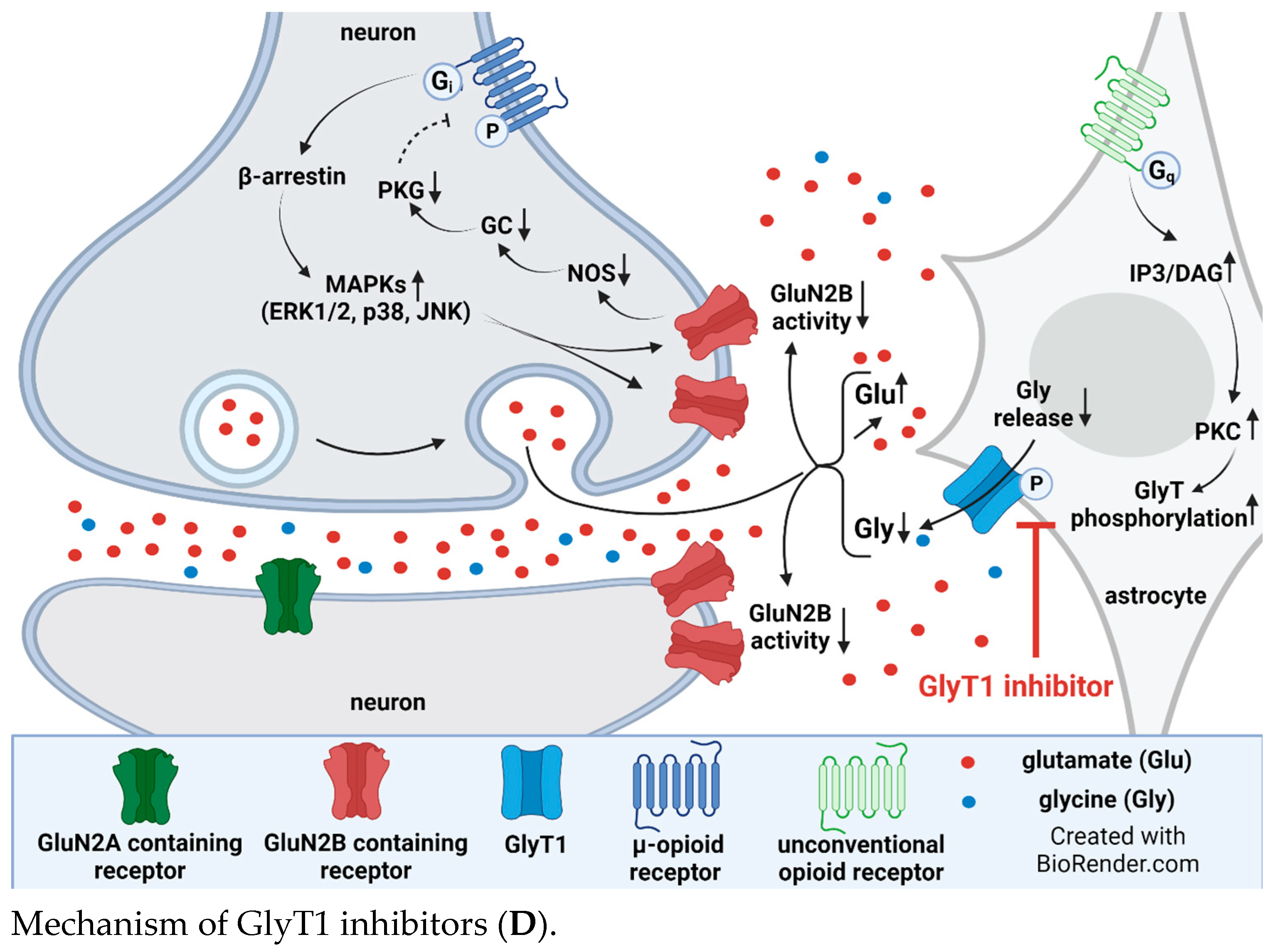
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Essmat, N.; Karádi, D.; Zádor, F.; Király, K.; Fürst, S.; Al-Khrasani, M. Insights into the Current and Possible Future Use of Opioid Antagonists in Relation to Opioid-Induced Constipation and Dysbiosis. Molecules 2023, 28, 7766. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, K.; Caputi, F.F.; Hanuska, A.; Kató, E.; Balogh, M.; Köles, L.; Palmisano, M.; Riba, P.; Hosztafi, S.; Romualdi, P.; et al. A new potent analgesic agent with reduced liability to produce morphine tolerance. Brain Res. Bull. 2015, 117, 32–38. [Google Scholar] [CrossRef]
- Cahill, C.M.; Walwyn, W.; Taylor, A.M.; Pradhan, A.A.; Evans, C.J. Allostatic Mechanisms of Opioid Tolerance Beyond Desensitization and Downregulation. Trends Pharmacol. Sci. 2016, 37, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Kaplovitch, E.; Gomes, T.; Camacho, X.; Dhalla, I.A.; Mamdani, M.M.; Juurlink, D.N. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0134550. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.J.; Krebs, E.E.; Hudson, T.; Brown, J.; Li, C.; Martin, B.C. Impact of opioid dose escalation on the development of substance use disorders, accidents, self-inflicted injuries, opioid overdoses and alcohol and non-opioid drug-related overdoses: A retrospective cohort study. Addiction 2020, 115, 1098–1112. [Google Scholar] [CrossRef]
- Henry, S.G.; Wilsey, B.L.; Melnikow, J.; Iosif, A.-M. Dose Escalation During the First Year of Long-Term Opioid Therapy for Chronic Pain. Pain Med. 2015, 16, 733–744. [Google Scholar] [CrossRef]
- Chen, S.-R.; Pan, H.-L. Blocking μ opioid receptors in the spinal cord prevents the analgesic action by subsequent systemic opioids. Brain Res. 2006, 1081, 119–125. [Google Scholar] [CrossRef]
- Ballantyne, J.C.; Koob, G.F. Allostasis theory in opioid tolerance. Pain 2021, 162, 2315–2319. [Google Scholar] [CrossRef]
- Fürst, S.; Zádori, Z.S.; Zádor, F.; Király, K.; Balogh, M.; László, S.B.; Hutka, B.; Mohammadzadeh, A.; Calabrese, C.; Galambos, A.R.; et al. On the Role of Peripheral Sensory and Gut Mu Opioid Receptors: Peripheral Analgesia and Tolerance. Molecules 2020, 25, 2473. [Google Scholar] [CrossRef]
- Gong, K.; Bhargava, A.; Jasmin, L. GluN2B N-methyl-D-aspartate receptor and excitatory amino acid transporter 3 are upregulated in primary sensory neurons after 7 days of morphine administration in rats: Implication for opiate-induced hyperalgesia. Pain 2016, 157, 147–158. [Google Scholar] [CrossRef]
- Manning, B.H.; Mao, J.; Frenk, H.; Price, D.D.; Mayer, D.J. Continuous co-administration of dextromethorphan or MK-801 with morphine: Attenuation of morphine dependence and naloxone-reversible attenuation of morphine tolerance. Pain 1996, 67, 79–88. [Google Scholar] [CrossRef]
- Trujillo, K.A.; Akil, H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 1991, 251, 85–87. [Google Scholar] [CrossRef] [PubMed]
- van der Staay, F.J.; Rutten, K.; Erb, C.; Blokland, A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav. Brain Res. 2011, 220, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.B.; Ho, R. Controversies of the Effect of Ketamine on Cognition. Front. Psychiatry 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, R.; Jin, Y.; Fang, J.; Du, J.; Shao, X.; Liang, Y.; Fang, J. Molecular mechanisms of opioid tolerance: From opioid receptors to inflammatory mediators (Review). Exp. Ther. Med. 2021, 22, 1004. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Ingram, S.L.; Henderson, G.; Chavkin, C.; von Zastrow, M.; Schulz, S.; Koch, T.; Evans, C.J.; Christie, M.J. Regulation of µ-Opioid Receptors: Desensitization, Phosphorylation, Internalization, and Tolerance. Pharmacol. Rev. 2013, 65, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Lemel, L.; Lane, J.R.; Canals, M. GRKs as Key Modulators of Opioid Receptor Function. Cells 2020, 9, 2400. [Google Scholar] [CrossRef]
- Duarte, M.L.; Devi, L.A. Post-translational Modifications of Opioid Receptors. Trends Neurosci. 2020, 43, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Liu, N.; Gintzler, A.R. Phosphorylation of unique C-terminal sites of the mu-opioid receptor variants 1B2 and 1C1 influences their Gs association following chronic morphine. J. Neurochem. 2020, 152, 449–467. [Google Scholar] [CrossRef]
- Bailey, C.P.; Smith, F.L.; Kelly, E.; Dewey, W.L.; Henderson, G. How important is protein kinase C in μ-opioid receptor desensitization and morphine tolerance? Trends Pharmacol. Sci. 2006, 27, 558–565. [Google Scholar] [CrossRef]
- Allouche, S.; Noble, F.; Marie, N. Opioid receptor desensitization: Mechanisms and its link to tolerance. Front. Pharmacol. 2014, 5, 280. [Google Scholar] [CrossRef]
- Dang, V.C.; Christie, M.J. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br. J. Pharmacol. 2012, 165, 1704–1716. [Google Scholar] [CrossRef]
- Deng, M.; Chen, S.; Chen, H.; Luo, Y.; Dong, Y.; Pan, H. Mitogen-activated protein kinase signaling mediates opioid-induced presynaptic NMDA receptor activation and analgesic tolerance. J. Neurochem. 2019, 148, 275–290. [Google Scholar] [CrossRef]
- Eulenburg, V.; Hülsmann, S. Synergistic Control of Transmitter Turnover at Glycinergic Synapses by GlyT1, GlyT2, and ASC-1. Int. J. Mol. Sci. 2022, 23, 2561. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bao, Y.; Zheng, H.; Qin, Y.; Hua, B. Can Src protein tyrosine kinase inhibitors be combined with opioid analgesics? Src and opioid-induced tolerance, hyperalgesia and addiction. Biomed. Pharmacother. 2021, 139, 111653. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.; A Kemp, J.; Priestley, T.; Knight, A.R.; Woodruff, G.N.; Iversen, L.L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc. Natl. Acad. Sci. USA 1986, 83, 7104–7108. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Price, D.; Mayer, D. Thermal hyperalgesia in association with the development of morphine tolerance in rats: Roles of excitatory amino acid receptors and protein kinase C. J. Neurosci. 1994, 14, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-S.; Cherng, C.-H.; Luk, H.-N.; Ho, S.-T.; Tung, C.-S. Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: Binding at μ-opioid receptors. Eur. J. Pharmacol. 1996, 297, 27–33. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Mathie, A.; Peters, J. G Protein-coupled receptors. Br. J. Pharmacol. 2011, 164, S5–S113. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 2009, 56, 2–5. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Das, B.; Yao, A.Y.; Yan, R. Metabotropic Glutamate Receptors in Alzheimer’s Disease Synaptic Dysfunction: Therapeutic Opportunities and Hope for the Future. J. Alzheimer’s Dis. 2020, 78, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Conn, P.J.; Pin, J.-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- Tamaru, Y.; Nomura, S.; Mizuno, N.; Shigemoto, R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: Differential location relative to pre- and postsynaptic sites. Neuroscience 2001, 106, 481–503. [Google Scholar] [CrossRef] [PubMed]
- Driessen, A.K. Vagal Afferent Processing by the Paratrigeminal Nucleus. Front. Physiol. 2019, 10, 475415. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Coderre, T.J. Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. NeuroReport 1996, 7, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Coderre, T.J. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain 1996, 68, 255–263. [Google Scholar] [CrossRef]
- Osikowicz, M.; Mika, J.; Przewlocka, B. The glutamatergic system as a target for neuropathic pain relief. Exp. Physiol. 2013, 98, 372–384. [Google Scholar] [CrossRef]
- Chiechio, S. Modulation of Chronic Pain by Metabotropic Glutamate Receptors. Adv. Pharmacol. 2016, 75, 63–89. [Google Scholar] [CrossRef]
- Carlton, S.M.; Hargett, G.L. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J. Comp. Neurol. 2007, 501, 780–789. [Google Scholar] [CrossRef]
- Okubo, M.; Yamanaka, H.; Kobayashi, K.; Noguchi, K. Differential expression of mGluRs in rat spinal dorsal horns and their modulatory effects on nocifensive behaviors. Mol. Pain 2019, 15, 1744806919875026. [Google Scholar] [CrossRef]
- Richardson-Burns, S.M.; Haroutunian, V.; Davis, K.L.; Watson, S.J.; Meador-Woodruff, J.H. Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol. Psychiatry 2000, 47, 22–28. [Google Scholar] [CrossRef]
- Neto, F.L.; Schadrack, J.; Berthele, A.; Zieglgänsberger, W.; Tölle, T.R.; Castro-Lopes, J.M. Differential distribution of metabotropic glutamate receptor subtype mRNAs in the thalamus of the rat. Brain Res. 2000, 854, 93–105. [Google Scholar] [CrossRef]
- Muly, E.C.; Maddox, M.; Smith, Y. Distribution of mGluR1α and mGluR5 immunolabeling in primate prefrontal cortex. J. Comp. Neurol. 2003, 467, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.M.; McQuail, J.A.; Schwabe, M.R.; Burke, S.N.; Setlow, B.; Bizon, J.L. Age-Related Declines in Prefrontal Cortical Expression of Metabotropic Glutamate Receptors that Support Working Memory. eNeuro 2018, 5, e0164-18. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Reeve, A.; Bowes, M.; Winter, J.; Wotherspoon, G.; Davis, A.; Schmid, P.; Gasparini, F.; Kuhn, R.; Urban, L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology 2001, 40, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; Rizzonelli, P.; Paterlini, M.; Moretto, G.; Knöpfel, T.; Kuhn, R.; Memo, M.; Spano, P. mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: A developmental study. Neurosci. Res. 1997, 28, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Azkue, J.J.; Knöpfel, T.; Kuhn, R.; Mateos, J.M.; Grandes, P. Distribution of the metabotropic glutamate receptor subtype mGluR5 in rat midbrain periaqueductal grey and relationship with ascending spinofugal afferents. Neurosci. Lett. 1997, 228, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, E.; Boccella, S.; Marabese, I.; Pierretti, G.; Guida, F.; Maione, S. The Cold Case of Metabotropic Glutamate Receptor 6: Unjust Detention in the Retina? Curr. Neuropharmacol. 2019, 18, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Govea, R.; Zhou, S.; Carlton, S. Group III metabotropic glutamate receptors and transient receptor potential vanilloid 1 co-localize and interact on nociceptors. Neuroscience 2012, 217, 130–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marabese, I.; de Novellis, V.; Palazzo, E.; Mariani, L.; Siniscalco, D.; Rodella, L.; Rossi, F.; Maione, S. Differential roles of mGlu8 receptors in the regulation of glutamate and γ-aminobutyric acid release at periaqueductal grey level. Neuropharmacology 2005, 49, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Karakas, E.; Furukawa, H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 2014, 344, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.W.; Wu, L.-J.; Shum, F.; Quan, J.; Zhuo, M. Cingulate NMDA NR2B receptors contribute to morphine-induced analgesic tolerance. Mol. Brain 2008, 1, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, M.; Shimoyama, N.; Inturrisi, C.E.; Elliott, K. Oral ketamine produces a dose-dependent CNS depression in the rat. Life Sci. 1996, 60, PL9–PL14. [Google Scholar] [CrossRef] [PubMed]
- Nakama-Kitamura, M. The N-Methyl-D-aspartate receptor antagonist dizocilpine inhibits associative antinociceptive tolerance to morphine in mice: Relation with memory. J. Pharmacol. Sci. 2005, 97, 75–82. [Google Scholar] [CrossRef]
- Laulin, J.-P.; Maurette, P.; Corcuff, J.-B.; Rivat, C.G.; Chauvin, M.; Simonnet, G. The Role of Ketamine in Preventing Fentanyl-Induced Hyperalgesia and Subsequent Acute Morphine Tolerance. Obstet. Anesth. Dig. 2002, 94, 1263–1269. [Google Scholar] [CrossRef]
- González, P.; Cabello, P.; Germany, A.; Norris, B.; Contreras, E. Decrease of tolerance to, and physical dependence on morphine by glutamate receptor antagonists. Eur. J. Pharmacol. 1997, 332, 257–262. [Google Scholar] [CrossRef]
- Elliott, K.; Minami, N.; Kolesnikov, Y.A.; Pasternak, G.W.; Inturrisi, C.E. The NMDA Receptor antagonists, LY274614 and MK-801, and the nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not to kappa opioids. Pain 1994, 56, 69–75. [Google Scholar] [CrossRef]
- Gutstein, H.B.; Trujillo, K.A. MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res. 1993, 626, 332–334. [Google Scholar] [CrossRef]
- Mendez, I.A.; Trujillo, K.A. NMDA receptor antagonists inhibit opiate antinociceptive tolerance and locomotor sensitization in rats. Psychopharmacology 2008, 196, 497–509. [Google Scholar] [CrossRef]
- Harris, L.D.; Regan, M.C.; Myers, S.J.; Nocilla, K.A.; Akins, N.S.; Tahirovic, Y.A.; Wilson, L.J.; Dingledine, R.; Furukawa, H.; Traynelis, S.F.; et al. Novel GluN2B-Selective NMDA Receptor Negative Allosteric Modulator Possesses Intrinsic Analgesic Properties and Enhances Analgesia of Morphine in a Rodent Tail Flick Pain Model. ACS Chem. Neurosci. 2023, 14, 917–935. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Laurie, D.; Seeburg, P. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J. Neurosci. 1994, 14 Pt 2, 3180–3194. [Google Scholar] [CrossRef]
- Tolle, T.; Berthele, A.; Zieglgansberger, W.; Seeburg, P.; Wisden, W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J. Neurosci. 1993, 13, 5009–5028. [Google Scholar] [CrossRef]
- Luque, J.; Bleuel, Z.; Malherbe, P.; Richards, J. Alternatively spliced isoforms of the N-methyl-d-aspartate receptor subunit 1 are differentially distributed within the rat spinal cord. Neuroscience 1994, 63, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Yung, K.K.L. Localization of glutamate receptors in dorsal horn of rat spinal cord. NeuroReport 1998, 9, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, C.; Shigemoto, R.; Bessho, Y.; Nakanishi, S.; Mizuno, N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J. Comp. Neurol. 1994, 347, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J. Physiol. 2000, 523, 621–628. [Google Scholar] [CrossRef]
- Ma, Q.-P.; Hargreaves, R. Localization of N-methyl-d-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience 2000, 101, 699–707. [Google Scholar] [CrossRef]
- Wong, H.; Liu, X.; Matos, M.F.; Chan, S.F.; Pérez-Otaño, I.; Boysen, M.; Cui, J.; Nakanishi, N.; Trimmer, J.S.; Jones, E.G.; et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J. Comp. Neurol. 2002, 450, 303–317. [Google Scholar] [CrossRef]
- Wee, K.S.; Zhang, Y.; Khanna, S.; Low, C. Immunolocalization of NMDA receptor subunit NR3B in selected structures in the rat forebrain, cerebellum, and lumbar spinal cord. J. Comp. Neurol. 2008, 509, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Bae, J.Y.; Nam, M.-H.; An, H.; Ju, Y.H.; Won, J.; Choi, J.H.; Hwang, E.M.; Han, K.-S.; Bae, Y.C.; et al. Activation of Astrocytic μ-opioid Receptor Elicits Fast Glutamate Release Through TREK-1-Containing K2P Channel in Hippocampal Astrocytes. Front. Cell. Neurosci. 2018, 12, 393968. [Google Scholar] [CrossRef]
- Cubelos, B. Localización del Transportador de Glicina GLYT1 en Sinapsis Glutamatérgicas y Caracterización de su Interacción con Proteínas Con Dominios PDZ. 2004. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=33450&info=resumen&idioma=SPA (accessed on 14 December 2023).
- Scimemi, A.; Fine, A.; Kullmann, D.M.; Rusakov, D.A. NR2B-Containing Receptors Mediate Cross Talk among Hippocampal Synapses. J. Neurosci. 2004, 24, 4767–4777. [Google Scholar] [CrossRef] [PubMed]
- Mony, L.; Kew, J.N.; Gunthorpe, M.J.; Paoletti, P. Allosteric modulators of NR2B-containing NMDA receptors: Molecular mechanisms and therapeutic potential. Br. J. Pharmacol. 2009, 157, 1301–1317. [Google Scholar] [CrossRef] [PubMed]
- Harsing, L.G., Jr.; Juranyi, Z.; Gacsalyi, I.; Tapolcsanyi, P.; Czompa, A.; Matyus, P. Glycine Transporter Type-1 and its Inhibitors. Curr. Med. Chem. 2006, 13, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Harsing, L.G.; Matyus, P. Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: The role of synaptic and non-synaptic glycine transporters. Brain Res. Bull. 2013, 93, 110–119. [Google Scholar] [CrossRef]
- Olivares, L.; Aragón, C.; Giménez, C.; Zafra, F. Analysis of the Transmembrane Topology of the Glycine Transporter GLYT1. J. Biol. Chem. 1997, 272, 1211–1217. [Google Scholar] [CrossRef]
- Raiteri, L.; Raiteri, M. Functional ‘glial’ GLYT1 glycine transporters expressed in neurons. J. Neurochem. 2010, 114, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Al-Khrasani, M.; Mohammadzadeh, A.; Balogh, M.; Király, K.; Barsi, S.; Hajnal, B.; Köles, L.; Zádori, Z.S.; Harsing, L.G. Glycine transporter inhibitors: A new avenue for managing neuropathic pain. Brain Res. Bull. 2019, 152, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.L. Inhibition of Glycine Re-Uptake: A Potential Approach for Treating Pain by Augmenting Glycine-Mediated Spinal Neurotransmission and Blunting Central Nociceptive Signaling. Biomolecules 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Łątka, K.; Bajda, M. Analysis of Binding Determinants for Different Classes of Competitive and Noncompetitive Inhibitors of Glycine Transporters. Int. J. Mol. Sci. 2022, 23, 8050. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Lakatos, P.P.; Balogh, M.; Zádor, F.; Karádi, D.; Zádori, Z.S.; Király, K.; Galambos, A.R.; Barsi, S.; Riba, P.; et al. Pharmacological Evidence on Augmented Antiallodynia Following Systemic Co-Treatment with GlyT-1 and GlyT-2 Inhibitors in Rat Neuropathic Pain Model. Int. J. Mol. Sci. 2021, 22, 2479. [Google Scholar] [CrossRef]
- Peiser-Oliver, J.M.; Evans, S.; Adams, D.J.; Christie, M.J.; Vandenberg, R.J.; Mohammadi, S.A. Glycinergic Modulation of Pain in Behavioral Animal Models. Front. Pharmacol. 2022, 13, 860903. [Google Scholar] [CrossRef]
- Li, X.-H.; Miao, H.-H.; Zhuo, M. NMDA Receptor Dependent Long-term Potentiation in Chronic Pain. Neurochem. Res. 2018, 44, 531–538. [Google Scholar] [CrossRef]
- Toth, E.; Weiss, B.; Banay-Schwartz, M. Effect of glycine derivatives on behavioral changes induced by 3-mercaptopropionic acid or phencyclidine in mice. Res. Commun. Psychol. Psychiatry Behav. 1986, 11, 1–9. [Google Scholar]
- Harsing, L.; Zsilla, G.; Matyus, P.; Nagy, K.; Marko, B.; Gyarmati, Z.; Timar, J. Interactions between glycine transporter type 1 (GlyT-1) and some inhibitor molecules—Glycine transporter type 1 and its inhibitors (Review). Acta Physiol. Hung. 2012, 99, 1–17. [Google Scholar] [CrossRef]
- Zhang, H.X.; Hyrc, K.; Thio, L.L. The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. J. Physiol. 2009, 587, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Carland, J.; Mansfield, R.; Ryan, R.; Vandenberg, R. Oleoyl-l-carnitine inhibits glycine transport by GlyT2. Br. J. Pharmacol. 2013, 168, 891–902. [Google Scholar] [CrossRef]
- Brown, A.; Carlyle, I.; Clark, J.; Hamilton, W.; Gibson, S.; McGarry, G.; McEachen, S.; Rae, D.; Thorn, S.; Walker, G. Discovery and SAR of Org 24598—A Selective Glycine Uptake Inhibitor. Bioorganic Med. Chem. Lett. 2001, 11, 2007–2009. [Google Scholar] [CrossRef] [PubMed]
- Herdon, H.J.; Godfrey, F.M.; Brown, A.M.; Coulton, S.; Evans, J.R.; Cairns, W.J. Pharmacological assessment of the role of the glycine transporter GlyT-1 in mediating high-affinity glycine uptake by rat cerebral cortex and cerebellum synaptosomes. Neuropharmacology 2001, 41, 88–96. [Google Scholar] [CrossRef]
- Harsing, L. An overview of Glyt-1 inhibitors under evaluation for the treatment of schizophrenia. Drugs Future 2013, 38, 555–568. [Google Scholar] [CrossRef]
- Gilfillan, R.; Kerr, J.; Walker, G.; Wishart, G. Glycine transporters and their inhibitors. Top. Med. Chem. 2009, 4, 223–247. [Google Scholar]
- McBain, C.J.; Kleckner, N.W.; Wyrick, S.; Dingledine, R. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol. Pharmacol. 1989, 36, 556–565. [Google Scholar] [PubMed]
- Lee, M.-Y.; Lin, Y.-R.; Tu, Y.-S.; Tseng, Y.J.; Chan, M.-H.; Chen, H.-H. Effects of sarcosine and N, N-dimethylglycine on NMDA receptor-mediated excitatory field potentials. J. Biomed. Sci. 2017, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Werdehausen, R.; Kremer, D.; Brandenburger, T.; Schlösser, L.; Jadasz, J.; Küry, P.; Bauer, I.; Aragón, C.; Eulenburg, V.; Hermanns, H. Lidocaine Metabolites Inhibit Glycine Transporter 1. Anesthesiology 2012, 116, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Long, K.D.; Mastropaolo, J.; Rosse, R.B.; Manaye, K.F.; Deutsch, S.I. Modulatory effects of d-serine and sarcosine on NMDA receptor-mediated neurotransmission are apparent after stress in the genetically inbred BALB/c mouse strain. Brain Res. Bull. 2006, 69, 626–630. [Google Scholar] [CrossRef]
- Watson, G.B.; Bolanowski, M.A.; Baganoff, M.P.; Deppeler, C.L.; Lanthorn, T.H. d-Cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed inXenopus oocytes. Brain Res. 1990, 510, 158–160. [Google Scholar] [CrossRef]
- Harrison, C.; Smart, D.; Lambert, D.G. Stimulatory effects of opioids. Br. J. Anaesth. 1998, 81, 20–28. [Google Scholar] [CrossRef]
- Corkrum, M.; Rothwell, P.E.; Thomas, M.J.; Kofuji, P.; Araque, A. Opioid-Mediated Astrocyte–Neuron Signaling in the Nucleus Accumbens. Cells 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- López-Corcuera, B.; Geerlings, A.; Aragón, C. Glycine neurotransmitter transporters: An update. Mol. Membr. Biol. 2001, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Adams, R.; Betz, H.; Schloss, P. Modulation of a Recombinant Glycine Transporter (GLYT1b) by Activation of Protein Kinase C. J. Neurochem. 1995, 65, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Miller, G.M. A Receptor Mechanism for Methamphetamine Action in Dopamine Transporter Regulation in Brain. J. Pharmacol. Exp. Ther. 2009, 330, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011, 116, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Hanuska, A.; Szénási, G.; Albert, M.; Koles, L.; Varga, A.; Szabo, A.; Matyus, P.; Harsing, L.G. Some Operational Characteristics of Glycine Release in Rat Retina: The Role of Reverse Mode Operation of Glycine Transporter Type-1 (GlyT-1) in Ischemic Conditions. Neurochem. Res. 2016, 41, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, L.J.; Babey, A.-M. Synthesis of the Mechanisms of Opioid Tolerance: Do We Still Say NO? Cell. Mol. Neurobiol. 2021, 41, 927–948. [Google Scholar] [CrossRef]
- Ibuki, T.; Marsala, M.; Masuyama, T.; Yaksh, T.L. Spinal amino acid release and repeated withdrawal in spinal morphine tolerant rats. Br. J. Pharmacol. 2003, 138, 689–697. [Google Scholar] [CrossRef]
- Cortese, K.; Gagliani, M.C.; Raiteri, L. Interactions between Glycine and Glutamate through Activation of Their Transporters in Hippocampal Nerve Terminals. Biomedicines 2023, 11, 3152. [Google Scholar] [CrossRef]
- Raiteri, L.; Stigliani, S.; Siri, A.; Passalacqua, M.; Melloni, E.; Raiteri, M.; Bonanno, G. Glycine taken up through GLYT1 and GLYT2 heterotransporters into glutamatergic axon terminals of mouse spinal cord elicits release of glutamate by homotransporter reversal and through anion channels. Biochem. Pharmacol. 2005, 69, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Chen, S.-R.; Chen, H.; Pan, H.-L. Chronic Opioid Potentiates Presynaptic but Impairs Postsynaptic N-Methyl-d-aspartic Acid Receptor Activity in Spinal Cords: Implications for opioid hyperalgesia and tolerance. J. Biol. Chem. 2012, 287, 25073–25085. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blázquez, P.; Rodríguez-Muñoz, M.; Garzón, J. Mu-Opioid Receptors Transiently Activate the Akt-nNOS Pathway to Produce Sustained Potentiation of PKC-Mediated NMDAR-CaMKII Signaling. PLoS ONE 2010, 5, e11278. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.L.; Oliveira-Lima, O.C.; Carvalho, G.A.; de Almeida Chiarelli, R.; Ribeiro, R.I.; Parreira, R.C.; da Madeira Freitas, E.M.; Resende, R.R.; Klempin, F.; Ulrich, H.; et al. Neurobiology of glycine transporters: From molecules to behavior. Neurosci. Biobehav. Rev. 2020, 118, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-J.; Lane, H.-Y.; Tsai, G.E. NMDA Pathology and Treatment of Schizophrenia. Curr. Pharm. Des. 2014, 20, 5118–5126. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Balla, A.; Sershen, H.; Lajtha, A. Reversal of phencyclidine-induced effects by glycine and glycine transport inhibitors. Biol. Psychiatry 1999, 45, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Adell, A. Brain NMDA Receptors in Schizophrenia and Depression. Biomolecules 2020, 10, 947. [Google Scholar] [CrossRef]
- Pei, J.-C.; Luo, D.-Z.; Gau, S.-S.; Chang, C.-Y.; Lai, W.-S. Directly and Indirectly Targeting the Glycine Modulatory Site to Modulate NMDA Receptor Function to Address Unmet Medical Needs of Patients with Schizophrenia. Front. Psychiatry 2021, 12, 742058. [Google Scholar] [CrossRef]
- Rosenbrock, H.; Desch, M.; Wunderlich, G. Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1557–1566. [Google Scholar] [CrossRef]
- Dang, Y.-H.; Ma, X.-C.; Zhang, J.-C.; Ren, Q.; Wu, J.; Gao, C.-G.; Hashimoto, K. Targeting of NMDA Receptors in the Treatment of Major Depression. Curr. Pharm. Des. 2014, 20, 5151–5159. [Google Scholar] [CrossRef]
- Newell, D.W.; Barth, A.; Ricciardi, T.N.; Malouf, A.T. Glycine Causes Increased Excitability and Neurotoxicity by Activation of NMDA Receptors in the Hippocampus. Exp. Neurol. 1997, 145, 235–244. [Google Scholar] [CrossRef]
- Ghasemi, M.; Schachter, S.C. The NMDA receptor complex as a therapeutic target in epilepsy: A review. Epilepsy Behav. 2011, 22, 617–640. [Google Scholar] [CrossRef] [PubMed]
- Ugale, V.; Deshmukh, R.; Lokwani, D.; Reddy, P.N.; Khadse, S.; Chaudhari, P.; Kulkarni, P.P. GluN2B subunit selective N-methyl-D-aspartate receptor ligands: Democratizing recent progress to assist the development of novel neurotherapeutics. Mol. Divers. 2023, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Autry, A.E.; Tolias, K.F.; Krishnan, V. Ketamine: Neuroprotective or Neurotoxic? Front. Neurosci. 2021, 15, 672526. [Google Scholar] [CrossRef]
- Myslobodsky, M.; Golovchinsky, V.; Mintz, M. Ketamine: Convulsant or anti-convulsant? Pharmacol. Biochem. Behav. 1981, 14, 27–33. [Google Scholar] [CrossRef]
- Pinto, M.C.X.; Lima, I.V.d.A.; da Costa, F.L.P.; Rosa, D.V.; Mendes-Goulart, V.A.; Resende, R.R.; Romano-Silva, M.A.; de Oliveira, A.C.P.; Gomez, M.V.; Gomez, R.S. Glycine transporters type 1 inhibitor promotes brain preconditioning against NMDA-induced excitotoxicity. Neuropharmacology 2015, 89, 274–281. [Google Scholar] [CrossRef]
- Shen, H.-Y.; van Vliet, E.A.; Bright, K.-A.; Hanthorn, M.; Lytle, N.K.; Gorter, J.; Aronica, E.; Boison, D. Glycine transporter 1 is a target for the treatment of epilepsy. Neuropharmacology 2015, 99, 554–565. [Google Scholar] [CrossRef]

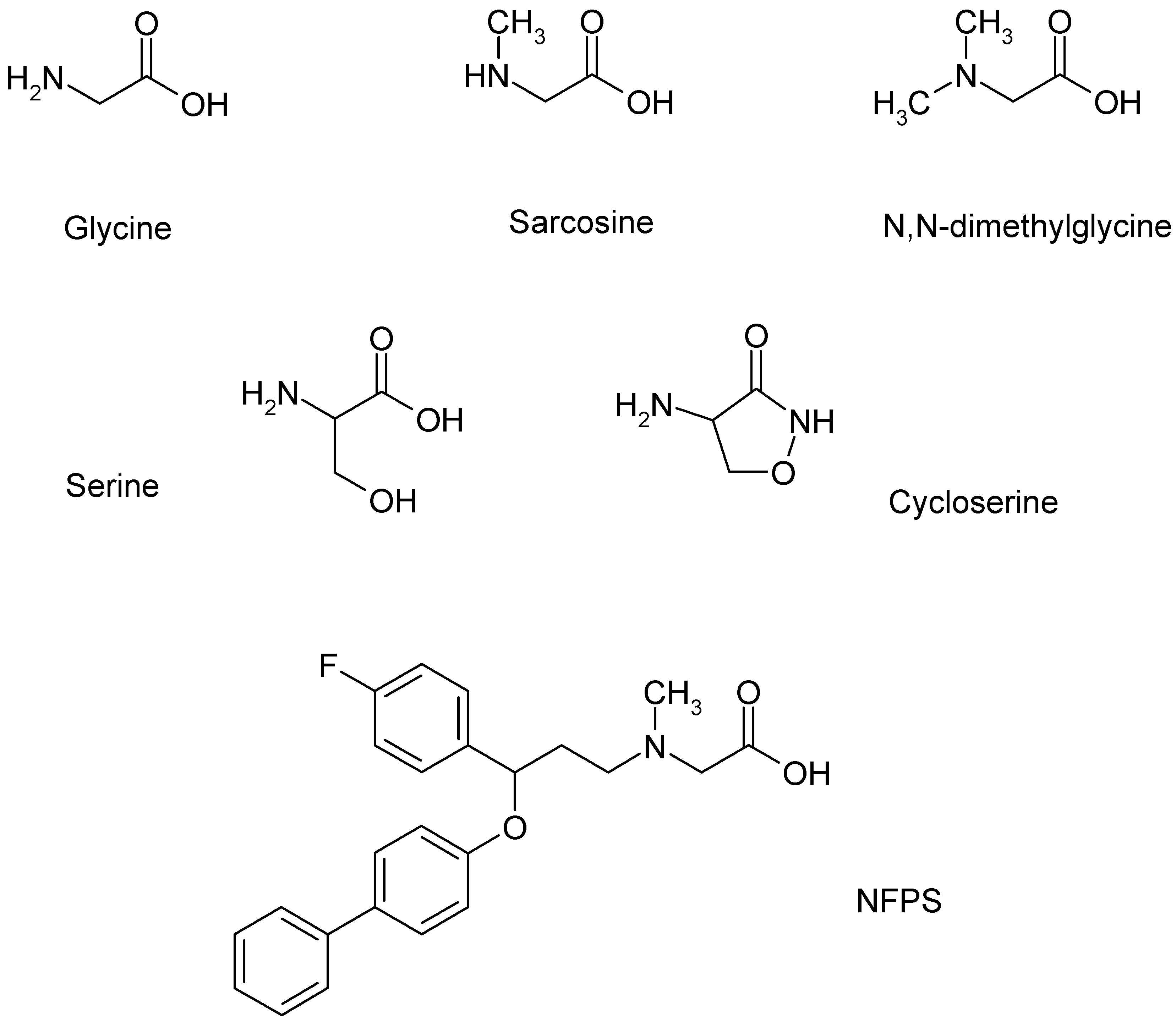
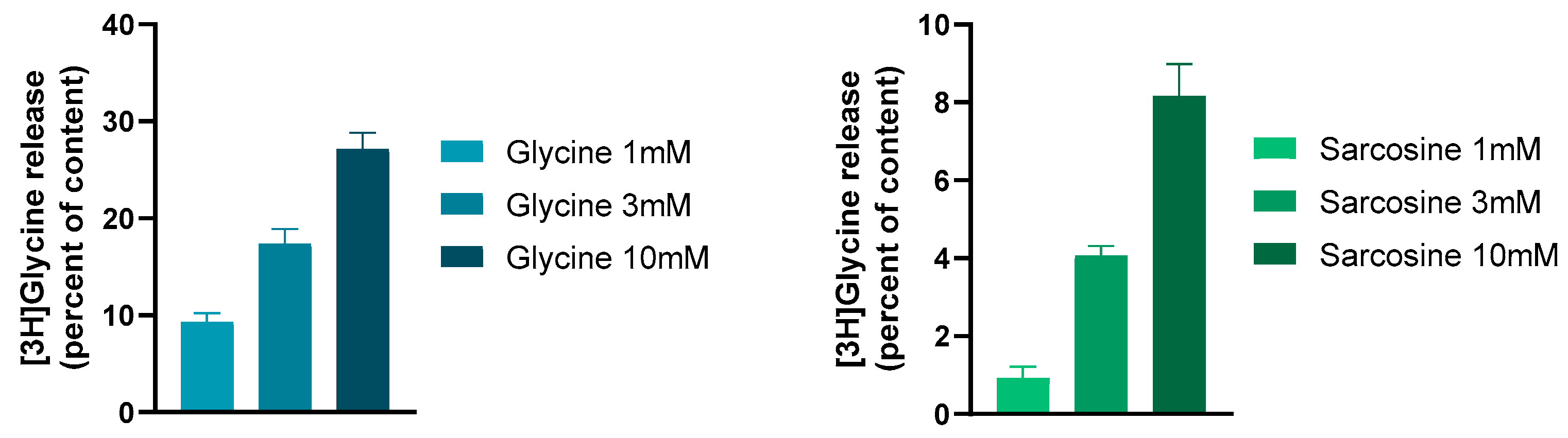
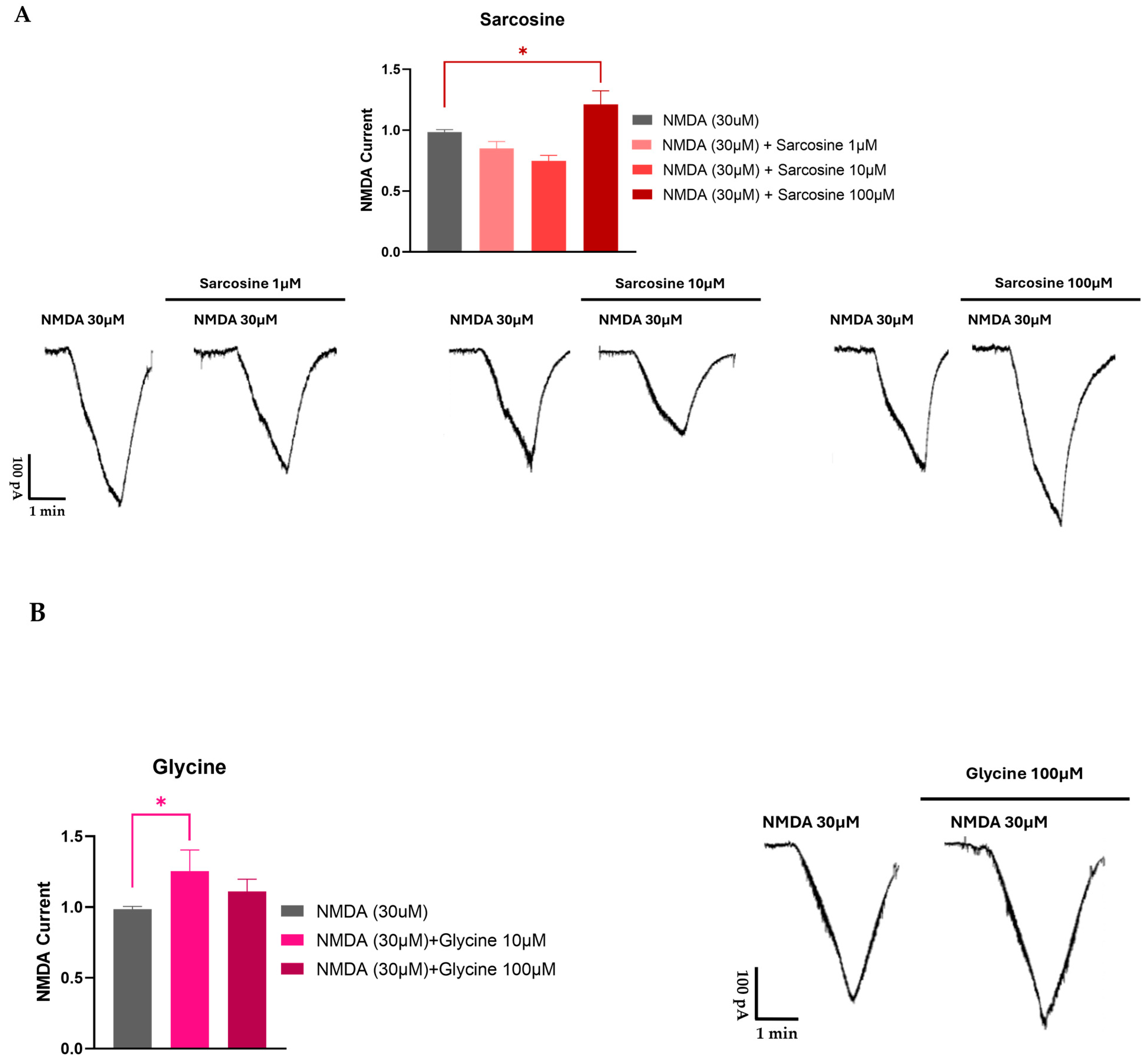
| Enzyme/Protein/Mechanism | Role in Opioid Tolerance | References |
|---|---|---|
| β-arrestin 1 and 2 | Receptor desensitization Receptor trafficking | [15,16] |
| GRK2, 3, 4, and 5 | Receptor desensitization and trafficking via receptor phosphorylation | [15,16,17,18] |
| PKCα, γ, and ε | Receptor desensitization and trafficking via receptor phosphorylation | [18,19,20,21] |
| CAMKII | Receptor desensitization via receptor phosphorylation | [16,18] |
| ERK1/2 | Receptor desensitization via arrestins | [16] |
| JNK | Receptor desensitization | [16,21] |
| Dynamin | Receptor trafficking | [16,22] |
| Rybophorin I (glycosylation) | Receptor trafficking | [18] |
| PATs (palmitoylation) | Receptor trafficking | [18] |
| Ubiquitination (participating proteins not described) | Receptor trafficking | [18] |
| Receptor Group | Receptor Subtype | Area (Region) | Distribution Pattern | Subject | Connection to Pain Sensation | References |
|---|---|---|---|---|---|---|
| Group I | mGluR1 | DRG | 6.8% | Rat, mouse, human, monkey | ↑ Nociceptive behaviors Inflammatory pain Neuropathy | [41,42,43,44,45,46] |
| PA | ||||||
| DH | ||||||
| VH | 90% | |||||
| Thalamus | ||||||
| PFC | ||||||
| mGluR5 | PA | Mouse, rat, human, monkey | [42,43,44,45,46,47,48,49] | |||
| DH | ||||||
| Thalamus | 90% | |||||
| PAG | ||||||
| PFC | ||||||
| Group II | mGluR2 | DRG | 51.6% | Rat, human | Activation induces analgesia in inflammatory and neuropathic pain | [41,43,46] |
| Thalamus | ||||||
| PFC | ||||||
| mGluR3 | DRG | [41,42,43,44,46] | ||||
| DH | ||||||
| Thalamus | ||||||
| PFC | ||||||
| Group III | mGluR4 | DH | Rat, human | Anti-hyperalgesic effect of mGlu group III activation | [42,43,44,46] | |
| Thalamus | ||||||
| VH | ||||||
| PFC | ||||||
| mGluR6 | Superior colliculus, hypothalamus, olfactory bulb | Mouse, rat | [50] | |||
| mGluR7 | DH | 90% | Rat, human | [42,43,44,46] | ||
| VH | ||||||
| Thalamus | ||||||
| PFC | ||||||
| mGluR8 | DRG | 75–80% | Rat, human | [41,42,43,46,51,52] | ||
| DH | ||||||
| Thalamus | ||||||
| PAG | ||||||
| PFC | ||||||
| Peripheral axons | 40% |
| Ligands | GlyT1 | NMDA Receptors |
|---|---|---|
| Glycine | Substrate | Co-agonist |
| Sarcosine | Substrate inhibitor Competitive antagonist | Co-agonist |
| N.N-Dimethylglycine | Ineffective | Partial agonist |
| N-Ethylglycine | Competitive inhibitor | |
| D-Serine | - | Co-agonist |
| D-Cycloserine | - | Partial agonist |
| NFPS | Competitive inhibitor Non-competitive inhibitor | No effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galambos, A.R.; Papp, Z.T.; Boldizsár, I.; Zádor, F.; Köles, L.; Harsing, L.G., Jr.; Al-Khrasani, M. Glycine Transporter 1 Inhibitors: Predictions on Their Possible Mechanisms in the Development of Opioid Analgesic Tolerance. Biomedicines 2024, 12, 421. https://doi.org/10.3390/biomedicines12020421
Galambos AR, Papp ZT, Boldizsár I, Zádor F, Köles L, Harsing LG Jr., Al-Khrasani M. Glycine Transporter 1 Inhibitors: Predictions on Their Possible Mechanisms in the Development of Opioid Analgesic Tolerance. Biomedicines. 2024; 12(2):421. https://doi.org/10.3390/biomedicines12020421
Chicago/Turabian StyleGalambos, Anna Rita, Zsolt Tamás Papp, Imre Boldizsár, Ferenc Zádor, László Köles, Laszlo G. Harsing, Jr., and Mahmoud Al-Khrasani. 2024. "Glycine Transporter 1 Inhibitors: Predictions on Their Possible Mechanisms in the Development of Opioid Analgesic Tolerance" Biomedicines 12, no. 2: 421. https://doi.org/10.3390/biomedicines12020421
APA StyleGalambos, A. R., Papp, Z. T., Boldizsár, I., Zádor, F., Köles, L., Harsing, L. G., Jr., & Al-Khrasani, M. (2024). Glycine Transporter 1 Inhibitors: Predictions on Their Possible Mechanisms in the Development of Opioid Analgesic Tolerance. Biomedicines, 12(2), 421. https://doi.org/10.3390/biomedicines12020421






