Cortisol Reactivity to Acute Psychosocial Stress in Physician Burnout
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Study Procedure
2.3. Trier Social Stress Test
2.4. Psychometric Assessment
2.5. Health Behavior Assessment
2.6. Biochemical Analyses
2.7. Data Analysis
3. Results
3.1. Sample Characteristics
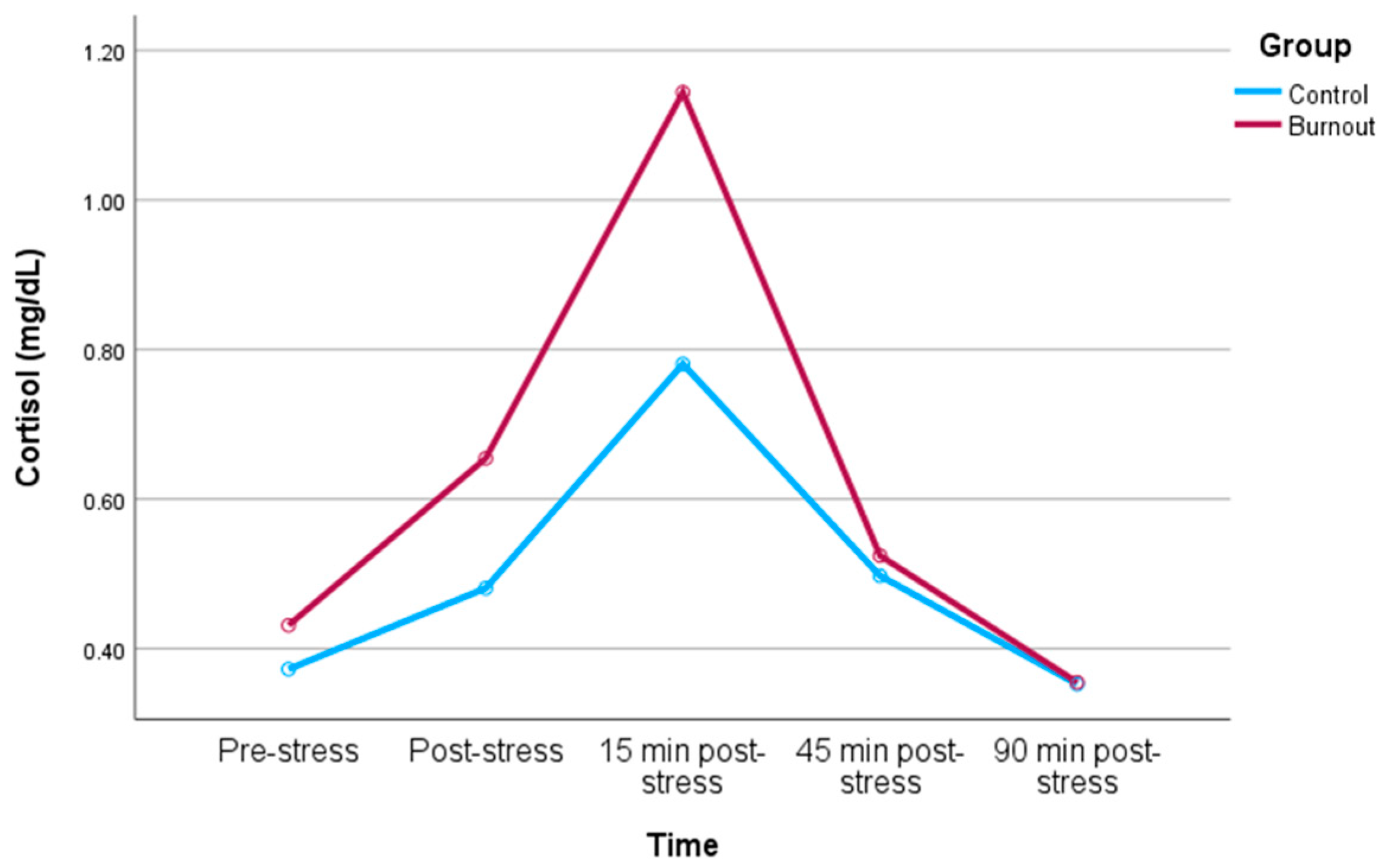
3.2. Cortisol Response to Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. ICD-11: International Classification of Diseases (11th Revision). Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/129180281 (accessed on 11 April 2023).
- Weilenmann, S.; Spiller, T.; Princip, M.; von Känel, R. 20 Jahre Forschung zu Burnout und anderen Belastungsindikatoren bei Schweizer Ärztinnen und Ärzten. Prim. Hosp. Care 2023, 23, 114–120. [Google Scholar] [CrossRef]
- Rotenstein, L.S.; Torre, M.; Ramos, M.A.; Rosales, R.C.; Guille, C.; Sen, S.; Mata, D.A. Prevalence of Burnout among Physicians: A Systematic Review. JAMA 2018, 320, 1131–1150. [Google Scholar] [CrossRef] [PubMed]
- West, C.P.; Huschka, M.M.; Novotny, P.J.; Sloan, J.A.; Kolars, J.C.; Habermann, T.M.; Shanafelt, T.D. Association of perceived medical errors with resident distress and empathy: A prospective longitudinal study. JAMA 2006, 296, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- West, C.P.; Dyrbye, L.N.; Shanafelt, T.D. Physician burnout: Contributors, consequences and solutions. J. Intern. Med. 2018, 283, 516–529. [Google Scholar] [CrossRef] [PubMed]
- West, C.P.; Tan, A.D.; Habermann, T.M.; Sloan, J.A.; Shanafelt, T.D. Association of resident fatigue and distress with perceived medical errors. JAMA 2009, 302, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Balch, C.M.; Bechamps, G.; Russell, T.; Dyrbye, L.; Satele, D.; Collicott, P.; Novotny, P.J.; Sloan, J.; Freischlag, J. Burnout and medical errors among American surgeons. Ann. Surg. 2010, 251, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Oreskovich, M.R.; Dyrbye, L.N.; Colaiano, J.M.; Satele, D.V.; Sloan, J.A.; Shanafelt, T.D. Personal consequences of malpractice lawsuits on American surgeons. J. Am. Coll. Surg. 2011, 213, 657–667. [Google Scholar] [CrossRef]
- Dewa, C.S.; Loong, D.; Bonato, S.; Thanh, N.X.; Jacobs, P. How does burnout affect physician productivity? A systematic literature review. BMC Health Serv. Res. 2014, 14, 325. [Google Scholar] [CrossRef]
- Patel, R.S.; Bachu, R.; Adikey, A.; Malik, M.; Shah, M. Factors Related to Physician Burnout and Its Consequences: A Review. Behav. Sci. 2018, 8, 98. [Google Scholar] [CrossRef]
- Shanafelt, T.; Sloan, J.; Satele, D.; Balch, C. Why do surgeons consider leaving practice? J. Am. Coll. Surg. 2011, 212, 421–422. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Raymond, M.; Kosty, M.; Satele, D.; Horn, L.; Pippen, J.; Chu, Q.; Chew, H.; Clark, W.B.; Hanley, A.E.; et al. Satisfaction with work-life balance and the career and retirement plans of US oncologists. J. Clin. Oncol. 2014, 32, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Marques-Pinto, A.; Moreira, S.; Costa-Lopes, R.; Zózimo, N.; Vala, J. Predictors of Burnout among Physicians: Evidence from a National Study in Portugal. Front. Psychol. 2021, 12, 699974. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Sloan, J.A.; Habermann, T.M. The well-being of physicians. Am. J. Med. 2003, 114, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Morita, T.; Akechi, T.; Sugawara, Y.; Fujimori, M.; Akizuki, N.; Nakano, T.; Uchitomi, Y. Burnout and psychiatric morbidity among physicians engaged in end-of-life care for cancer patients: A cross-sectional nationwide survey in Japan. Psychooncology 2007, 16, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Salvagioni, D.A.J.; Melanda, F.N.; Mesas, A.E.; González, A.D.; Gabani, F.L.; Andrade, S.M. Physical, psychological and occupational consequences of job burnout: A systematic review of prospective studies. PLoS ONE 2017, 12, e0185781. [Google Scholar] [CrossRef]
- Toker, S.; Melamed, S.; Berliner, S.; Zeltser, D.; Shapira, I. Burnout and risk of coronary heart disease: A prospective study of 8838 employees. Psychosom. Med. 2012, 74, 840–847. [Google Scholar] [CrossRef]
- Sara, J.D.; Prasad, M.; Eleid, M.F.; Zhang, M.; Widmer, R.J.; Lerman, A. Association between Work-Related Stress and Coronary Heart Disease: A Review of Prospective Studies through the Job Strain, Effort-Reward Balance, and Organizational Justice Models. J. Am. Heart Assoc. 2018, 7, e008073. [Google Scholar] [CrossRef]
- Bayes, A.; Tavella, G.; Parker, G. The biology of burnout: Causes and consequences. World J. Biol. Psychiatry 2021, 22, 686–698. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Wüst, S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress 2010, 13, 1–14. [Google Scholar] [CrossRef]
- Rothe, N.; Steffen, J.; Penz, M.; Kirschbaum, C.; Walther, A. Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: A systematic review. Neurosci. Biobehav. Rev. 2020, 114, 232–270. [Google Scholar] [CrossRef]
- De Vente, W.; Olff, M.; Van Amsterdam, J.G.; Kamphuis, J.H.; Emmelkamp, P.M. Physiological differences between burnout patients and healthy controls: Blood pressure, heart rate, and cortisol responses. Occup. Environ. Med. 2003, 60 (Suppl. S1), i54–i61. [Google Scholar] [CrossRef] [PubMed]
- De Vente, W.; van Amsterdam, J.G.; Olff, M.; Kamphuis, J.H.; Emmelkamp, P.M. Burnout Is Associated with Reduced Parasympathetic Activity and Reduced HPA Axis Responsiveness, Predominantly in Males. BioMed Res. Int. 2015, 2015, 431725. [Google Scholar] [CrossRef]
- Jönsson, P.; Österberg, K.; Wallergård, M.; Hansen, Å.M.; Garde, A.H.; Johansson, G.; Karlson, B. Exhaustion-related changes in cardiovascular and cortisol reactivity to acute psychosocial stress. Physiol. Behav. 2015, 151, 327–337. [Google Scholar] [CrossRef]
- Lennartsson, A.K.; Sjörs, A.; Währborg, P.; Ljung, T.; Jonsdottir, I.H. Burnout and Hypocortisolism—A Matter of Severity? A Study on ACTH and Cortisol Responses to Acute Psychosocial Stress. Front. Psychiatry 2015, 6, 8. [Google Scholar] [CrossRef]
- Wekenborg, M.K.; von Dawans, B.; Hill, L.K.; Thayer, J.F.; Penz, M.; Kirschbaum, C. Examining reactivity patterns in burnout and other indicators of chronic stress. Psychoneuroendocrinology 2019, 106, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A.; Perrar, K.M. Die Messung von Burnout. Untersuchung einer deutschen Fassung des Maslach Burnout Inventory (MBI-D) [Measuring burnout: A study of a German version of the Maslach Burnout Inventory (MBI-D)]. Diagnostica 1992, 38, 328–353. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Kennedy, P.J.; Dockray, S.; Cryan, J.F.; Dinan, T.G.; Clarke, G. The Trier Social Stress Test: Principles and practice. Neurobiol. Stress 2016, 6, 113–126. [Google Scholar] [CrossRef]
- Siegrist, J.; Wege, N.; Pühlhofer, F.; Wahrendorf, M. A short generic measure of work stress in the era of globalization: Effort-reward imbalance. Int. Arch. Occup. Environ. Health 2009, 82, 1005–1013. [Google Scholar] [CrossRef]
- Gräfe, K.; Zipfel, S.; Herzog, W.; Löwe, B. Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”. Diagnostica 2004, 50, 171–181. [Google Scholar] [CrossRef]
- Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 4. [Google Scholar] [CrossRef]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of Regular Physical Activity and Fitness on Stress Reactivity as Measured with the Trier Social Stress Test Protocol: A Systematic Review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef] [PubMed]
- Herhaus, B.; Petrowski, K. Cortisol Stress Reactivity to the Trier Social Stress Test in Obese Adults. Obes. Facts 2018, 11, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge, Academic: New York, NY, USA, 1988. [Google Scholar]
- Turner, A.I.; Smyth, N.; Hall, S.J.; Torres, S.J.; Hussein, M.; Jayasinghe, S.U.; Ball, K.; Clow, A.J. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 2020, 114, 104599. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Burnout, hair cortisol, and timing: Hyper- or hypocortisolism? Psychoneuroendocrinology 2018, 87, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Penz, M.; Siegrist, J.; Wekenborg, M.K.; Rothe, N.; Walther, A.; Kirschbaum, C. Effort-reward imbalance at work is associated with hair cortisol concentrations: Prospective evidence from the Dresden Burnout Study. Psychoneuroendocrinology 2019, 109, 104399. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Seeman, T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Von Känel, R.; Princip, M.; Holzgang, S.A.; Sivakumar, S.; Pazhenkottil, A.P.; Gomez Vieito, D.; Zuccarella-Hackl, C. Sympathetic nervous system responses to acute psychosocial stress in male physicians with clinical burnout. Biol. Psychol. 2023, 183, 108687. [Google Scholar] [CrossRef]
- Marchand, A.; Durand, P.; Juster, R.P.; Lupien, S.J. Workers’ psychological distress, depression, and burnout symptoms: Associations with diurnal cortisol profiles. Scand. J. Work. Environ. Health. 2014, 40, 305–314. [Google Scholar] [CrossRef]
- Lim, G.Y.; Jang, T.W.; Sim, C.S.; Ahn, Y.S.; Jeong, K.S. Comparison of Cortisol level by Shift Cycle in Korean Firefighters. Int. J. Environ. Res. Public Health 2020, 17, 4760. [Google Scholar] [CrossRef]
- Dienes, K.; Gartland, N.; Ferguson, E. The relationship between the cortisol awakening response and cortisol reactivity to a laboratory stressor. Br. J. Health Psychol. 2019, 24, 265–281. [Google Scholar] [CrossRef]
- Liu, J.J.W.; Ein, N.; Peck, K.; Huang, V.; Pruessner, J.C.; Vickers, K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology 2017, 82, 26–37. [Google Scholar] [CrossRef]
- Stephens, M.A.; Mahon, P.B.; McCaul, M.E.; Wand, G.S. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology 2016, 66, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Grossi, G.; Perski, A.; Ekstedt, M.; Johansson, T.; Lindström, M.; Holm, K. The morning salivary cortisol response in burnout. J. Psychosom. Res. 2005, 59, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Garbellotto, G.I.; Reis, F.J.; Feoli, A.M.P.; Piovesan, C.H.; Gustavo, A.D.S.; Oliveira, M.D.S.; Macagnan, F.E.; Ferreira, C.A.S.; Bauer, M.E.; Wietzycoski, C.R. Salivary cortisol and metabolic syndrome components association. Arq. Bras. Cir. Dig. 2018, 31, e1351. [Google Scholar] [CrossRef]
- De Nys, L.; Anderson, K.; Ofosu, E.F.; Ryde, G.C.; Connelly, J.; Whittaker, A.C. The effects of physical activity on cortisol and sleep: A systematic review and meta-analysis. Psychoneuroendocrinology 2022, 143, 105843. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
| Variable | Burnout Group (n = 30) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| Age, years | 46.77 (10.56) | 52.93 (7.48) | 0.012 |
| Body mass index, kg/m2 | 25.63 (3.08) | 24.35 (2.72) | 0.095 |
| Exercise, times/week | 1.99 (1.62) | 2.67 (1.92) | 0.147 |
| Emotional exhaustion, score | 29.17 (7.13) | 6.67 (3.99) | <0.001 |
| Depersonalization, score | 11.33 (7.00) | 3.07 (3.60) | <0.001 |
| Personal accomplishments, score | 12.03 (6.74) | 5.67 (4.37) | <0.001 |
| Effort, score | 10.65 (1.36) | 8.07 (2.13) | <0.001 |
| Reward, score | 19.58 (4.03) | 22.24 (2.96) | 0.005 |
| Effort-Reward ratio | 1.34 (0.41) | 0.87 (0.27) | <0.001 |
| Patient Health Questionnaire-9, score | 7.40 (3.13) | 2.20 (1.97) | <0.001 |
| Shift workers | 18 | 17 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccarella-Hackl, C.; Princip, M.; Holzgang, S.A.; Sivakumar, S.; Kuenburg, A.; Pazhenkottil, A.P.; Gomez Vieito, D.; von Känel, R. Cortisol Reactivity to Acute Psychosocial Stress in Physician Burnout. Biomedicines 2024, 12, 335. https://doi.org/10.3390/biomedicines12020335
Zuccarella-Hackl C, Princip M, Holzgang SA, Sivakumar S, Kuenburg A, Pazhenkottil AP, Gomez Vieito D, von Känel R. Cortisol Reactivity to Acute Psychosocial Stress in Physician Burnout. Biomedicines. 2024; 12(2):335. https://doi.org/10.3390/biomedicines12020335
Chicago/Turabian StyleZuccarella-Hackl, Claudia, Mary Princip, Sarah A. Holzgang, Sinthujan Sivakumar, Alexa Kuenburg, Aju P. Pazhenkottil, Diego Gomez Vieito, and Roland von Känel. 2024. "Cortisol Reactivity to Acute Psychosocial Stress in Physician Burnout" Biomedicines 12, no. 2: 335. https://doi.org/10.3390/biomedicines12020335
APA StyleZuccarella-Hackl, C., Princip, M., Holzgang, S. A., Sivakumar, S., Kuenburg, A., Pazhenkottil, A. P., Gomez Vieito, D., & von Känel, R. (2024). Cortisol Reactivity to Acute Psychosocial Stress in Physician Burnout. Biomedicines, 12(2), 335. https://doi.org/10.3390/biomedicines12020335








