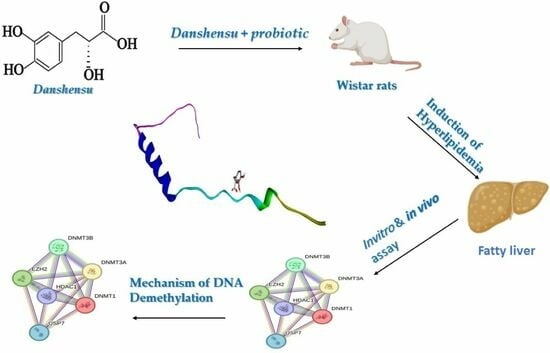
Synergistic Differential DNA Demethylation Activity of Danshensu (Salvia miltiorrhiza) Associated with Different Probiotics in Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Materials and Methods
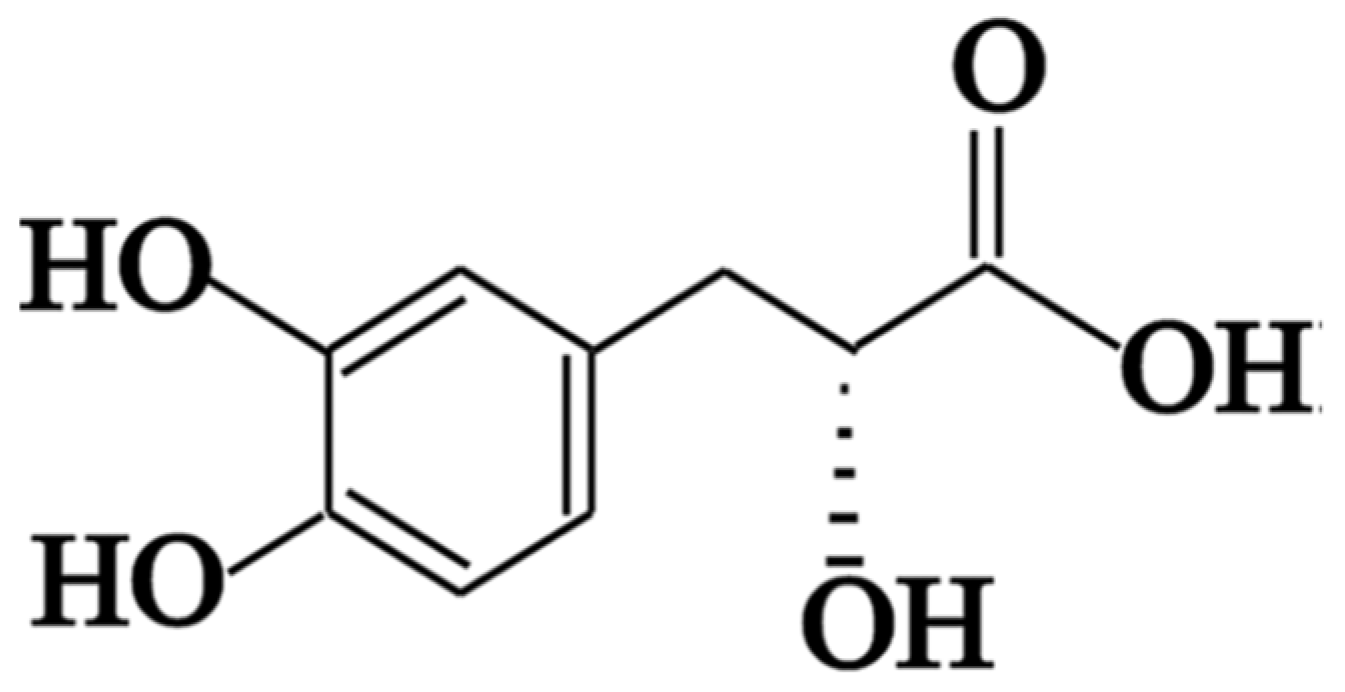
2.1. In Vitro Study of Danshensu Extracts
2.2. Total Polyphenol Content (TPC)
2.3. Determination of Total Flavonoids (TFs)
2.4. Measurement of Antioxidant Properties
2.5. Total Reduction Capability
2.6. ABTS Radical Cation Decolorization Assay
2.7. Hydrogen Peroxide (H2O2) Radical Scavenging Activity
2.8. Nitric Oxide (NO) Radical Scavenging Assay
2.9. In Vitro Lipid Peroxidation (LPO) Assay
2.9.1. Preparation of Rat Liver Homogenate
2.9.2. TBARS Assay
2.10. In Vivo Study
2.10.1. Animals and Experimental Design
2.10.2. Acute Toxicity Study
2.10.3. Induction of Hyperlipidemia
2.10.4. Collection of the Body Organs (Heart, Liver, and Serum)
2.10.5. Preparation of Tissue Homogenate
2.11. Biochemical Analysis
2.11.1. Blood Lipid Profile Analysis
2.11.2. Determination of HMG-CoA Reductase Activity
2.11.3. Determination of Hepatic and Fecal Lipids
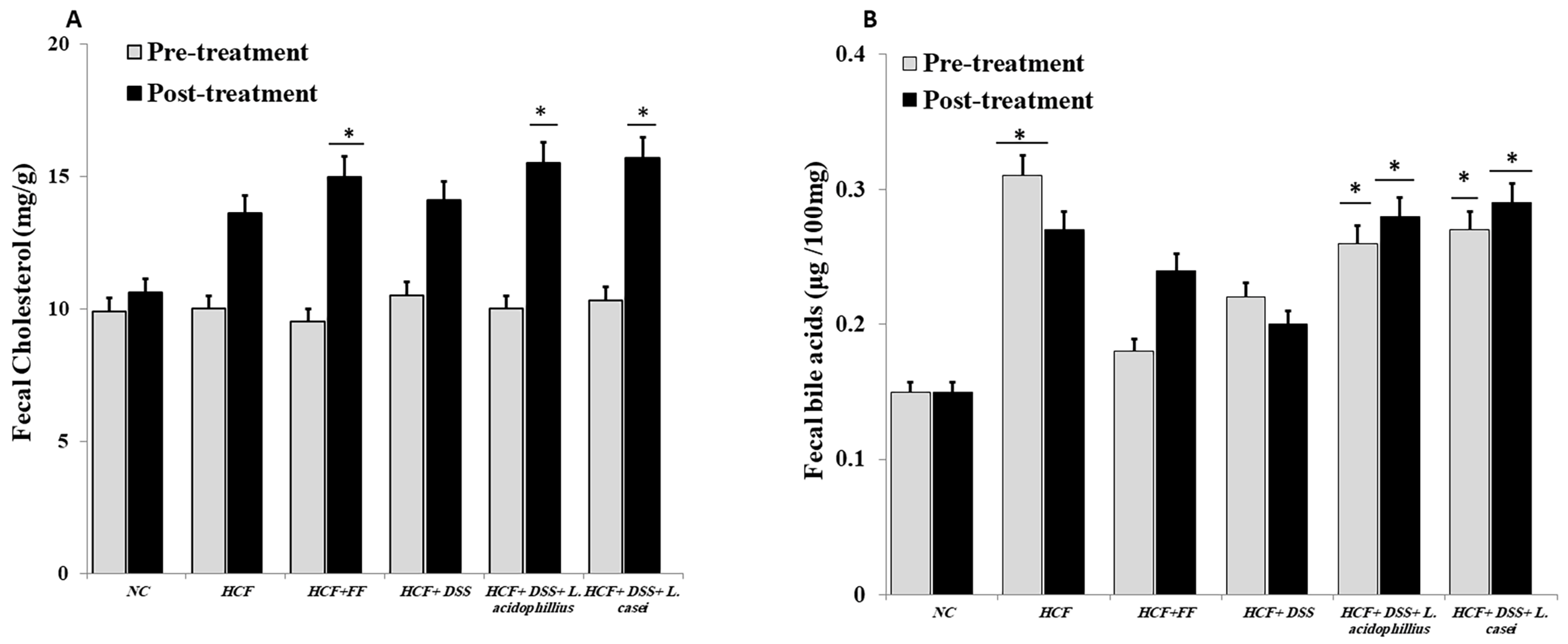
2.11.4. Measurement of Fecal Bile Acids
2.11.5. Evaluation of Tissue Markers of Oxidative Stress
2.12. Quantitative RT-PCR
2.13. In Silico Study
2.13.1. Ligand Preparation
2.13.2. Receptor Preparation
2.13.3. Molecular Docking
2.13.4. ADMET and Drug-Likeness Properties
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
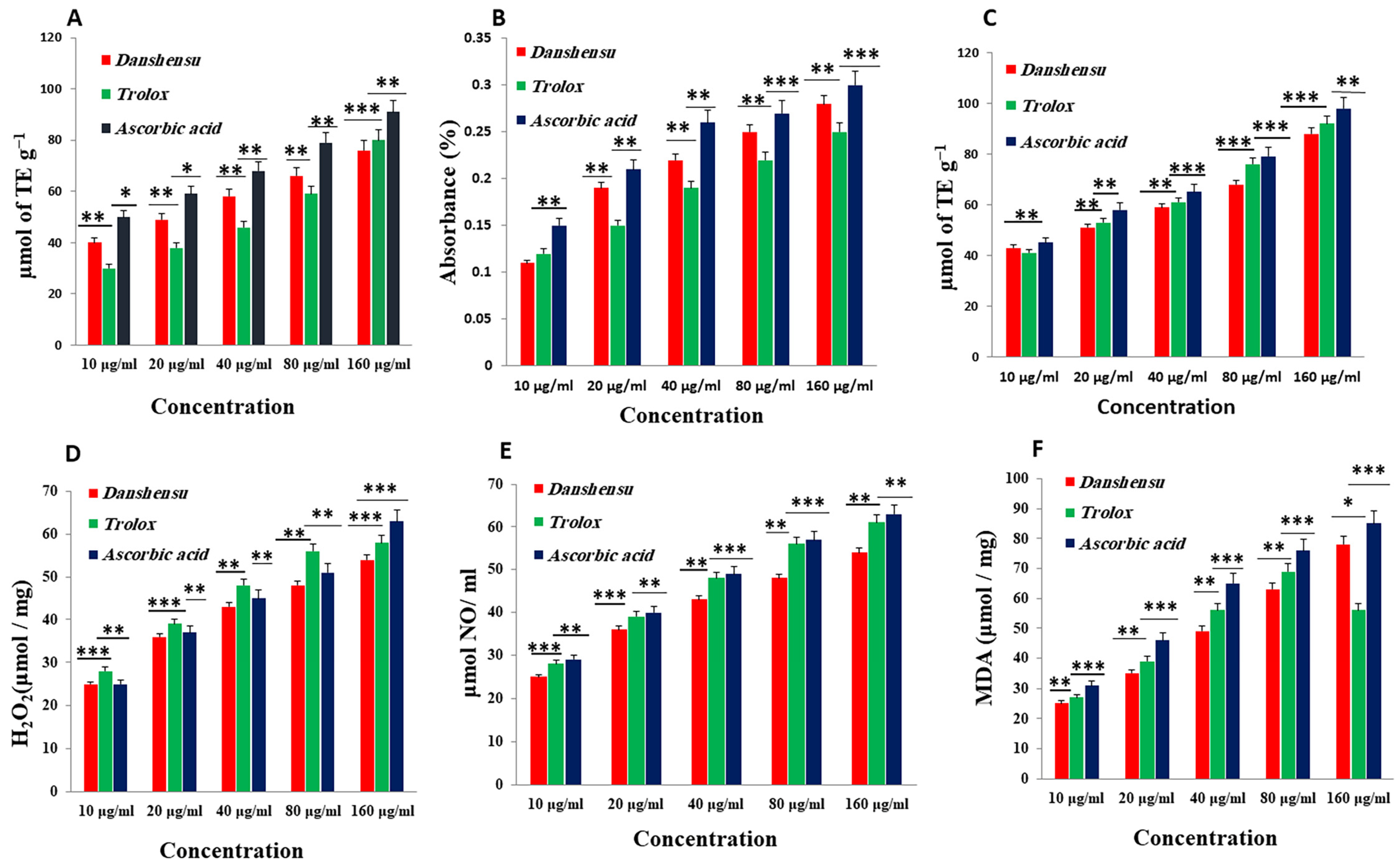
3.2. In Vitro Antioxidant Activities of Danshensu
3.2.1. DPPH Radical Scavenging Activity
3.2.2. Total Reduction Capability
3.2.3. ABTS Radical Cation Decolorization Assay
3.2.4. Hydrogen Peroxide (H2O2) Radical Scavenging Activity
3.2.5. Nitric Oxide (NO) Radical Scavenging Activity
3.2.6. Inhibition of Lipid Peroxidation (LPO)
3.3. In Vivo Study
3.3.1. Acute Toxicity of Danshensu on Animals
3.3.2. Danshensu Combined with PROBIOTICS—Effect on Body Weight
3.3.3. Biochemical Parameters
3.3.4. Oxidative Stress Markers
3.3.5. Serum
3.3.6. Heart
3.3.7. Liver
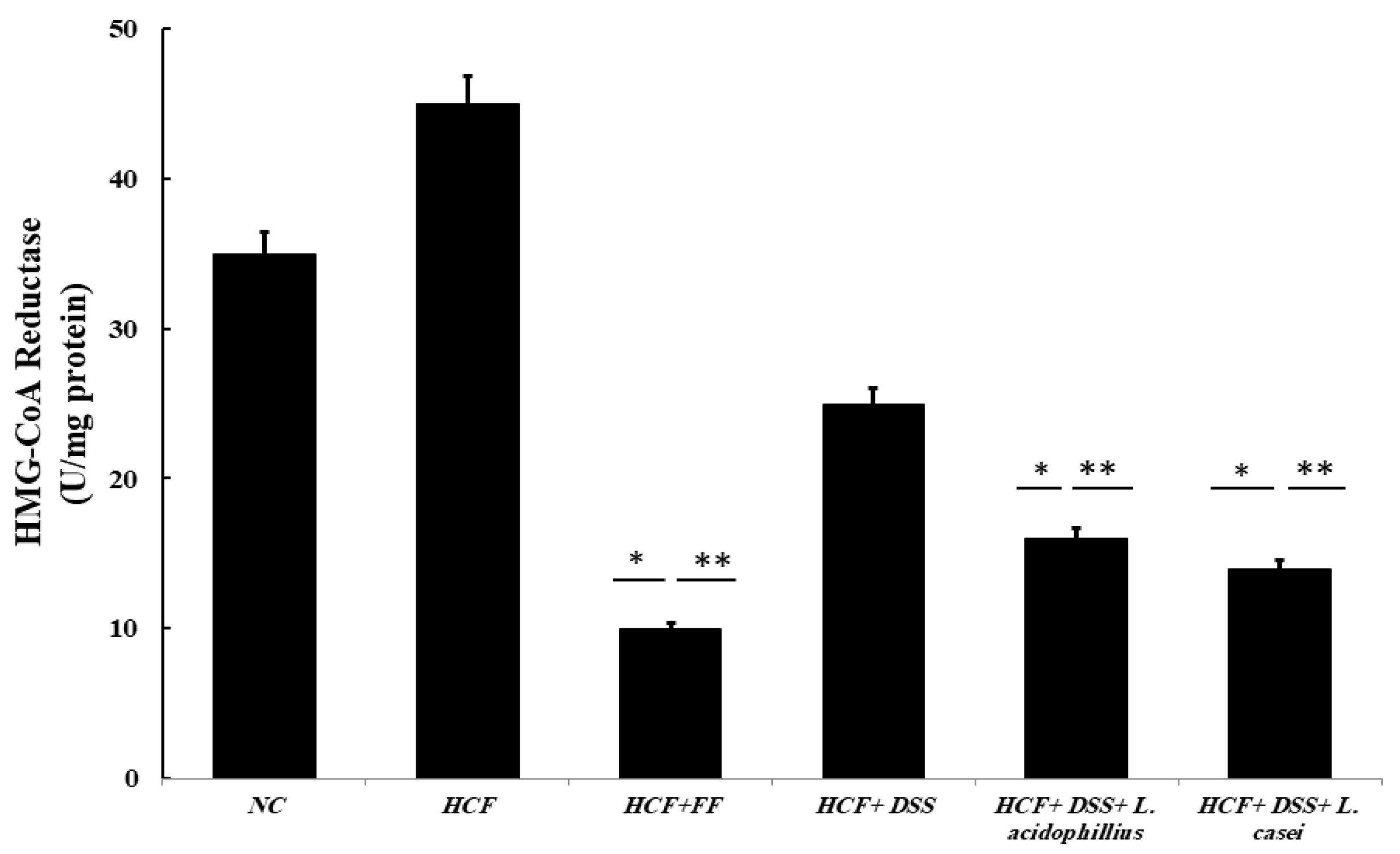
3.3.8. Determination of HMG-CoA Reductase Activity
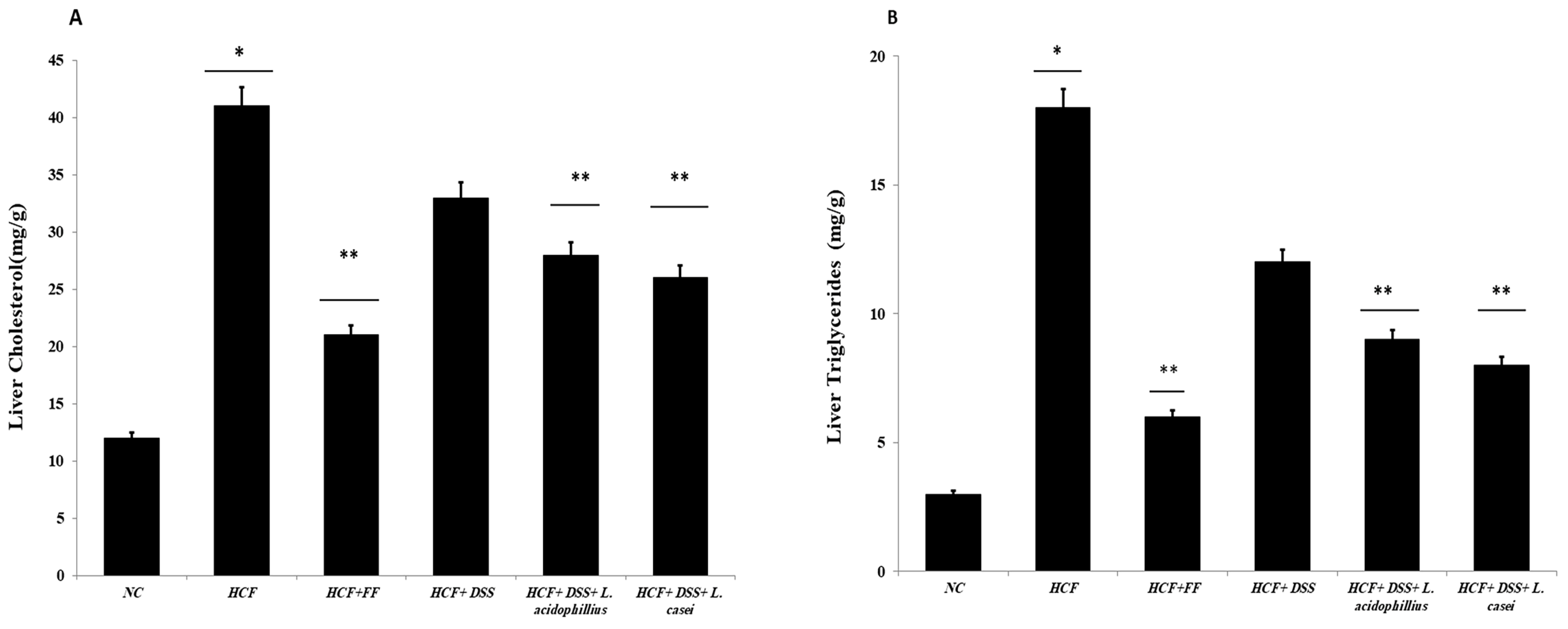
3.3.9. Determination of Hepatic and Fecal Lipids and Fecal Bile Acids
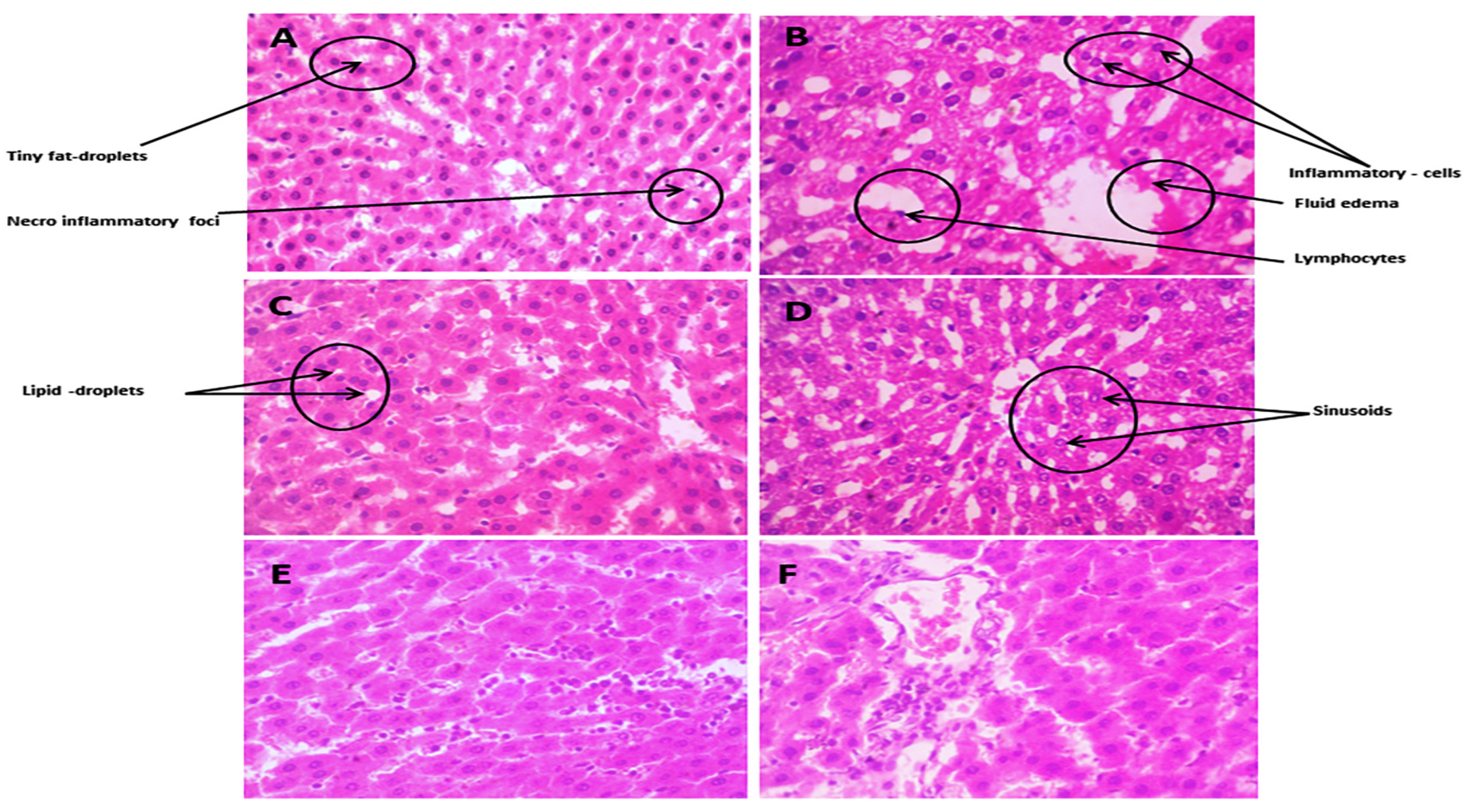
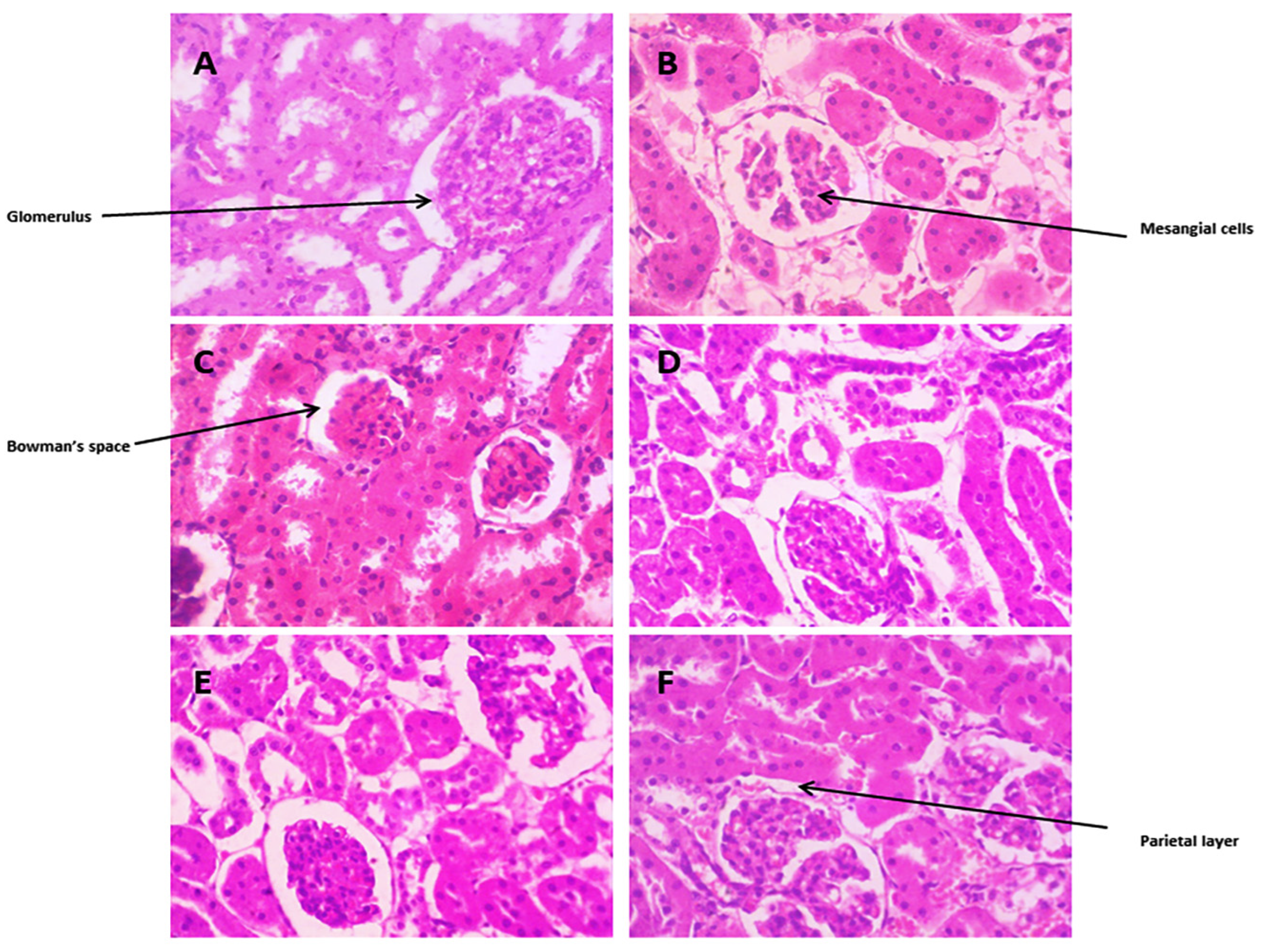
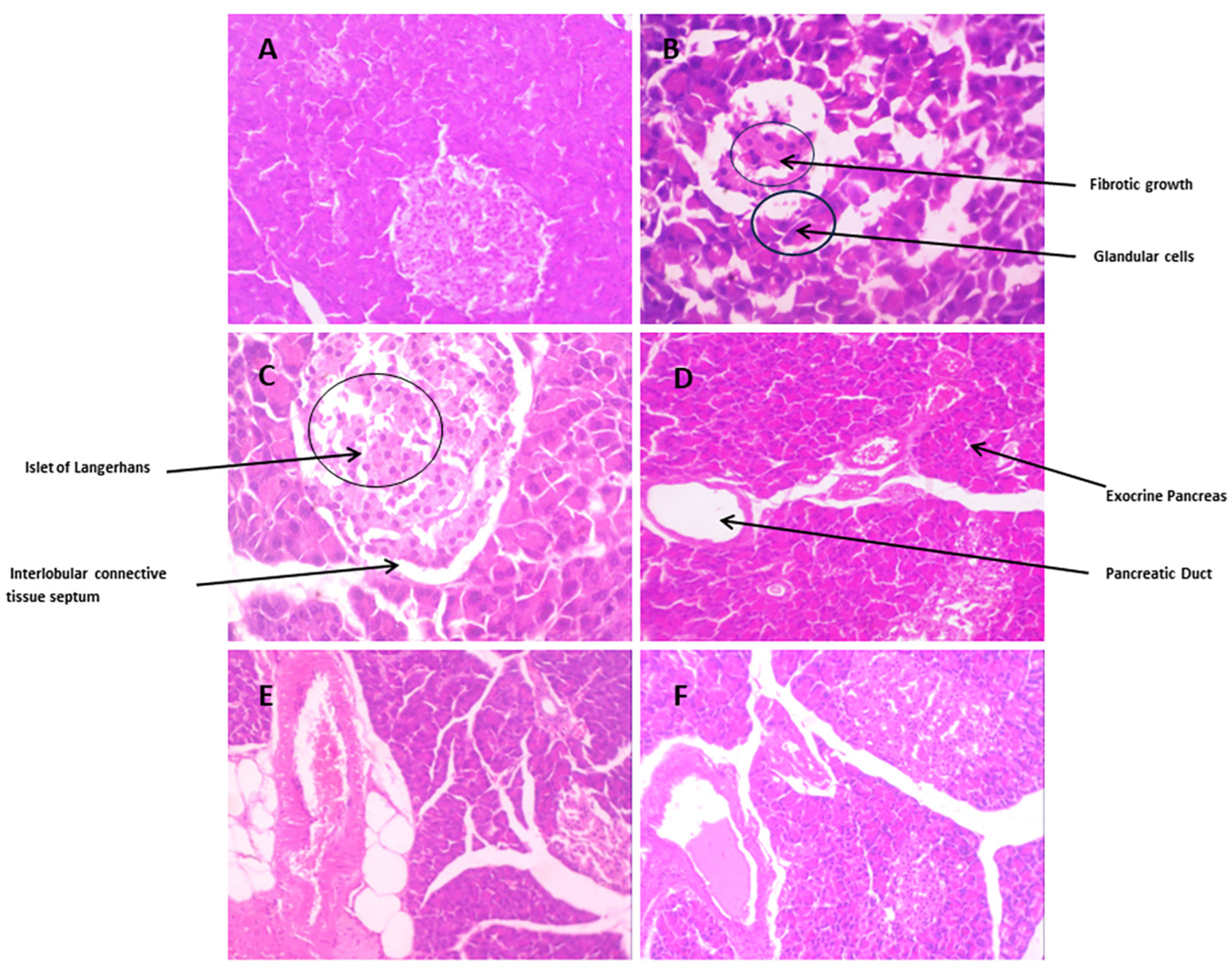
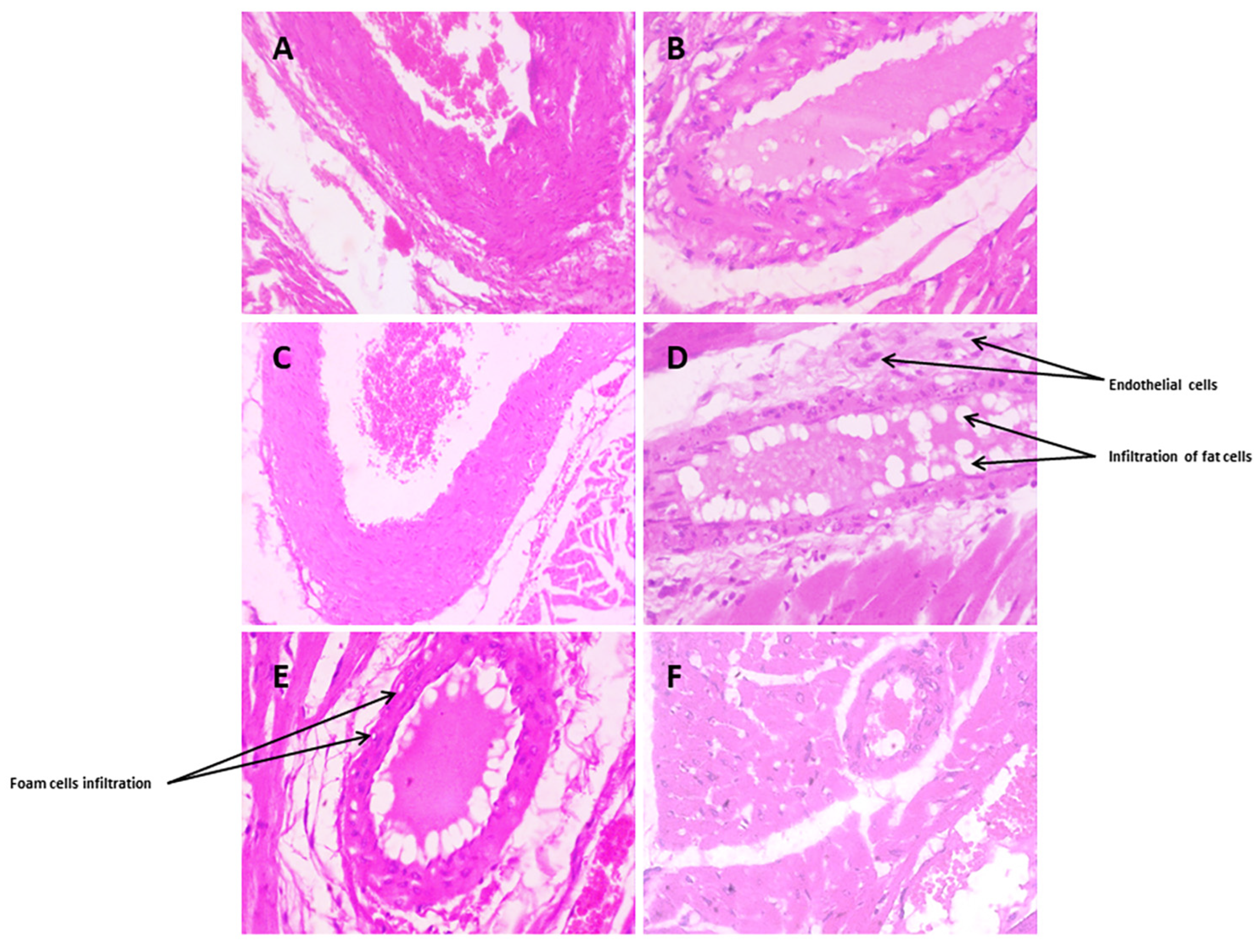
3.3.10. Histopathological Changes
Liver
Kidney
Pancreatic
Coronary Blood Vessels
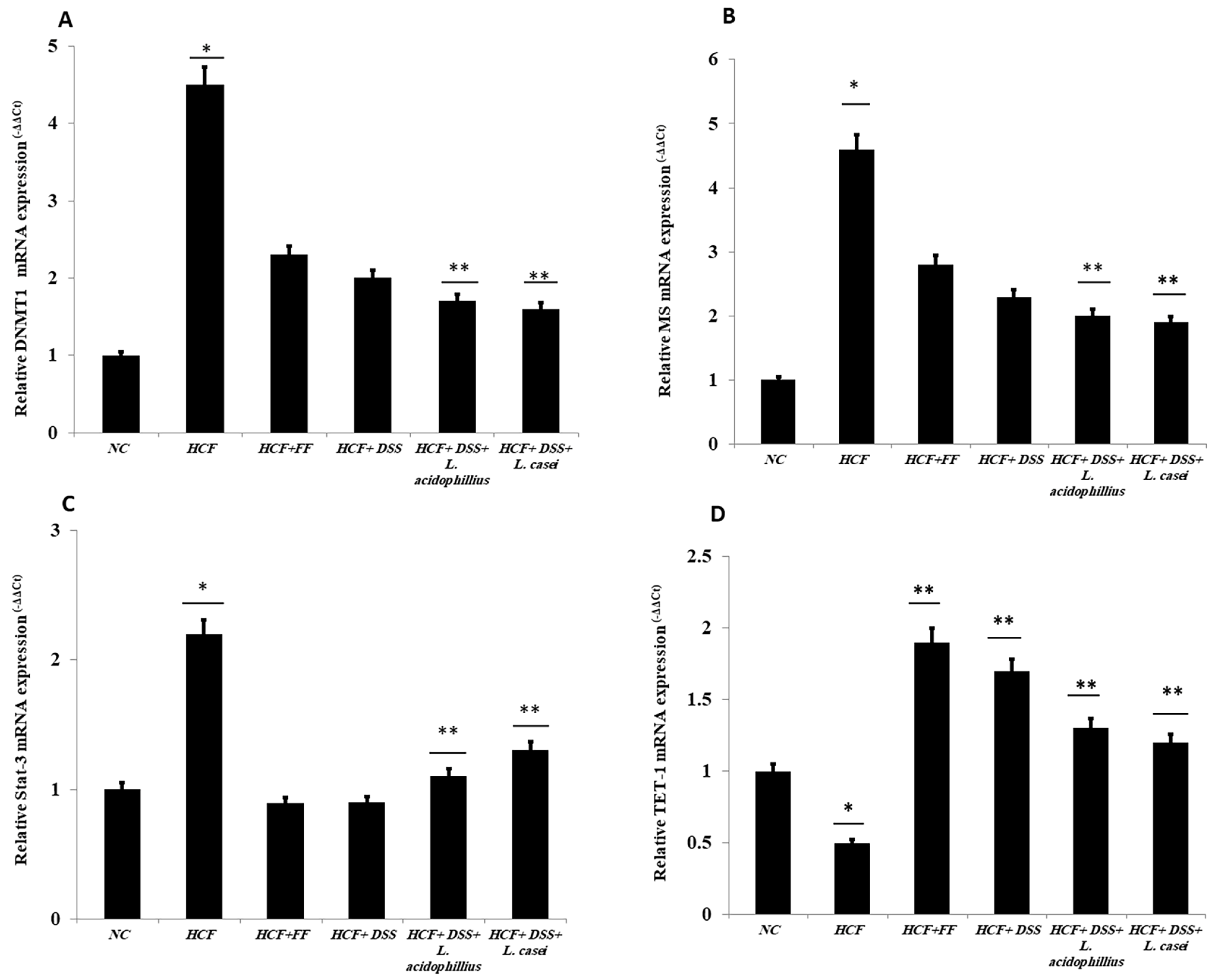
3.4. Quantitative RT-PCR
3.5. In Silico Study
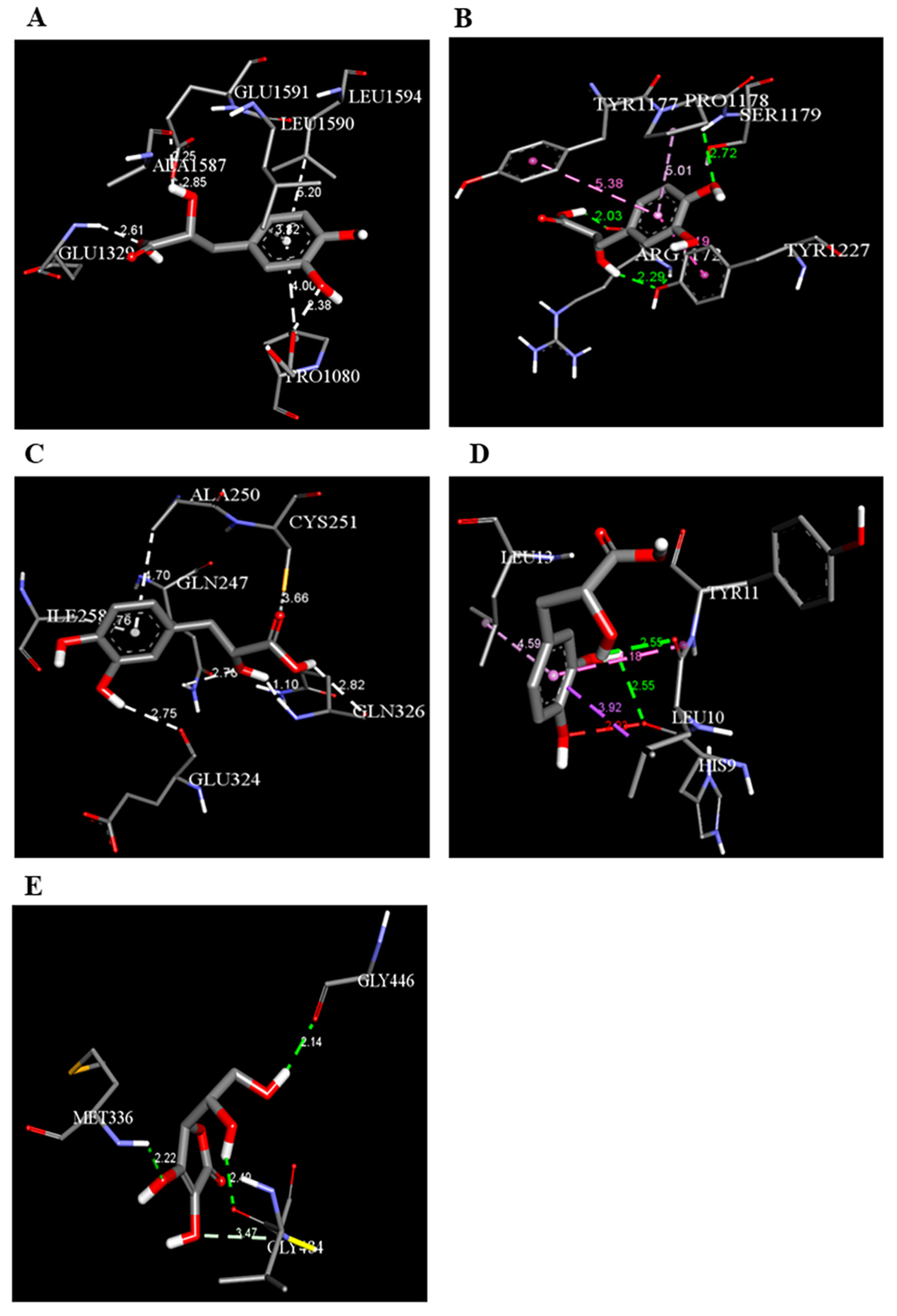
3.5.1. Molecular Docking
3.5.2. ADMET and Drug-Likeness Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyun, J.; Jung, Y. DNA methylation in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2020, 21, 8138. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Gallego-Durán, R.; Gallego, P.; Grande, L. Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Zhang, J.Y.; Song, S.D.; Qu, K.; Xu, X.S.; Liu, S.S.; Liu, C. Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World J. Gastroenterol. 2015, 21, 1650. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E.; Dhawan, A. Childhood and adolescent nonalcoholic fatty liver disease: Is it different from adults? J. Clin. Exp. Hepatol. 2019, 9, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Slomko, H.; Heo, H.J.; Einstein, F.H. Minireview: Epigenetics of obesity and diabetes in humans. Endocrinology 2012, 153, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, R.S.; Bird, A.P. CpG islands—‘A rough guide’. FEBS Lett. 2009, 583, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Reik, W.; Dean, W. DNA methylation and mammalian epigenetics. Electrophoresis 2001, 22, 2838–2843. [Google Scholar] [CrossRef]
- Guo, J.U.; Su, Y.; Zhong, C.; Ming, G.L.; Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 2011, 145, 423–434. [Google Scholar] [CrossRef]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- Jiao, J.; Sanchez, J.I.; Saldarriaga, O.A.; Solis, L.M.; Tweardy, D.J.; Maru, D.M.; Stevenson, H.L.; Beretta, L. Spatial molecular and cellular determinants of STAT3 activation in liver fibrosis progression in non-alcoholic fatty liver disease. JHEP Rep. 2023, 5, 100628. [Google Scholar] [CrossRef]
- Mendoza, J.; Purchal, M.; Yamada, K.; Koutmos, M. Structure of full-length cobalamin-dependent methionine synthase and cofactor loading captured in crystallo. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hernández, N.E.; Tereschuk, M.L.; Abdala, L.R. Antimicrobial activity of flavonoids in medicinal plants from Tafı del Valle (Tucuman, Argentina). J. Ethnopharmacol. 2000, 73, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Polya, G. Biochemical Targets of Plant Bioactive Compounds: A Pharmacological Reference Guide to Sites of Action and Biological Effects; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Maruthanila, V.L.; Poornima, J.; Mirunalini, S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3, 3′-diindolylmethane: A therapeutic marvel. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 832161. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Yamamoto, K.; Katrich, E.; Leontowicz, H.; Lojek, A.; Leontowicz, M.; Cíz, M.; Goshev, I.; Shalev, U.; Trakhtenberg, S. Antioxidative properties of Jaffa sweeties and grapefruit and their influence on lipid metabolism and plasma antioxidative potential in rats. Biosci. Biotechnol. Biochem. 2003, 67, 907–910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bao, X.Y.; Zheng, Q.; Tong, Q.; Zhu, P.C.; Zhuang, Z.; Zheng, G.Q.; Wang, Y. Danshensu for myocardial ischemic injury: Preclinical evidence and novel methodology of quality assessment tool. Front. Pharmacol. 2018, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Wu, L.; Tang, Y.; Zhou, G.; Qu, C.; Duan, J.A. Chemical analysis of the herbal medicine Salviae miltiorrhizae Radix et Rhizoma (Danshen). Molecules 2016, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Jovanović Stojanov, S.; Ntungwe, E.N.; Dinić, J.; Podolski-Renić, A.; Pajović, M.; Rijo, P.; Pešić, M. Coleon U, Isolated from Plectranthus mutabilis Codd., Decreases P-Glycoprotein Activity Due to Mitochondrial Inhibition. Pharmaceutics 2023, 15, 1942. [Google Scholar] [CrossRef]
- Won, G.; Choi, S.I.; Park, N.; Kim, J.E.; Kang, C.H.; Kim, G.H. In vitro antidiabetic, antioxidant activity, and probiotic activities of Lactiplantibacillus plantarum and Lacticaseibacillus paracasei strains. Curr. Microbiol. 2021, 78, 3181–3191. [Google Scholar] [CrossRef]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef]
- Hadi, A.; Sepandi, M.; Marx, W.; Moradi, S.; Parastouei, K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102216. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Isbilen, A.; Eyupoglu, I.; Sag, E.; ÖREM, A.; Sen, T.; Kaklikkaya, N.; Kaya, G. Effects of long-term synbiotic supplementation in addition to lifestyle changes in children with obesity-related non-alcoholic fatty liver disease. Turk. J. Gastroenterol. 2017, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chan, J.Y.; Lee, C.T.; Tain, Y.L. Hypertension programmed by perinatal high-fat diet: Effect of maternal gut microbiota-targeted therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Li, J.X.; Chen, J.Y.; Chen, P.Y.; Ma, L.R.; Zhang, G.L.; Yan, P.Y. Gut microbiota: A potential target for traditional Chinese medicine intervention in coronary heart disease. Chin. Med. 2021, 16, 108. [Google Scholar] [CrossRef]
- Nsimba, R.Y.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kumari, S.; Deori, M.; Elancheran, R.; Kotoky, J.; Devi, R. In vitro and in vivo antioxidant, anti-hyperlipidemic properties and chemical characterization of Centella asiatica (L.) extract. Front. Pharmacol. 2016, 7, 400. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Sreejayan, X.X.; Rao, M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; Elsayed, A.F.M.; Abd, E.L.; Maksoud, A.I. Investigation of Angiogenesis and Wound Healing Potential Mechanisms of Zinc Oxide Nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef]
- Hassan, A.; Al-Salmi, F.A.; Abuamara, T.M.M.; Matar, E.R.; Amer, M.E.; Fayed, E.M.M.; Hablas, M.G.A.; Mohammed, T.S.; Ali, H.E.; EL-fattah, F.M.A.; et al. Ultrastructural analysis of zinc oxide nanospheres enhances anti-tumor efficacy against Hepatoma. Front. Oncol. 2022, 12, 933750. [Google Scholar] [CrossRef]
- Feng, J.; Fitz, Y.; Li, Y.; Fernandez, M.; Puch, I.C.; Wang, D.; Solomon, S.B. Catheterization of the carotid artery and jugular vein to perform hemodynamic measures, infusions and blood sampling in a conscious rat model. JoVE (J. Vis. Exp.) 2015, 95, e51881. [Google Scholar]
- Friedwald’s, W.T.; Levy, I.R.; Frederickson, S.D. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of superoxide anion radical in auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Hassan, A.; Al-Salmi, F.A.; Saleh, M.A.; Sabatier, J.M.; Alatawi, F.A.; Alenezi, M.A.; Albalwe, F.M.; Meteq, R.; Albalawi, H.; Darwish, D.B.E.; et al. Inhibition Mechanism of Methicillin-Resistant Staphylococcus aureus by Zinc Oxide Nanorods via Suppresses Penicillin-Binding Protein 2a. ACS Omega 2023, 8, 9969–9977. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.C.; Ho, C.T. Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Sharaf, E.M.; Hassan, A.; Al-Salmi, F.A.; Albalwe, F.M.; Albalawi, H.M.R.; Darwish, D.B.; Fayad, E. Synergistic antibacterial activity of compact silver/magnetite core-shell nanoparticles core shell against Gram-negative foodborne pathogens. Front. Microbiol. 2022, 13, 929491. [Google Scholar] [CrossRef]
- Khella, K.F.; El Maksoud, A.I.A.; Hassan, A.; Abdel-Ghany, S.E.; Elsanhoty, R.M.; Aladhadh, M.A.; Abdel-Hakeem, M.A. Carnosic acid encapsulated in albumin nanoparticles induces apoptosis in breast and colorectal cancer cells. Molecules 2022, 27, 4102. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Cândido, T.M.; Ariede, M.B.; Pinto, C.A.S.d.O.; Lima, F.V.; Magalhães, W.V.; Pedro, N.M.E.; Padovani, G.; Sufi, B.d.S.; Rijo, P.; Velasco, M.V.R.; et al. Rosmarinic Acid Multifunctional Sunscreen: Comet Assay and In Vivo Establishment of Cutaneous Attributes. Cosmetics 2022, 9, 141. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Zhang, X. On changes of activity of antioxidases in hippocampus of rats with multi-infarct dementia and the intervention effects of acupuncture. China J. Tradit. Chin. Med. Pharm. 2005, 20, 724–726. [Google Scholar]
- Kumari, S.; Elancheran, R.; Kotoky, J.; Devi, R. Rapid screening and identification of phenolic antioxidants in Hydrocotyle sibthorpioides Lam. by UPLC–ESI-MS/MS. Food Chem. 2016, 203, 521–529. [Google Scholar] [CrossRef]
- Petitjean, S.J.; Lecocq, M.; Lelong, C.; Denis, R.; Defrère, S.; Mariage, P.A.; Alsteens, D.; Pilette, C. Salvia miltiorrhiza Bunge as a potential natural compound against COVID-19. Cells 2022, 11, 1311. [Google Scholar] [CrossRef]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G. Salvia miltiorrhiza in treating cardiovascular diseases: A review on its pharmacological and clinical applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Danford, C.J.; Yao, Z.; Jiang, Z.G. Non-alcoholic fatty liver disease: A narrative review of genetics. J. Biomed. Res. 2018, 32, 389. [Google Scholar]
- Melton, P.E.; Burton, M.A.; Lillycrop, K.A.; Godfrey, K.M.; Rauschert, S.; Anderson, D.; Burdge, G.C.; Mori, T.A.; Beilin, L.J.; Ayonrinde, O.T.; et al. Differential DNA methylation of steatosis and non-alcoholic fatty liver disease in adolescence. Hepatol. Int. 2023, 17, 584–594. [Google Scholar] [CrossRef]
- Matias, D.; Nicolai, M.; Fernandes, A.S.; Saraiva, N.; Almeida, J.; Saraiva, L.; Faustino, C.; Díaz-Lanza, A.M.; Reis, C.P.; Rijo, P. Comparison Study of Different Extracts of Plectranthus madagascariensis, P. neochilus and the Rare P. porcatus (Lamiaceae): Chemical Characterization, Antioxidant, Antimicrobial and Cytotoxic Activities. Biomolecules 2019, 9, 179. [Google Scholar] [CrossRef]
- Ntungwe, E.; Domínguez-Martín, E.M.; Bangay, G.; Garcia, C.; Guerreiro, I.; Colombo, E.; Saraiva, L.; Díaz-Lanza, A.M.; Rosatella, A.; Alves, M.M.; et al. Self-Assembly Nanoparticles of Natural Bioactive Abietane Diterpenes. Int. J. Mol. Sci. 2021, 22, 10210. [Google Scholar] [CrossRef] [PubMed]
- Ntungwe, E.; Domínguez-Martín, E.M.; Teodósio, C.; Teixidó-Trujillo, S.; Armas Capote, N.; Saraiva, L.; Díaz-Lanza, A.M.; Duarte, N.; Rijo, P. Preliminary Biological Activity Screening of Plectranthus spp. Extracts for the Search of Anticancer Lead Molecules. Pharmaceuticals 2021, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, M.; Mota, J.; Fernandes, A.S.; Pereira, F.; Pereira, P.; Reis, C.P.; Robles Velasco, M.V.; Baby, A.R.; Rosado, C.; Rijo, P. Assessment of the Potential Skin Application of Plectranthus ecklonii Benth. Pharmaceuticals 2020, 13, 120. [Google Scholar] [CrossRef]
- Potential Mechanisms Underlying the Hepatic–Protective Effects of Danshensu on Iron Overload Mice. Biol. Pharm. Bull. 2020, 43, 968–975. [CrossRef]
- Gao, Y.; Wang, N.; Zhang, Y.; Ma, Z.; Guan, P.; Ma, J.; Zhang, Y.; Zhang, X.; Wang, J.; Zhang, J.; et al. Mechanism of protective effects of Danshen against iron overload-induced injury in mice. J. Ethnopharmacol. 2013, 145, 254–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xie, Y.; Gao, Y.; Ma, J.; Yuan, J.; Li, J.; Wang, J.; Li, L.; Zhang, J.; et al. Multitargeted inhibition of hepatic fibrosis in chronic iron-overloaded mice by Salvia miltiorrhiza. J. Ethnopharmacol. 2013, 148, 671–681. [Google Scholar] [CrossRef]
- Aigner, E.; Weiss, G.; Datz, C. Dysregulation of iron and copper homeostasis in nonalcoholic fatty liver. World J. Hepatol. 2015, 7, 177–188. [Google Scholar] [CrossRef]
- Sitarek, P.; Synowiec, E.; Kowalczyk, T.; Bangay, G.; Śliwiński, T.; Picot, L.; Princiotto, S.; Rijo, P. Anticancer Properties of Plectranthus ornatus-Derived Phytochemicals Inducing Apoptosis via Mitochondrial Pathway. Int. J. Mol. Sci. 2022, 23, 11653. [Google Scholar] [CrossRef]
- Farouk, F.; Zarka, M.A.; Al-Sawahli, M.M.; Hassan, A.; Mohamed, A.F.; Ibrahim, I.M.; Mohammed, F.A.E.-R.; Shebl, R.I. Rosmarinic acid inhibits Rift Valley fever virus: In vitro, computational and analytical studies. Future Virol. 2023, 18, 1001–1019. [Google Scholar] [CrossRef]
- Domínguez-Martín, E.M.; Magalhães, M.; Díaz-Lanza, A.M.; Marques, M.P.; Princiotto, S.; Gómez, A.M.; Efferth, T.; Cabral, C.; Rijo, P. Phytochemical Study and Antiglioblastoma Activity Assessment of Plectranthus hadiensis (Forssk.) Schweinf. ex Sprenger var. hadiensis Stems. Molecules 2022, 27, 3813. [Google Scholar] [CrossRef]
- Devi, R.; Sharma, D. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol. 2004, 90, 63–68. [Google Scholar] [CrossRef]
- Stein, O.; Stein, Y. Atheroprotective mechanisms of HDL. Atherosclerosis 1999, 144, 285–301. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Hanif, S.; Iftkhar, T. Phytochemical profiling with antioxidant and antimicrobial screening of Amaranthus viridis L. leaf and seed extracts. Open J. Med. Microbiol. 2013, 3, 37050. [Google Scholar] [CrossRef]
- Pal, R.; Girhepunje, K.; Shrivastav, N.; Hussain, M.M.; Thirumoorthy, N. Antioxidant and free radical scavenging activity of ethanolic extract of Morinda citrifolia. Ann. Biol. Res. 2011, 2, 127–131. [Google Scholar]
- Deori, M.; Boruah, D.C.; Devi, D.; Devi, R. Antioxidant and antigenotoxic effects of pupae of the muga silkworm Antheraea assamensis. Food Biosci. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Kang, S.C.; Baek, K.H. Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pac. J. Trop. Med. 2014, 7, 9–15. [Google Scholar] [CrossRef]
- Chidambaram, U.; Pachamuthu, V.; Natarajan, S.; Elango, B.; Ramkumar, K.M. In vitro evaluation of free radical scavenging activity of Codariocalyx motorius root extract. Asian Pac. J. Trop. Med. 2013, 6, 188–194. [Google Scholar] [CrossRef]
- Bode, J.G.; Albrecht, U.; Häussinger, D.; Heinrich, P.C.; Schaper, F. Hepatic acute phase proteins–regulation by IL-6-and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 2012, 91, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qi, Y.F.; Yu, Y.R. STAT3: A key regulator in liver fibrosis. Ann. Hepatol. 2021, 21, 100224. [Google Scholar] [CrossRef]
- Seo, H.Y.; Kim, M.K.; Lee, S.H.; Hwang, J.S.; Park, K.G.; Jang, B.K. Kahweol ameliorates the liver inflammation through the inhibition of NF-κB and STAT3 activation in primary Kupffer cells and primary hepatocytes. Nutrients 2018, 10, 863. [Google Scholar] [CrossRef]
- Khalil, H.; Nada, A.H.; Mahrous, H.; Hassan, A.; Rijo, P.; Ibrahim, I.A.; Moahmed, D.D.; AL-Salmi, F.A.; Mohamed, D.D.; Elmaksoud, A.I.A. Amelioration effect of 18β-Glycyrrhetinic acid on methylation inhibitors in hepatocarcinogenesis -induced by diethylnitrosamine. Front. Immunol. 2024, 14, 1206990. [Google Scholar] [CrossRef]

| Primer | Sequence (5′-3′) |
|---|---|
| DNMT-1 | F: AGGAATGTGTGAAGGAGAAATTG |
| R: CTTGAACGCTTAGCCTCTCCATC | |
| MS | F: AGAAGAGGATTATGGTGCTGGATG |
| R: TCTTAATTCCTGTCTGGAGAGTT | |
| STAT-3 | F: ACCCAACAGCCGCCGTAG |
| R: CAGACTGGTTGTTTCCATTCAGAT | |
| TET1 | F: ACTCCCTGAGGTCTGTCCTGGGA |
| R: GGATCGAGACATAGCTACAGAGT | |
| GAPDH | F: CAGGTTGTCTCCTGCGACTT |
| R: TATGGG GGTCTGGGATGGAA |
| Parameter | Danshensu Extracts |
|---|---|
| Total Polyphenol Content (TPC) | 111.9 ± 216 mg GAE g−1 |
| Total flavonoids (TF) | 33.79 ± 1.89 mg QE g−1 |
| Group | Initial Body Weight-1st Day (g) | Final Body Weight-28th Day (g) |
|---|---|---|
| NC | 170.5 ± 2.31 | 182 ± 2.8 |
| HCF | 185.2 ± 2.75 | 225 ± 3.22 * |
| HCF + FF | 176.2 ± 3.93 | 181 ± 2.89 |
| HCF + DSS | 175.3 ± 3.43 | 202 ± 3.11 * |
| HCF + DSS + L. acidophillius | 177.22 ± 2.31 | 190 ± 3.61 |
| HCF + DSS + L. casei | 174.22 ± 3.91 | 187 ± 2.3 |
| Parameters (mg/dL) | NC | HCF | HCF + FF | HCF + DSS | HCF + DSS + L. acidophillius | HCF + DSS + L. casei |
|---|---|---|---|---|---|---|
| TC | 73.04 ± 2.21 | 153.44 ± 3.21 | 126.12 ± 5.1 * | 112 ± 3.7 * | 94 ± 2.9 | 91 ± 1.67 |
| TG | 61.43 ± 2.31 | 121.21 ± 2.1 | 103.31 ± 3.97 * | 94.18 ± 2.3 | 81.47 ± 2.1 | 77.31 ± 3.6 |
| HDL | 62.59 ± 3.62 | 33.54 ± 4.85 | 41.18 ± 2.1 | 50.45 ± 3.6 | 56.12 ± 3.4 | 61.11 ± 1.9 |
| LDL | 51 ± 2.33 ** | 141.32 ± 3.66 | 89.37 ± 5.9 | 63.17 ± 2.8 | 56.41 ± 1.6 | 52.17 ± 3.6 |
| VLDL | 15.86 ± 2.73 | 23.37 ± 3.97 | 20.47 ± 4.1 | 18.3 ± 1.09 | 16.8 ± 1.03 * | 15.9 ± 0.97 * |
| Parameters | NC | HCF | HCF + FF | HCF + DSS | HCF + DSS + L. acidophillius | HCF + DSS + L. casei | |
|---|---|---|---|---|---|---|---|
| Serum | SOD a | 39.1 ± 1.14 | 17.2 ± 2 ++ | 25 ± 3.6 | 29.6 ± 4.5 | 34.1 ± 3.4 | 38.6 ± 2.6 ++ |
| GSH b | 137 ± 4.5 | 203 ± 5.7 | 113 ± 3.1 | 121 ± 1.6 | 129 ± 4.3 | 135 ± 6.5 | |
| TBARS c | 26.2 ± 1.1 | 41.8 ± 4.1 | 36.3 ± 3.81 | 31.23 ± 2.2 | 29.1 ± 1.8 | 27.3 ± 2.1 | |
| NO d | 28.12 ± 1.32 | 51.4 ± 1.36 | 22.91 ± 1.51 | 36.23 ± 2.6 | 30.19 ± 1.23 | 25.31 ± 3.1 | |
| Liver | SOD a | 88 ± 1.3 | 51 ± 1.2 + | 69 ± 1.4 | 73 ± 1.2 | 79 ± 1.1 | 84 ± 1.3 ** |
| GSH b | 339 ± 8.6 | 265 ± 6.1 | 302 ± 5.7 | 309 ± 4.4 | 329 ± 5.7 | 343 ± 6.4 | |
| TBARS c | 96.4 ± 5.2 | 161 ± 7.6 ++ | 142 ± 8.39 | 116 ± 5.6 | 104 ± 6.5 | 89.2 ± 7.4 ** | |
| NO d | 21.3 ± 2.3 | 43.2 ± 2.1 | 27.1 ± 3.4 | 21.2 ± 2.1 | 16.1 ± 2.3 | 14.3 ± 0.6 | |
| Heart | SOD a | 69.4 ± 3.6 | 35.1 ± 1.4 + | 61.1 ± 2.5 | 62.2 ± 1.5 | 68.13 ± 0.98 | 69.1 ± 1.31 * |
| GSH b | 123 ± 4.3 | 81 ± 3.4 ++ | 95 ± 4.3 | 101 ± 2.3 * | 112 ± 5.8 ** | 117 ± 6.4 *** | |
| TBARS c | 36.2 ± 1.4 | 53.4 ± 4.3 | 41.1 ± 2.1 | 34.1 ± 3.2 | 30 ± 2.3 | 29 ± 2.1 | |
| NO d | 15.1 ± 1.3 | 25.2 ± 1.4 | 21.16 ± 2.2 | 17.14 ± 1.6 | 15.10 ± 1.5 | 12.12 ± 1.1 | |
| Compound | Protein | ∆G (kcal/mol) | 2D | Interactions |
|---|---|---|---|---|
| Danshensu | DNAT-1 | 5.9 | 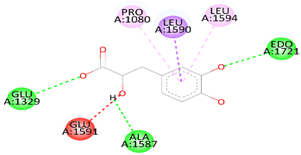 | Hydrogen bond (GLU 1329, ALA 1587 and, EDO1721), Pi-sigma LEU1591), Pi-Alkyl (LEU 1594 and, PRO1080), unfavorable acceptor -acceptor bond (GLU 1591) |
| Danshensu | MS | −5.7 | 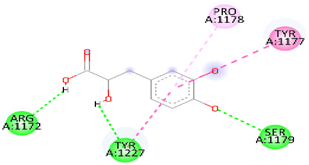 | Hydrogen bond (ARG 1172, TYR1227 and, SER1179), Pi-sigma (LEU1591), Pi-Alkyl(PRO1178), and Pi-Pi Stacked (TYR 1177) |
| Danshensu | STAT-3 | −5.3 | 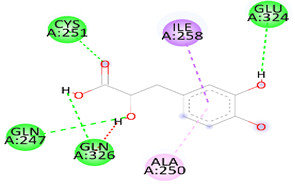 | Hydrogen bond (GLN 247, GLN326 GLU 324 and, CYS 251), Pi-sigma (LEU1591), Pi-Alkyl(ALA 250), and Pi-Pi Sigma (ILE 258) Unfavorable Donor -Donor bond (GLN 326) |
| Danshensu | TET-1 | −4.0 | 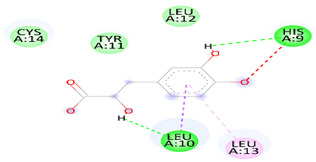 | Van der Waals bond (CYS 14, LEU 12, TYR 12), Hydrogen bond (HIS 9, LEU 10)), Pi-sigma (LEU10), Pi-Alkyl (LEU 13) and, Unfavorable acceptor -acceptor bond (HIS 9) |
| Danshensu | HMG | −5.0 | 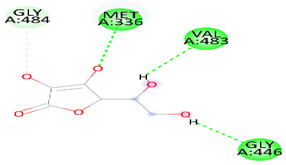 | Hydrogen bond (MET 336, VAL483 GLY 446) and, Carbon Hydrogen Bond (CLY 484) |
| Compound | Absorption Intestinal Human Absorption | Distribution | Metabolism | AMES Toxicity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log p | Log S | CYP2D6 Substrate | CYP3A4 Substrate | CYP1A2 Inhibitior | CYP2C19 Inhibitior | CYP2C9 Inhibitior | CYP2D6 Inhibitior | CYP3A4 Inhibitior | Total Clearance | |||
| Danshensu | 41.771 | 0.0858 | −1.40 | No | No | No | No | No | No | No | 0.444 | No |
| Compound | Molecular Weight | HBA | HBD | mlogP | Synthetic Accessibility | Bioavailability | Lipinski Violation | Drug Likeness |
|---|---|---|---|---|---|---|---|---|
| Danshensu | 470.518 g/mol | 5 | 4 | −0.04 | 1.91 | 0.56 | 0 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.; Rijo, P.; Abuamara, T.M.M.; Ali Lashin, L.S.; Kamar, S.A.; Bangay, G.; Al-Sawahli, M.M.; Fouad, M.K.; Zoair, M.A.; Abdalrhman, T.I.; et al. Synergistic Differential DNA Demethylation Activity of Danshensu (Salvia miltiorrhiza) Associated with Different Probiotics in Nonalcoholic Fatty Liver Disease. Biomedicines 2024, 12, 279. https://doi.org/10.3390/biomedicines12020279
Hassan A, Rijo P, Abuamara TMM, Ali Lashin LS, Kamar SA, Bangay G, Al-Sawahli MM, Fouad MK, Zoair MA, Abdalrhman TI, et al. Synergistic Differential DNA Demethylation Activity of Danshensu (Salvia miltiorrhiza) Associated with Different Probiotics in Nonalcoholic Fatty Liver Disease. Biomedicines. 2024; 12(2):279. https://doi.org/10.3390/biomedicines12020279
Chicago/Turabian StyleHassan, Amr, Patrícia Rijo, Tamer M. M. Abuamara, Lashin Saad Ali Lashin, Sherif A. Kamar, Gabrielle Bangay, Majid Mohammed Al-Sawahli, Marina K. Fouad, Mohammad A. Zoair, Tamer I. Abdalrhman, and et al. 2024. "Synergistic Differential DNA Demethylation Activity of Danshensu (Salvia miltiorrhiza) Associated with Different Probiotics in Nonalcoholic Fatty Liver Disease" Biomedicines 12, no. 2: 279. https://doi.org/10.3390/biomedicines12020279
APA StyleHassan, A., Rijo, P., Abuamara, T. M. M., Ali Lashin, L. S., Kamar, S. A., Bangay, G., Al-Sawahli, M. M., Fouad, M. K., Zoair, M. A., Abdalrhman, T. I., Elebeedy, D., Ibrahim, I. A., Mohamed, A. F., & Abd El Maksoud, A. I. (2024). Synergistic Differential DNA Demethylation Activity of Danshensu (Salvia miltiorrhiza) Associated with Different Probiotics in Nonalcoholic Fatty Liver Disease. Biomedicines, 12(2), 279. https://doi.org/10.3390/biomedicines12020279